Abstract
CD34+Thy-1+Lin− cells are enriched for primitive hematopoietic progenitor cells (PHP), as defined by the cobblestone area-forming cell (CAFC) assay, and for bone marrow (BM) repopulating hematopoietic stem cells (HSC), as defined by the in vivo SCID-hu bone assay. We evaluated the effects of different cytokine combinations on BM-derived PKH26-labeled CD34+Thy-1+Lin− cells in 6-day stroma-free cultures. Nearly all (>95%) of the CD34+Thy-1+Lin− cells divided by day 6 when cultured in thrombopoietin (TPO), c-kit ligand (KL), and flk2/flt3 ligand (FL). The resulting CD34hiPKHlo (postdivision) cell population retained a high CAFC frequency, a mean 3.2-fold increase of CAFC numbers, as well as a capacity for in vivo marrow repopulation similar to freshly isolated CD34+Thy-1+Lin− cells. Initial cell division of the majority of cells occurred between day 2 and day 4, with minimal loss of CD34 and Thy-1 expression. In contrast, cultures containing interleukin-3 (IL-3), IL-6, and leukemia inhibitory factor contained a mean of 75% of undivided cells at day 6. These CD34hi PKHhi cells retained a high frequency of CAFC, whereas the small population of CD34hiPKHlo postdivision cells contained a decreased frequency of CAFC. These data suggest that use of a combination of TPO, KL, and FL for short-term culture of CD34+Thy-1+Lin− cells increases the number of postdivision PHP, measured as CAFC, while preserving the capacity for in vivo engraftment.
PLURIPOTENT hematopoietic stem cells (HSC) are considered to be ideal targets for gene therapy. Use of retroviral vectors for gene transduction requires that the target cells pass through mitosis.1,2 Because the majority of freshly isolated HSC are thought to be quiescent,3 it is necessary to provide appropriate ex vivo conditions to stimulate HSC division without differentiation and subsequent loss of multilineage potential to achieve efficient clinical therapy with gene-manipulated HSC. Stroma appears to be required to provide such conditions,4 but, due to the technical difficulties of using stromal cultures for clinical gene therapy trials, the appropriate culture conditions that stimulate ex vivo replication of human HSC in the absence of stroma must be defined.
To attempt retroviral gene transduction of HSC in the absence of stroma, interleukin-3 (IL-3) and IL-6 in combination with c-kit ligand (KL)4 or leukemia inhibitory factor (LIF)5 are usually added to stroma-free cultures. However, the efficiency of gene transduction into pluripotent HSC remains low. Ex vivo culture of HSC with IL-3 can be detrimental to maintenance of primitive HSC function, as was shown by decreased reconstituting ability of HSC in lethally irradiated mice.6,7 Retrovirus-mediated gene expression in human hematopoietic cells correlated inversely with growth factor stimulation when cultures included IL-3.8 In addition, IL-3 can abrogate B-lymphoid potential and is a positive regulator of early myelopoiesis.9 We have previously shown that 3 to 6 days of culture in the presence of IL-3 induces not only cell division of primitive human CD34+Lin−Rhodamine (Rh123)lo cells, but also differentiation (loss of CD34 expression).10,11 There is now increasing evidence that inclusion of IL-3 in cultures results in loss of the long-term reconstituting ability of HSC.6,7,12 13
We, therefore, wished to investigate combinations of early acting stromal-derived cytokines for stimulation of CD34+Thy-1+Lin− cell division without differentiation. Signaling through tyrosine kinase receptors (TKR) is important to induce HSC division. The ligand for the TKR c-kit (kit ligand) plays an important role in stimulation of proliferation of HSC, usually in synergy with other cytokines.14,15 Another important HSC-associated TKR, flk2/flt3, was first identified in the mouse.16,17 Flk2/flt3 ligand stimulates proliferation of both murine16,18-21 and human HSC.18,19,22-24In addition to these two factors, thrombopoietin (TPO), although originally believed to be a megakaryocyte (MK) lineage-specific cytokine,25 has been shown to stimulate proliferation of HSC of both mouse26-28 and human.10,11 29-31
The cobblestone area-forming cell (CAFC) assay allows in vitro estimation of the frequency of primitive hematopoietic progenitor cells (PHP) within a population, whereas the SCID-hu bone assay measures the in vivo bone marrow (BM) repopulating ability of HSC. Both CAFC and SCID-hu bone repopulating HSC are enriched among CD34+Thy-1+Lin−cells.32-34 In the present study, we have compared different cytokine combinations added to short-term cultures of adult BM CD34+Thy-1+Lin− cells for retention of in vitro CAFC and in vivo SCID-hu bone repopulating ability within the population of divided human CD34hicells. One condition used for gene transduction, ie, IL-3, IL-6, and LIF, was compared with TPO plus KL11 and with TPO, KL, plus flk2/flt3 ligand (FL).35 Newly generated CD34hicells could be identified by the loss of the fluorescent membrane dye PKH26.36-38 The optimal timepoint for maximal division with minimal differentiation was determined. TPO, KL, and FL in combination were found to stimulate division of a majority of CD34+Thy-1+Lin− cells by day 4, with minimal loss of CD34 or Thy-1 expression.
