Abstract
Ceramide is a product of agonist-induced sphingolipid metabolism in several cell types, including polymorphonuclear leukocytes (PMNs). In adherent PMNs, the kinetics of ceramide production correspond with the termination of fMLP-stimulated H2O2 release. Furthermore, short chain ceramides inhibit fMLP-mediated H2O2 release in adherent PMNs. In the present study, we investigated the effects of short chain ceramides and sphingoid bases on phagocytosis of IgG-opsonized erythrocytes (EIgG) by suspended PMNs activated with fMLP. N-Acetylsphingosine, N-acetylphytosphingosine, phytosphingosine, sphingosine, and dihydrosphingosine, but not N-acetyldihydrosphingosine, inhibited phagocytosis of EIgG. In contrast, these same lipids did not inhibit fMLP-mediated chemotaxis. Endogenous ceramide levels increased within the first few minutes of phagocytosis, with a significant (P < .05) accumulation by 30 minutes, the time by which phagocytosis was terminated. Neutral sphingomyelinase activity paralleled the increase in ceramide, consistent with the generation of ceramide by the hydrolysis of sphingomyelin. The N-acetyl-conjugated sphingols (C2 ceramides) blocked phosphatidylethanol formation indicating that phospholipase D (PLD) is an intracellular target of ceramide action. These data suggest that ceramides, generated through activation of the sphingomyelin cycle, act as negative regulators of FcγR-mediated phagocytosis.
POLYMORPHONUCLEAR leukocytes (PMNs) are recruited to sites of tissue injury and inflammation where they ingest and destroy foreign pathogens, remove immune complexes, clear away damaged tissue, and initiate wound repair. Pathogens and other “foreign particles” at these sites are ingested by phagocytosis and are contained within phagosomes. Lysosomal enzymes and reactive oxygen metabolites are delivered to nascent phagosomes where they combine to destroy the pathogen. This highly regulated and directed delivery of destructive products into phagosomes minimizes damage to surrounding tissues. Unactivated PMNs are inefficient at particle engulfment. This inefficiency restricts the release of damaging products that accompany phagocytosis to sites of PMN activation. However, upon cell contact with extracellular matrix proteins, chemoattractants, and cytokines, molecules abundant at sites of infection and tissue injury, PMN phagocytosis of opsonized targets is greatly enhanced.1 The enhanced phagocytosis is reflected in both the number of targets ingested per cell, as well as in the total number of PMNs engaged in phagocytosis.
Phagocytosis, whether in unactivated or activated PMNs, generally involves two different receptor classes: (1) Fcγ receptors recognizing the Fc domain of IgG and (2) complement receptors recognizing the C3b/C3bi fragments of the complement system.2 The signaling pathways involved in phagocytosis appear to be determined by the activation state of the PMNs, as well as the type of agonist used for activation. For example, ingestion of IgG-opsonized targets by unstimulated PMNs requires phospholipase D (PLD) activation and is sensitive to elevated levels of adenosine-3′,5′-cyclic monophosphate (cAMP).3,4 This response does not require elevated [Ca2+]i or protein kinase C (PKC) activation and is insensitive to treatment with pertussis toxin.4-6 In PMNs activated with tumor necrosis factor (TNF )α, phagocytosis is sensitive to both pertussis and cholera toxins, as well as inhibitors of PKC.6,7 In contrast, increased phagocytosis in response to fMLP is independent of PKC and requires an increase in [Ca2+]i associated with the ligation of Fcγ receptors.8 Thus, although phagocytosis involves many different signaling pathways, the molecular details of these signal transduction pathways have not been clearly defined.
PLD activation coincides with phagocytosis in both unactivated and activated PMNs.3,4,9,10 The phosphatidic acid (PA), formed by the action of PLD on phosphatidylcholine, can act as a Ca2+ ionophore and possesses fusogenic properties.11 Thus, the PA generated during phagocytosis may promote the fusion of secretory granules with newly formed phagosomes. However, in adherent PMNs activated with fMLP, an increase in PA alone is not sufficient to stimulate degranulation.12 In these cells, it is the conversion of PA to diradylglycerol (DRG) that is critical for the fusion of secretory granules with the plasma membrane. Another downstream target of DRG may be the activation of PKC. Fällman et al9 reported that complement receptor-mediated phagocytosis leads to PLD activation, which results in DRG formation and the activation of PKC. In turn, PKC phosphorylates the MARCKS protein, a protein that modulates membrane-cytoskeleton interactions, and they speculate that this may regulate pseudopod formation during phagocytosis.9 Despite the speculation about the role of these second messengers in phagocytosis, very little is actually known.
Sphingolipid metabolism, like glycerophospholipid metabolism, gives rise to products that function as second messengers.13 Over the last several years, ceramide has emerged as a second messenger mediating proliferation, differentiation, gene expression, and apoptosis.14-17 In the present study, we examined the role of ceramides in the phagocytosis of IgG-opsonized erythrocytes by PMNs. We observed that both C2 ceramides and related sphingoid bases were inhibitors of Fc-mediated phagocytosis by PMNs in suspension activated with fMLP. N-acetylsphingosine and N-acetylphytosphingosine blocked phosphatidylethanol and phosphatidic acid formation, demonstrating that PLD was an intracellular target of ceramide action. Ceramide levels increased within the first few minutes of phagocytosis, consistent with a role for ceramide in the regulation of phagocytosis by fMLP-activated PMNs in suspension. Neutral sphingomyelinase activity increased in parallel with ceramide, suggesting that ceramide was being generated by the hydrolysis of sphingomyelin. Our data suggest that ceramide is an important signaling molecule in PMN phagocytosis.
MATERIALS AND METHODS
Reagents.fMLP, imidazole, 4β-phorbol 12β-myristate 13α-acetate (PMA), diethylene-triaminepentaacetic acid (DETAPAC), PLD (cabbage type I), O-phenylenediamine hydrochloride, cardiolipin from bovine heart, n-octyl β-D-glucopyranoside, 1,2-dioleoyl-sn-glycerol, 1,2-dioleoyl-sn-phosphatidyl choline, sphingosine, DL-erythro-dihydrosphingosine, stearylamine, and phytosphingosine hydrochloride were purchased from Sigma Chemical Co (St Louis, MO). sn-1,2-Diacylglycerol kinase from Escherichia coli and dithiothreitol were purchased from Calbiochem (San Diego, CA). Silica gel 60 thin layer chromatography plates were purchased from EM Separations (Gibbstown, NJ); N-6-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino hexanoyl sphingosylphosphocholine (NBD-C6-sphingomyelin) was from Molecular Probes (Eugene, OR); [γ-32P]-adenosine-5′-triphosphate was from ICN Pharmaceuticals, Inc (Irvine, CA); and 1-O-[3H]-octadecyl-sn-glycero-3-phosphocholine was from Amersham Corp (Arlington Heights, IL). Eagle's Minimum Essential Medium (EMEM) was purchased from Biowhittaker (Walkersville, MD). N-acetylsphingosine was synthesized from sphingosine, N-acetyldihydrosphingosine from DL-erythro-dihydrosphingosine, and N-acetylphytosphingosine from phytosphingosine-HCl as previously described.18
Cells.Human PMNs were isolated from human peripheral blood as described previously.19 Briefly, fresh whole blood was obtained by venipuncture from healthy volunteers and immediately added to acid citrate dextrose. The PMNs were purified by dextran sedimentation followed by hypotonic lysis to remove the majority of erythrocytes and then centrifuged through Ficoll-Paque (Pharmacia LKB Biotechnology Inc, Piscataway, NJ) to remove contaminating mononuclear cells. This purification scheme yields at least 98% PMNs with few contaminating platelets and a viability of >95% as determined by trypan blue exclusion.
Phagocytic targets.Sheep erythrocytes were purchased from Biowhittaker. One milliliter of stock erythrocytes was washed three times with buffer containing 2.5% dextrose, 0.05% gelatin, 2.5 mmol/L sodium barbital (pH 7.5), 75 mmol/L NaCl, 0.15 mmol/L CaCl2 , and 0.5 mmol/L MgCl2 . Erythrocytes (109/mL) were incubated for 30 minutes at 37°C with antisheep erythrocyte IgG (1:350 to 1:500 dilution; Diamedix, Miami, FL), followed by a 30-minute incubation on ice. The dilution of antibody used was that which caused slight aggregation of erythrocytes as determined by titration. Antibody-treated erythrocytes (EIgG) were washed three times and suspended in the same buffer at 2 to 3 × 109.
Phagocytosis assays.Phagocytosis assays were conducted essentially as outlined by Pommier et al.20 For studies with ceramides and sphingoid bases, lipid stocks (10 mmol/L in ethanol) were diluted to 50 μmol/L in EMEM and probe sonicated for 1 minute. PMNs, suspended at 2 × 106/mL in EMEM, were incubated with varying concentrations of lipid for 30 minutes at 22°C. Following incubation, PMNs were activated with fMLP (100 nmol/L) or PMA (15 ng/mL) for 10 minutes at 37°C, and then EIgG (1 × 108/mL) were added to the activated PMNs and incubation continued for an additional 30 minutes at 37°C. EIgG that were not internalized were lysed with distilled water, returned to isotonicity by the addition of 0.6 mol/L KCl, and fixed with 1% glutaraldehyde. Phagocytosis was quantitated microscopically and expressed as the number of particles ingested per 100 cells (phagocytic index). Controls consisted of fMLP-activated PMNs that had not been exposed to lipids. Inhibition of phagocytosis in the presence of lipids is expressed as percent control, with control being phagocytosis by fMLP-treated PMNs in the absence of lipid treatment. One-sample, two-tailed Student's t-tests were used to assess statistical significance.
Assays for ceramide formation and sphingomyelinase activity.For these experiments, PMNs were batch incubated with EIgG and aliquots of PMNs removed at the indicated time points. The cell aliquots were centrifuged at 10,000×g for 10 seconds, EIgG not ingested were lysed with distilled water, the cells returned to isotonicity, and the samples centrifuged at 10,000×g for 10 seconds. The cell pellets were resuspended in the appropriate buffers for the sphingomyelinase assay and ceramide extraction.
Lipids were extracted by the method of Van Veldhoven and Bell21 as recently described.22 Specifically, PMNs were pelleted, extracted with methanol and each sample mixed with chloroform and water to obtain a chloroform:methanol:water ratio of (1:2:0.8). These samples were then vortexed and incubated at room temperature for 1 hour. These samples were centrifuged for 10 minutes at 2,000×g and the supernatants transferred to a clean tube. The pellet was reextracted and both supernatants combined. To obtain two phases, chloroform and water were added so that the final ratio of chloroform:methanol:water was 10:10:9. After vortexing and centrifugation, the lower phase was removed, washed with an equal volume of 1 mol/L NaCl:methanol (9:1) and centrifuged. The lower phase was then transferred to a clean tube, stored at −20°C and analyzed within 48 hours. Phosphates were determined by the method of Ames.23
Total cellular ceramide was assayed by the method of Preiss et al24 as previously reported.18 Lipid analyses were also performed on EIgG and ice cold PMNs plus EIgG to rule out the contribution of ceramide by the opsonized red cell targets.
Acid and neutral sphingomyelinase assays were based on Gatt et al,25 using liposomes containing NBD-sphingomyelin (10 μmol/L substrate and 30 μmol/L 1,2 dioleoyl-sn-glycerol-3 phosphocholine). The pH optimum for sphingomyelinase activity was determined using overlapping buffer systems containing 125 mmol/L NaOAc (pH 4.0-5.6), 50 mmol/L mono[tris(hydroxymethyl)aminomethane]maleate (pH 5.6-7.2), or 50 mmol/L Tris-HCl (pH 7.2-8.0). Assays for the Mg2+-dependent, neutral enzyme contained 50 mmol/L Tris-HCl (pH 7.4), 25 mmol/L KCl, 5 mmol/L MgCl2 and 106 cells in a total volume of 0.25 mL. Samples were probe sonicated and incubated at 37°C for 30 minutes in a temperature-regulated bath sonicator. The fluorescent product, NBD-ceramide, was isolated by partitioning the assay mixture with 0.45 mL 2-propanol, 1.5 mL heptane, and 0.2 mL water. Samples were centrifuged at 2,000×g, and 0.9 mL of the upper phase transferred to a clean tube containing 0.35 mL of water to remove traces of contaminating NBD-SM. After centrifuging again, the upper layer was analyzed on a fluorimeter (460 nm excitation and 515 nm emission). Sphingomyelinase activity was expressed as activity per 106 cells. The reaction was linear over 40 minutes and with cell concentrations ranging from 4 × 105 to 4 × 106. As with the ceramide determinations, control samples containing EIgG and ice cold PMNs plus EIgG were used to rule out the contribution of these targets in the sphingomyelinase assays. Paired, one-tailed Student's t-tests were used to determine statistical significance.