MATERIALS AND METHODS
Antibodies.
To enrich for CD34+Thy-1+Lin−cells, we used Tuk3 (anti-CD34 obtained from Dr A. Ziegler, University of Berlin, Berlin, Germany) directly conjugated to sulphorhodamine (SR) and GM201 (antihuman Thy-1 from Dr W. Rettig, Ludwig Cancer Research Institute, New York, NY) directly conjugated to phycoerythrin (PE; SyStemix, Palo Alto, CA). As an isotype control for anti-CD34 (Tuk3) staining, we obtained FLOPC 21 mouse IgG3 (Sigma, St Louis, MO) conjugated to SR (SyStemix). As a control for anti–Thy-1 staining, we used purified mouse IgG1 (Becton Dickinson, Mountain View, CA) conjugated to PE (SyStemix). The lineage panel of fluorescein isothiocyanate (FITC)-conjugated antibodies Leu-5b (anti-CD2), Leu-M3 (anti-CD14), Leu-M1 (anti-CD15), Leu-11a (anti-CD16), SJ25C1 (anti-CD19), FITC-conjugated mouse IgG1 and IgG2a, PE- and FITC-conjugated HPCA-2 (anti-CD34), and PE-conjugated Leu-12 (anti-CD19) and Leu-M9 (anti-CD33) were purchased from Becton Dickinson. FITC-conjugated antibody D2.10 (antiglycophorin A) was purchased from AMAC (Westbrook, ME). Hybridomas that produce monoclonal antibodies to monomorphic or polymorphic determinants of HLA molecules were obtained from American Type Culture Collection (ATCC; Rockville, MD).39
Purification of CD34+Thy-1+Lin−cells from BM.
Human adult BM (ABM) cells from normal donors were pre-enriched for CD34+ cells using a magnetic bead selection device (SyStemix). CD34+ cells were also selected from BM from two multiorgan donors and frozen before use. CD34+ cells were incubated for 10 minutes on ice with 2 mg/mL heat-inactivated human gamma globulin (Gamimune; Miles Inc, Elkhart, IN) to block nonspecific Fc binding. Subsequently, the cells were washed with staining buffer (SB). SB contained Hank's Balanced Saline Solution (JRH Biosciences, Lenexa, KS), 0.5% bovine serum albumin (Sigma), and 10 mmol/L HEPES (Sigma). Cells were stained for 30 minutes on ice with anti-CD34-SR (6 μg/mL), anti–Thy-1-PE (10 μg/mL), and the lineage panel of FITC-conjugated antibodies. Appropriate isotype controls were used, as described above. Cells were then washed with SB and resuspended at a concentration of 106/mL in SB containing 1 μg/mL propidium iodide (PI; Molecular Probes Inc, Eugene, OR). A Vantage fluorescence-activated cell sorter (FACS; Becton Dickinson Immunocytometry Systems, San Jose, CA) was used to sort live (PIlo) CD34+Thy-1+Lin− cells. The sorts were reanalyzed to assure clean separation of cell subpopulations.
PKH26 fluorescent dye labeling.