PA and PEt formation.PMNs were resuspended at 1 × 107/mL in phosphate-buffered saline (PBS) and labeled with 1-O-[3H]octadecyl-sn-glycero-3-phosphocholine (10−8 mol/L; Amersham) for 30 minutes at 37°C. The labeled cells were washed with PBS and resuspended at 2 × 106 cells/mL in PBS containing 1 mmol/L Ca2+ and 1 mmol/L Mg2+. Cells were incubated with 10 μmol/L lipid or buffer (control) for 30 minutes at 22°C, followed by the addition of 200 mmol/L EtOH for 5 minutes at 37°C. PMNs were activated with fMLP and phagocytosis initiated as outlined above. At each time point after addition of EIgG, cell aliquots were removed, EIgG that were not internalized were lysed, and PMNs were resuspended in buffer. The lipids of each sample were extracted according to the method of Van Veldhoven and Bell.21 Assays for [3H]-labeled PEt and PA were performed as previously described.12 Samples to evaluate the kinetics of phagocytosis were taken at the same time points. Paired, two-tailed Student's t-tests were used to determine statistical significance.
Chemotaxis assay.PMNs were suspended at 106/mL in EMEM containing 5 or 10 μmol/L of the indicated lipids and incubated for 30 minutes at 22°C. fMLP was diluted in EMEM in the presence or absence of lipid and placed in the lower wells of a 48-well microBoyden chamber (Neuroprobe, Cabin John, MD). The lower wells were separated from upper wells by a 3-μm-pore diameter polycarbonate membrane that had been soaked in EMEM containing 0.1% bovine serum albumin (BSA). PMNs were added to the upper wells of the chamber, and the chamber placed in a 37°C humidified incubator with 5% CO2 for 30 minutes. Filters were fixed, stained, and mounted on slides. The number of PMNs migrating through the filter was determined microscopically by counting five 400× fields and presented as migration index.
Measurement of lactate dehydrogenase (LDH).PMNs were suspended at 2 × 106/mL in PBS containing 1 mmol/L Ca2+, 1 mmol/L Mg2+, and 5 mmol/L glucose and incubated with 5 or 10 μmol/L of the indicated lipid, or 0.05 or 0.1% ethanol (no lipid control), respectively, for 30 minutes at 22°C. After incubation, cells were centrifuged at 400×g for 6 minutes and the cell supernatants assayed for the release of LDH. An aliquot of supernatant was added to 0.1 mol/L Na-phosphate buffer (pH 7.3) containing 2 mmol/L Na pyruvate and 65 μg/mL β-nicotinamide adenosine dinucleotide, reduced (NADH), and the decrease in absorbance at 340 nm measured immediately. Triton X-100 was added to an aliquot of PMNs and the supernatant assayed to determine total LDH content. This value was used to calculate the percent total release for all samples. The results are expressed as the mean of the percent total cellular content ± standard deviation (SD) for three experiments. Unpaired, two-tailed Student's t-tests were used to determine statistical significance.
RESULTS
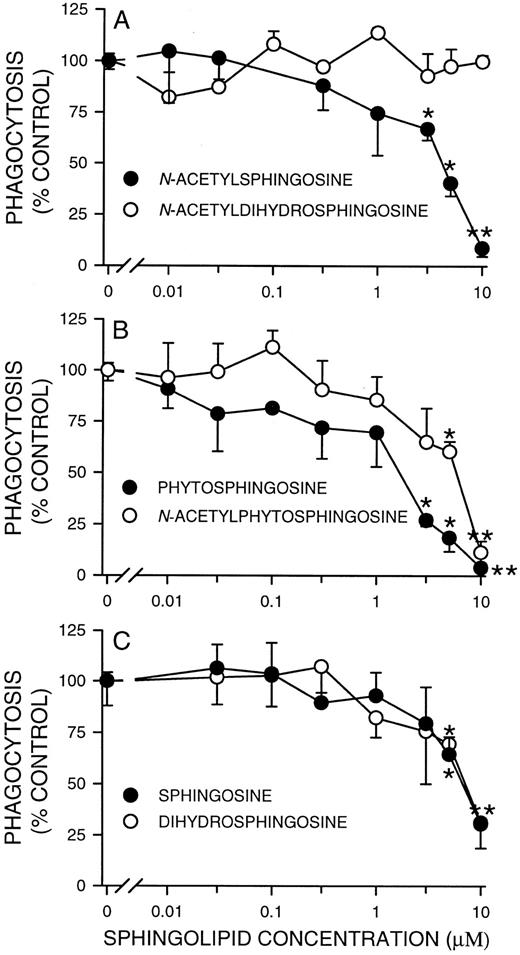
Effects of short chain (C2 ) ceramides, sphingoid bases, and their analogs on phagocytosis by fMLP-stimulated PMNs.We previously showed that N-acetylsphingosine was a potent inhibitor of H2O2 release in adherent PMNs activated with fMLP.18 Because adherent and phagocytosing cells share some of the same signaling pathways, we determined if N-acetylsphingosine had a similar inhibitory effect on Fc receptor-mediated phagocytosis. In the presence of 5 and 10 μmol/L N-acetylsphingosine, phagocytosis of EIgG by fMLP-activated PMNs was significantly reduced by about 50% and 90% (from a phagocytic index of 102.3 ± 22.8 SD to 40.8 ± 6.3 SD and 8.9 ± 2.6, n = 3), respectively (Fig 1A). Inhibition was not the result of decreased target binding to the cell surface, as EIgG binding was the same in the presence or absence of N-acetylsphingosine (data not shown).
Concentration dependence of inhibition of phagocytosis by FMLP-activated PMNs by C2 ceramides and sphingoid bases. Suspended PMNs (2 × 106/mL) were incubated with the indicated doses of lipids for 30 minutes at 22°C, followed by activation with FMLP (100 nmol/L) for 10 minutes at 37°C, and phagocytosis measured as outlined in Materials and Methods. Values represent the mean ± SD for three experiments. The phagocytic index in the absence of lipid (100% control) was 102.3 ± 22.8.
Concentration dependence of inhibition of phagocytosis by FMLP-activated PMNs by C2 ceramides and sphingoid bases. Suspended PMNs (2 × 106/mL) were incubated with the indicated doses of lipids for 30 minutes at 22°C, followed by activation with FMLP (100 nmol/L) for 10 minutes at 37°C, and phagocytosis measured as outlined in Materials and Methods. Values represent the mean ± SD for three experiments. The phagocytic index in the absence of lipid (100% control) was 102.3 ± 22.8.
In addition to N-acetylsphingosine, other sphingolipid metabolites have been implicated as cellular second messengers. For example, sphingosine, but not N-acetylsphingosine, is a potent inhibitor of PKC activity and can activate PLD.26,27 In contrast, N-acetylsphingosine inhibits PLD and has no effect on PKC activity.28,29 Because the metabolism of ceramide, and possibly C2 ceramides, can lead to the formation of other lipid effector molecules such as sphingosine, it is important to determine which metabolite is responsible for a particular biological response. Like N-acetylsphingosine, phytosphingosine, N-acetylphytosphingosine, sphingosine and dihydrosphingosine significantly (P < .05) inhibited phagocytosis of EIgG at 5 μmol/L (Fig 1). At the same concentration, however, N-acetyldihydrosphingosine had no effect. At 10 μmol/L, all the ceramides and sphingoid bases tested, with the exception of N-acetyldihydrosphingosine, significantly (P < .01) inhibited phagocytosis. The ID50s of N-acetylsphingosine and phytosphingosine were 4.7 and 1.7 μmol/L, respectively. The ID50s of N-acetylphytosphingosine, sphingosine and dihydrosphingosine were all about 8.0 μmol/L. The rank order for inhibition of phagocytosis was phytosphingosine > N-acetylsphingosine > N-acetylphytosphingosine = sphingosine = dihydrosphingosine. This differs from the rank order observed for inhibition of oxidant release in fMLP-stimulated adherent PMNs where N-acetylsphingosine ≥ N-acetylphytosphingosine > phytosphingosine ≥ sphingosine > dihydrosphingosine.18
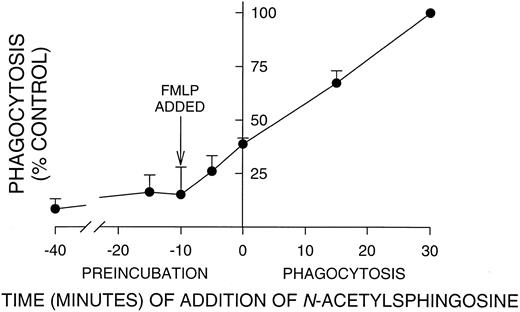
Incubation time with N-acetylsphingosine required for significant inhibition of PMN phagocytosis. PMNs were aliquoted into tubes and N-acetylsphingosine (10 μmol/L) added at the indicated time points. In all cases, PMNs were activated with FMLP 10 minutes before the addition of EIgG as indicated by the arrow. Time 0 is the time of EIgG addition; times < 0 are after EIgG addition, and times < 0 are during preincubation with N-acetylsphingosine. Significant inhibition occurred even when N-acetylsphingosine was added after phagocytosis had been initiated by the addition of EIgG. Values represent the mean ± SD for three experiments. The phagocytic index in the absence of N-acetylsphingosine (100% control) was 145 ± 46.
Incubation time with N-acetylsphingosine required for significant inhibition of PMN phagocytosis. PMNs were aliquoted into tubes and N-acetylsphingosine (10 μmol/L) added at the indicated time points. In all cases, PMNs were activated with FMLP 10 minutes before the addition of EIgG as indicated by the arrow. Time 0 is the time of EIgG addition; times < 0 are after EIgG addition, and times < 0 are during preincubation with N-acetylsphingosine. Significant inhibition occurred even when N-acetylsphingosine was added after phagocytosis had been initiated by the addition of EIgG. Values represent the mean ± SD for three experiments. The phagocytic index in the absence of N-acetylsphingosine (100% control) was 145 ± 46.
To determine whether the effects of sphingosine and N-acetylsphingosine on phagocytosis were due to the known effects of sphingosine on inhibiting PKC, we examined the effects of these sphingols on phagocytosis in PMA-stimulated PMNs. Sphingosine completely suppressed PMA-enhanced phagocytosis; ie, the phagocytic index was 39.5 ± 6.4 SD in the absence of sphingosine and 5.0 ± 0.9 SD in the presence of sphingosine (n = 3). However, N-acetylsphingosine had no effect on PMA-stimulated phagocytosis (phagocytic index = 37.3 ± 6.4 SD). This data indicates that N-acetylsphingosine inhibits phagocytosis by a PKC-independent mechanism.
Because cell number and incubation conditions are critical in determining the ID50 of cell-permeable ceramides,30 and because these agents can be cytotoxic,31 we examined the possibility that the inhibition of oxidant release and phagocytosis was the result of cell injury or death. Phytosphingosine caused slightly more release of LDH at the highest concentration of lipid (at 10 μmol/L percent release was 4.0 ± 1.4 SD for controls v 8.8 ± 0.1 SD for phytosphingosine, P < .05; n = 3), but the other lipids were similar to control values at both concentrations of lipid (data not shown), indicating that the inhibitory effects seen in Fig 1 were not the result of cell lysis.