Cells were washed with protein-free PBS. The PKH26 dye (Sigma) was diluted 1:250 in the kit diluent. The cell pellet was resuspended at a concentration of 107/mL. This cell suspension was then added to an equal volume of PKH26 and incubated for exactly 4 minutes at room temperature (RT). An equal volume of fetal bovine serum (FBS; Gemini BioProducts, Calabasas, CA) was then added and incubated for an additional 1 minute at RT. An equal volume of Iscove's modified Dulbecco's medium (IMDM) containing 10% FBS was then added. The cells were counted and then centrifuged. The pellet was resuspended at a concentration of 105/mL in IMDM/10% FBS with and without cytokines for short-term suspension culture. The cells were plated in round-bottom 96-well plates at 100 μL/well.
Short-term suspension culture.
PKH26-labeled cells were cultured for 6 days at 104cells/100 μL of medium (IMDM, 10% FBS) in round-bottom 96-well plates in suspension cultures containing different cytokine combinations. The cytokines used included IL-3 (10 ng/mL), IL-6 (10 ng/mL), LIF (50 ng/mL; Novartis, Basel, Switzerland), TPO (10 to 15 ng/mL; R&D Systems, Minneapolis, MN), KL (50 to 75 ng/mL), and FL (50 to 75 ng/mL; SyStemix). Cell numbers were determined using a hemocytometer and trypan blue to exclude dead cells.
FACS analysis of cultured cells.
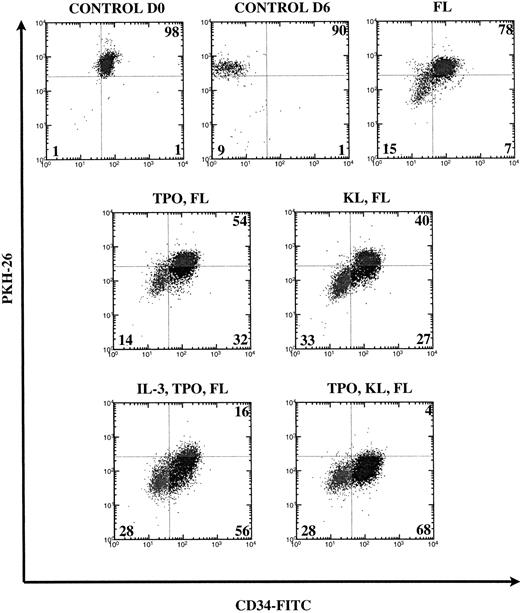
A fraction of PKH26-labeled cells to be used as control was kept overnight at 37°C in the absence of cytokines to remove unstably incorporated dye as well as antibodies bound to the surface and was then stained with anti-CD34-FITC. The settings (PKH26 vCD34-FITC) of the Vantage cell sorter were determined using these cells that we called control day 0 (D0). At day 6 (D6), the wells were pooled and cells were counted and stained with anti-CD34-FITC antibody after incubation with Gamimune. Cell division was measured by loss of PKH26 dye fluorescence and primitiveness by retention of the CD34 cell surface marker (Fig 1).
FACS analysis of CD34+Thy-1+Lin− cells cultured for 6 days in various cytokine combinations containing FL. Numbers are percentages of cells in each quadrant from a representative experiment. Loss of PKH26 fluorescence indicates cell division. Loss of CD34 expression indicates differentiation. Control cells were cultured overnight (D0) or for 6 days (D6) without cytokines and quadrants were set using live-gated undivided cells. The percentages of undivided cells (UR and UL quadrants) are combined.
FACS analysis of CD34+Thy-1+Lin− cells cultured for 6 days in various cytokine combinations containing FL. Numbers are percentages of cells in each quadrant from a representative experiment. Loss of PKH26 fluorescence indicates cell division. Loss of CD34 expression indicates differentiation. Control cells were cultured overnight (D0) or for 6 days (D6) without cytokines and quadrants were set using live-gated undivided cells. The percentages of undivided cells (UR and UL quadrants) are combined.