Preincubation time required for C2-ceramide inhibition of phagocytosis.In contrast to oxidant release by adherent PMNs, which has a lag of 30 to 45 minutes,12 the onset of phagocytosis was very rapid, 1 to 2 minutes. Therefore, we determined how long PMNs must be preincubated with N-acetylsphingosine to obtain maximal inhibition. PMNs were incubated with N-acetylsphingosine for varying times before and after fMLP activation and the addition of EIgG (Fig 2). The time of incubation is indicated on the X-axis, with 0 corresponding to the time of EIgG addition to cells. Note that PMNs were activated with fMLP 10 minutes before the initiation of phagocytosis (arrow, Fig 2). It was necessary to add N-acetylsphingosine at or before fMLP activation to inhibit phagocytosis by ∼85%. However, significant inhibition (P < .05) occurred even when N-acetylsphingosine was added 15 minutes after phagocytosis was initiated. In all subsequent experiments, PMNs were preincubated with lipids for 30 minutes at 22°C to insure maximal inhibition.
Effect of ceramides and sphingoid bases on PMN chemotaxis to fMLP.To determine if ceramide or its structural analogs interfere with other fMLP-mediated responses, we examined the effects of C2 ceramides and sphingoid bases on fMLP-mediated chemotaxis. Interestingly, chemotaxis to fMLP (10 nmol/L) was not inhibited or enhanced by any of the lipids we tested (data not shown). This indicates that the observed effects on phagocytosis were specific, and further supports the conclusion that preincubation of PMNs with lipids did not inhibit PMN responses by causing cell damage or interfering with fMLP activation.
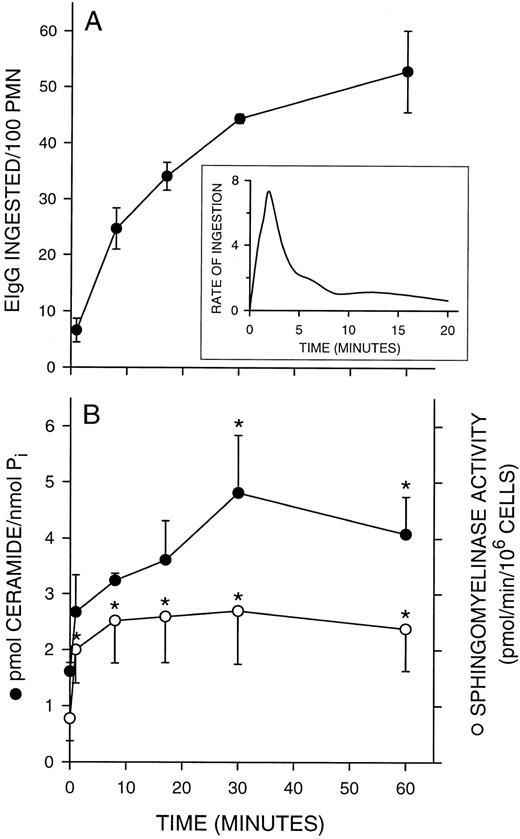
Sphingomyelinase activation and ceramide formation during phagocytosis.We previously observed that ceramide levels increased in adherent PMNs at a time when the respiratory burst was terminated, suggesting that these endogenous ceramides are involved in the regulation of oxidant release.18 To implicate a role for endogenous ceramides in phagocytosis by fMLP-stimulated PMNs, we measured ceramide levels. FMLP activation, in the absence of phagocytic targets, did not stimulate ceramide formation (2.4 ± 0.9 pmol/nmol Pi for control cells v 1.6 ± 0.2 pmol/nmol Pi for FMLP-activated cells). In addition, there was no measurable increase in ceramide levels during phagocytosis in the absence of fMLP activation (2.1 ± 0.5 pmol/nmol Pi at time 0 v 2.3 ± 0.4 pmol/nmol Pi following 30 minutes of phagocytosis). Phagocytosis in fMLP-stimulated PMNs is initiated within 1 minute after the addition of EIgG and completed by 30 minutes (Fig 3A). Ceramide levels increased within the first minute of EIgG addition, with a significant (P < .05) accumulation seen by 30 minutes (Fig 3B). The increase in endogenous ceramide coincided with a steep drop in the rate of phagocytosis (Fig 3A, inset), suggesting that ceramide may be a negative modulator of EIgG-mediated phagocytosis by fMLP-activated PMNs. Consistent with this finding, preincubation with N-acetylsphingosine reduces the rate of EIgG ingestion (data not shown). Erythrocytes, at the same concentration as those used in our phagocytosis assays, did not contain detectable levels of ceramide (data not shown).
Kinetics of sphingomyelinase activation and ceramide formation during PMN phagocytosis of EIgG. PMNs (2 × 106/mL) were activated with FMLP (100 nmol/L) for 10 minutes at 37°C. EIgG (1 × 108/mL) were added and aliquots of cells removed at the indicated time points. EIgG not ingested were lysed and PMNs resuspended in buffer. Samples were probe sonicated and assayed for ceramide and sphingomyelinase activity. (A) Kinetics of phagocytosis. Inset: Rate of phagocytosis was determined by removing aliquots of PMNs at 30-second intervals for the first 2.5 minutes after addition of EIgG. Subsequent time points were at 60-second intervals for the next 3.5 minutes and at 4-minute intervals thereafter. Rate of ingestion was calculated by dividing the number of EIgG ingested in a given time interval by the number of minutes in that interval. The rates were plotted over the midpoint for each interval. (B) Kinetics of ceramide formation and sphingomyelinase activation. Values represent the mean ± SEM of three experiments. * = Significantly different from the zero time point, P < .05.
Kinetics of sphingomyelinase activation and ceramide formation during PMN phagocytosis of EIgG. PMNs (2 × 106/mL) were activated with FMLP (100 nmol/L) for 10 minutes at 37°C. EIgG (1 × 108/mL) were added and aliquots of cells removed at the indicated time points. EIgG not ingested were lysed and PMNs resuspended in buffer. Samples were probe sonicated and assayed for ceramide and sphingomyelinase activity. (A) Kinetics of phagocytosis. Inset: Rate of phagocytosis was determined by removing aliquots of PMNs at 30-second intervals for the first 2.5 minutes after addition of EIgG. Subsequent time points were at 60-second intervals for the next 3.5 minutes and at 4-minute intervals thereafter. Rate of ingestion was calculated by dividing the number of EIgG ingested in a given time interval by the number of minutes in that interval. The rates were plotted over the midpoint for each interval. (B) Kinetics of ceramide formation and sphingomyelinase activation. Values represent the mean ± SEM of three experiments. * = Significantly different from the zero time point, P < .05.
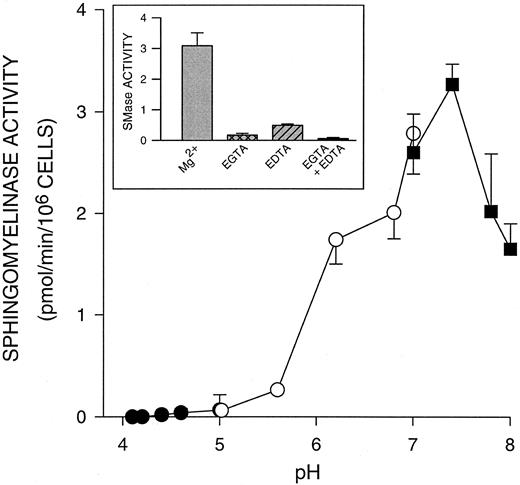
We next evaluated whether ceramide was being generated by the hydrolysis of sphingomyelin. Although the sphingomyelinase cycle has been linked to cell activation in leukemic cell lines,31 this pathway has not been demonstrated in human PMNs. An initial characterization of PMN sphingomyelinase indicated that the major sphingomyelinase in unactivated PMNs was a Mg2+-dependent (Fig 4, inset), neutral SMase, with a pH optimum of 7.4 (Fig 4). A Lineweaver-Burke plot of activity versus substrate concentration indicated an apparent Km of 7.3 × 10−6 mol/L and a Vmax of 5.8 nmol/min/mg protein. During PMN phagocytosis of EIgG, we saw significant increases (P < .05) in this neutral sphingomyelinase activity that paralleled the increase in ceramide formation (Fig 3B). Sphingomyelinase activity remained elevated over the 60 minutes of this assay. The EIgG preparation or surface bound EIgG did not contribute to the observed increase in sphingomyelinase. In the presence of cytochalasin D (5 μg/mL), which results in EIgG binding without internalization, we saw similar increases in sphingomyelinase activity and ceramide accumulation (data not shown). This finding indicates that Fc receptor ligation is sufficient to trigger the activation of sphingomyelinase and accumulation of ceramide in fMLP-activated PMNs.
pH dependence of sphingomyelinase activity in PMNs. Sphingomyelinase activity was determined using liposomes containing NBD-sphingomyelin. Each sample consisted of PMNs (1 × 106) in 0.25 mL Tris-HCl (pH 7.2 to 7.8; ▪) or Tris-maleate buffer (pH 5.6 to 7.2; ○) containing 25 mmol/L Mg2+ or Na acetate buffer (pH 4.0 to 5.6; •), with liposomes containing 10 μmol/L substrate and 30 μmol/L lecithin. Samples were probe sonicated and incubated at 37°C for 30 minutes in a bath sonicator. The fluorescent product, NBD-ceramide, was isolated by partitioning the assay mixture with 2-propanol, heptane and water, and was analyzed on a fluorimeter. Values represent the mean ± SD for three experiments. Inset: Sphingomyelinase assays were conducted at pH 7.4 in the presence or absence of 25 mmol/L Mg2+, 5 mmol/L EGTA or 5 mmol/L EDTA. Values represent the mean ± SD for three experiments.
pH dependence of sphingomyelinase activity in PMNs. Sphingomyelinase activity was determined using liposomes containing NBD-sphingomyelin. Each sample consisted of PMNs (1 × 106) in 0.25 mL Tris-HCl (pH 7.2 to 7.8; ▪) or Tris-maleate buffer (pH 5.6 to 7.2; ○) containing 25 mmol/L Mg2+ or Na acetate buffer (pH 4.0 to 5.6; •), with liposomes containing 10 μmol/L substrate and 30 μmol/L lecithin. Samples were probe sonicated and incubated at 37°C for 30 minutes in a bath sonicator. The fluorescent product, NBD-ceramide, was isolated by partitioning the assay mixture with 2-propanol, heptane and water, and was analyzed on a fluorimeter. Values represent the mean ± SD for three experiments. Inset: Sphingomyelinase assays were conducted at pH 7.4 in the presence or absence of 25 mmol/L Mg2+, 5 mmol/L EGTA or 5 mmol/L EDTA. Values represent the mean ± SD for three experiments.
Effects of C2 ceramides and sphingoid bases on PEt and PA formation during phagocytosis of EIgG by fMLP-activated PMNs.PLD activation is a principal signal in PMN activation and has been associated with the uptake of both complement- and IgG-opsonized particles.3,9,10 Furthermore, PLD is one of the intracellular targets of ceramide action.18 30 Initially, we evaluated whether ceramide was inhibiting EIgG-mediated phagocytosis by blocking the activation of PLD. N-acetylsphingosine blocked both phagocytosis and the activation of PLD in PMNs challenged with EIgG. In the absence of N-acetylsphingosine, phosphatidylethanol (PEt) and phosphatidic acid (PA) levels increased following fMLP activation (time 0). No further increases were seen in the absence of EIgG (data not shown). Significant increases (P < .05) in PEt and PA formation were seen following the addition of EIgG to fMLP-activated PMNs (Fig 5). These increases paralleled the uptake of EIgG targets (data not shown). In the absence of fMLP activation, the addition of EIgG to PMNs did not cause an increase in PEt. At concentrations of N-acetylsphingosine (10 μmol/L) that inhibited phagocytosis, both PEt and PA formation were significantly inhibited (Fig 5).
Effect of N-acetylsphingosine on PEt and PA formation during EIgG-induced phagocytosis. PMNs were labeled with 1-O-[3H]octadecyl-sn-glycero-3-phosphocholine as previously described.18 Labeled cells (2 × 106/mL) were incubated in the presence or absence of 10 μmol/L N-acetylsphingosine for 30 minutes at 22°C followed by activation with fMLP (100 nmol/L) for 10 minutes at 37°C. EIgG (108/mL) were added to the activated PMN and phagocytosis allowed to proceed for 0.5 to 60 minutes at 37°C. At the indicated time points, PMNs were removed, EIgG not internalized removed by lysis, and lipids extracted and analyzed as described in Materials and Methods. Values represent the mean ± SD for three experiments. * = Significantly different from the zero time point (FMLP activation alone), P < .05.
Effect of N-acetylsphingosine on PEt and PA formation during EIgG-induced phagocytosis. PMNs were labeled with 1-O-[3H]octadecyl-sn-glycero-3-phosphocholine as previously described.18 Labeled cells (2 × 106/mL) were incubated in the presence or absence of 10 μmol/L N-acetylsphingosine for 30 minutes at 22°C followed by activation with fMLP (100 nmol/L) for 10 minutes at 37°C. EIgG (108/mL) were added to the activated PMN and phagocytosis allowed to proceed for 0.5 to 60 minutes at 37°C. At the indicated time points, PMNs were removed, EIgG not internalized removed by lysis, and lipids extracted and analyzed as described in Materials and Methods. Values represent the mean ± SD for three experiments. * = Significantly different from the zero time point (FMLP activation alone), P < .05.
We looked at the effect of the other C2 ceramides and sphingoid bases on PEt and PA formation during phagocytosis. N-acetylphytosphingosine inhibited both PEt and PA formation (Table 1), but N-acetyldihydrosphingosine did not have a significant effect on either PEt or PA formation. By contrast, the sphingoid bases markedly stimulated both PEt and PA formation. Recently, we reported that N-acetylsphingosine and N-acetylphytosphingosine inhibited PEt formation while sphingosine, dihydrosphingosine, and phytosphingosine activated PEt formation in fMLP-activated adherent PMNs.18 Gomez-Muñoz et al30 also reported that C2 and C6 ceramides inhibited PLD activation in response to PA or lyso-PA in rat fibroblasts. The activation of PLD by sphingoid bases also occurs in other cell types, suggesting that this is a general phenomenon.32
DISCUSSION
Evidence implicating a role for ceramides in signal transduction has come from studies using short chain ceramides to duplicate receptor-mediated activation. Treating HL-60 cells with C2 or C6 ceramide induces HL-60 cell differentiation at subthreshold concentrations of vitamin D3 .33 Higher concentrations of ceramide, in the absence of vitamin D3 , induces early downregulation of c-myc and subsequent HL-60 cell differentiation.33,34 These results indicate that ceramide formation may be an important signal for HL-60 cell differentiation. Extending these studies to human PMNs, we have shown that ceramide formation accompanies oxidant release in adherent PMNs stimulated with fMLP.18 Ceramide accumulates 90 to 120 minutes following stimulation, correlating with the termination of the respiratory burst. The kinetics of ceramide formation in adherent PMNs indicate that it might be a negative regulator of oxidant release. Consistent with this hypothesis, we and others have found that short chain ceramide analogs are potent inhibitors of fMLP-induced oxidant release in both PMNs in suspension and adherent to fibrinogen.18 35
We now report that ceramides may also act as negative regulators of IgG-dependent phagocytosis by PMNs in suspension activated with fMLP. We saw an increase in endogenous ceramide levels at a time when phagocytosis was rapidly declining. In contrast, phagocytosis by PMA-activated PMNs did not result in a similar increase in endogenous ceramide (data not shown). Consistent with a role for ceramides as negative regulators of phagocytosis in fMLP-activated PMNs, C2 ceramides were potent inhibitors of phagocytosis. The inhibitory effects of N-acetylsphingosine were observed under conditions in which phagocytosis was increased following activation with fMLP, but not phorbol ester. This later observation is consistent with a PKC-independent effect of N-acetylsphingosine on phagocytosis of EIgG by fMLP-activated PMNs.
During cell activation, ceramide is most frequently derived from the hydrolysis of sphingomyelin by the action of a neutral sphingomyelinase, resulting in the generation of ceramide and phosphorylcholine.13 The sphingomyelin cycle was originally described in the HL-60 promyelocytic leukemia cell line. The addition of 1α,25-dihydroxyvitamin D3 , TNF-α or interferon (IFN)-γ, inducers of monocytic differentiation, to undifferentiated HL-60 cells increases neutral sphingomyelinase activity.13,34,36 In U937 cells, treatment with TNFα is reported to activate both neutral and acidic sphingomyelinases.37 These sphingomyelinases are contained within different intracellular compartments, suggesting that neutral and acidic sphingomyelinases hydrolyze sphingomyelin at different sites within the cell. There are at least three sphingomyelinases that have been implicated in ceramide formation: (1) a membrane-associated, Mg2+-dependent, neutral sphingomyelinase,38 (2) a cytosolic, Mg2+-independent, neutral sphingomyelinase,39 and (3) an endosomal, acid sphingomyelinase.37
The activity of a Mg2+-dependent, neutral sphingomyelinase increases within the first few minutes of addition of EIgG to fMLP-activated PMNs in suspension. Similar results were seen in human fibroblasts where a membrane-associated, Mg2+-dependent, neutral sphingomyelinase is activated within 5 minutes of addition of TNFα.40 In HL-60 cells, the activity of a Mg2+-independent, neutral SMase increases within 2 hours of vitamin D3 treatment.39 However, a Mg2+-dependent, neutral SMase is also activated by vitamin D3 treatment of HL-60 cells and may also participate in this process. In U937 cells, both a Mg2+-dependent, neutral sphingomyelinase and an acidic sphingomyelinase are activated within the first few minutes of addition of TNFα.37 NF-κB activation may be controlled by the acid sphingomyelinase, whereas a proline-directed protein kinase and PLA2 activation are regulated by the neutral sphingomyelinase. If there is acid sphingomyelinase activity in PMNs, it is at such low levels that it was not easily detected in our assay system. Thus, it appears that a neutral, Mg2+-dependent sphingomyelinase is a key enzyme that is activated during Fc receptor-mediated phagocytosis in fMLP-activated PMNs.
Presently, very little is known about the intracellular targets of ceramide. Rosenwald and Pagano41 have hypothesized that changes in intracellular ceramide levels may alter normal membrane traffic in cells. Specifically, their studies showed that C6 ceramide inhibits the movement of vesicular stomatitis virus glycoprotein through the medial and trans compartments of the Golgi apparatus subsequent to its transport to the cell surface.41 They also showed that both fluid-phase and receptor-mediated endocytosis were inhibited by C6 ceramide,42 suggesting a more general role for ceramide in regulating membrane trafficking. Our data showing that N-acetylsphingosine blocked phagocytosis are consistent with ceramide being a negative regulator of membrane trafficking.
The mechanisms by which ceramides exert their effects are unknown. MAP kinase activation and inhibition of PLD are potential downstream signaling pathways that are regulated by ceramide. For example, TNF-α activation of HL-60 cells leads to the tyrosine phosphorylation and activation of MAP kinase, p42MAPK,43 with kinetics parallel to the kinetics of sphingomyelin hydrolysis. Furthermore, the addition of exogenous sphingomyelinase or C2-ceramide leads to p42MAPK activation, implicating the involvement of ceramides in this pathway. In contrast to the HL-60 cell system where receptor-mediated ceramide generation leads to cell activation, ceramides inhibit receptor-stimulated activation of PLD in fibroblasts treated with bradykinin or phosphatidate.29,30 Likewise, we recently reported that ceramides inhibited PLD activation in PMNs adherent to fibrinogen and activated with fMLP.18
PLD is one of the signaling pathways thought to regulate PMN phagocytosis.10 Therefore, it was not surprising to find that C2 ceramides inhibited PLD activity in response to the phagocytic targets in fMLP-activated PMNs. In contrast, the sphingoid bases increased PLD activity. The marked differences between C2 ceramides and sphingoid bases on PLD activity and PA levels suggest that they are targeting different intracellular pathways in fMLP-activated PMNs. This is consistent with our previous findings on fMLP-activated adherent PMNs where N-acetylsphingosine and N-acetylphytosphingosine inhibited PEt formation and had minimal effect on PA while sphingosine, dihydrosphingosine and phytosphingosine stimulated both PEt and PA accumulation.18 In all the systems studied to date, there is divergence in the effects of ceramides and sphingoid bases on PLD activity and PA formation. Ceramides inhibit receptor-stimulated activation of PLD in fibroblasts and basophilic leukemia cells.28-30 Ceramide can also block receptor-mediated translocation of PKC, which is the upstream signal for PLD activation in some systems.28,29 However, PMA-induced translocation of PKC is not affected by N-acetylsphingosine.28 It is not presently clear what, if any, role PKC plays in Fc receptor-mediated phagocytosis by fMLP-activated PMNs. Even though sphingosine, phytosphingosine, and dihydrosphingosine are all reported to inhibit PKC activity, these compounds increase PEt formation in fMLP-activated PMNs,18 27 indicating that there are alternative pathways for PLD activation in these cells. Based on differences in their intracellular targets, it is likely that C2 ceramides and sphingoid bases inhibit phagocytosis by targeting divergent intracellular pathways.
Even though N-acetylsphingosine (10 μmol/L) completely inhibited the phagocytosis of EIgG and PLD activity, treatment of these cells with 1% ethanol only partially (50%) inhibited phagocytosis (data not shown). These findings suggest that other signaling pathways, in addition to PLD, are susceptible to ceramide-mediated inhibition. Identification of other targets of ceramide action are currently ongoing.
Supported by National Institutes of Health Grants No. HL53074 (to S.J.S.), AI20065 (to L.A.B.), and DK41487 and DK39255 (to J.A.S.), and a grant from the American Heart Association of Michigan (to S.J.S.). J.A.S. is an Established Investigator of the American Heart Association.
Address reprint requests to Suzanne J. Suchard, PhD, Department of Pediatrics, University of Michigan, Room 7510A, MSRB-1, Box 0684, Ann Arbor, MI 48109.

![Fig. 5. Effect of N-acetylsphingosine on PEt and PA formation during EIgG-induced phagocytosis. PMNs were labeled with 1-O-[3H]octadecyl-sn-glycero-3-phosphocholine as previously described.18 Labeled cells (2 × 106/mL) were incubated in the presence or absence of 10 μmol/L N-acetylsphingosine for 30 minutes at 22°C followed by activation with fMLP (100 nmol/L) for 10 minutes at 37°C. EIgG (108/mL) were added to the activated PMN and phagocytosis allowed to proceed for 0.5 to 60 minutes at 37°C. At the indicated time points, PMNs were removed, EIgG not internalized removed by lysis, and lipids extracted and analyzed as described in Materials and Methods. Values represent the mean ± SD for three experiments. * = Significantly different from the zero time point (FMLP activation alone), P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/6/10.1182_blood.v89.6.2139/3/m_bl_0036f5.jpeg?Expires=1769099718&Signature=Z373tiUps5Nwh~jJEPAWqmiVMgiZh4guOsl9x6af1ViLLEg2CRyRlu0h1gZYkGTTJGDl3VuCJdBibrjGitOga-YEaQZHm2ws3JIfqHTTatmWBRdft-AovvF9KOxv6thoDXgJsm~MaNV0iercZhziQ63VT5kGZvnXoJSzZMRtNdk2w9FVWhRexFfdmVjaulXY-7U4tyefS--FnEMaZYx0zIYDTKUvcgzakpkijA-oM7cFQqrJqPZT-byyrzt6mqWNT10NzmBUjS8uJSQ94mEXesltw5lQ~pzHyPt~PD2xY1-a0BOBs8zApM6An2EguxLprLYULE0JKuJp~~vXVUfMLg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