Undivided (PKHhi) and divided (PKHlo) subpopulations of CD34hi cells were purified from 6-day cultures containing IL-3, IL-6, and LIF or TPO and KL to determine if PHP numbers were maintained or increased within the population of CD34hi cells that had undergone division. Figure 2 shows typical gates used for FACS sorting from representative experiments. In each experiment, control cells were cultured without cytokines and then stained with the irrelevant mouse IgG1-FITC to set the gates. One example of a control stain is shown for the TPO, KL, and FL combination. For this cytokine combination, all cells were PKHlo and these were divided into CD34hi and CD34lo/− subsets, which were placed into the CAFC assay to determine the PHP frequency and multilineage potential of the cells postdivision.
FACS sort gates based on PKH26 versus CD34 fluorescence. After 6 days of culture of CD34+Thy-1+Lin− cells in different combinations of cytokines, cells were stained with anti-CD34-FITC. Sort gates shown are on live (PI low) cells. These were set based on the PKH26 profile of live unstimulated control cells. Each cytokine condition required a different tissue for sorting and therefore sort gates varied accordingly.
FACS sort gates based on PKH26 versus CD34 fluorescence. After 6 days of culture of CD34+Thy-1+Lin− cells in different combinations of cytokines, cells were stained with anti-CD34-FITC. Sort gates shown are on live (PI low) cells. These were set based on the PKH26 profile of live unstimulated control cells. Each cytokine condition required a different tissue for sorting and therefore sort gates varied accordingly.
CAFC assay.
A proportion of the cells was cultured at limiting dilution in the CAFC assay as described previously.34 Briefly, cells were seeded in 96-well plates preseeded with a murine stromal cell line (Sys-1) in 1:1 IMDM/RPMI medium (JRH BioSciences, Woodland, CA) containing 1 mmol/L sodium pyruvate (JRH BioSciences), 5 × 10−5 mol/L 2-mercaptoethanol (Sigma), and 10% FBS. Limiting dilution ranged from 100 cells per well to 0.78 cells per well. After 5 weeks, wells containing cobblestone areas were enumerated and CAFC frequency of the cell population was calculated using maximum likelihood estimation with SAS software.40 The statistical significance of CAFC frequency difference between cultured cell populations was determined by ANOVA. Statistical significance of CAFC number difference between cultured and starting cell populations was determined using the Student's t-test. Representative wells containing cobblestone areas (at least 10 per sample group) were individually analyzed by FACS for the presence of CD33+immature myeloid, CD19+ B-lymphoid, and CD34+progenitor cell populations to estimate the multilineage potential of the original cells.
SCID-hu bone assay.
The SCID-hu bone assay was performed as previously described.34 39 C.B-17 scid/scid mice were used as recipients of human fetal bone grafts. First, limiting dilution analysis was performed to determine the dose of CD34+Thy-1+Lin− cells that reliably gives donor reconstitution in the SCID-hu bone model. HLA-mismatched fetal bone grafts were injected with cell doses ranging from 1,000 to 30,000 CD34+Thy-1+Lin− cells per graft into mice that received whole body irradiation (400 rad) shortly before cell injection. To achieve a sufficient number of grafts at each dose, four tissue donors were used in four separate experiments. Eight weeks after injection, the bone grafts were recovered and the BM cells harvested and analyzed for donor cell engraftment using FITC conjugates of allotype-specific HLA antibodies versus PE-conjugated anti-CD19, anti-CD33, and anti-CD34. Total human cells were detected with W6/32-PE (antihuman HLA class I major histocompatibility complex [MHC] molecule monomorphic determinant). Cells were analyzed on a FACScan analyzer (Becton Dickinson Immunocytometry Systems). Grafts having at least 1% of hematopoietic cells bearing donor HLA antigen were considered positive. The percentage of grafts showing donor reconstitution was assayed for each cell dose tested. At five times the limit dose, or 10,000 cells, donor reconstitution was observed in all grafts.
Uncultured BM CD34+Thy-1+Lin−as well as CD34hi PKHlo and CD34lo/− PKHlo cells from D6 cultures in TPO, KL, and FL were sorted and injected (10,000 cells per graft) into SCID-hu bone grafts. Eight weeks after injection, the bone grafts were analyzed for engraftment of donor CD33+, CD19+, and CD34+ cells.
Kinetics of cell division.
A fraction of PKH26-labeled CD34+Thy-1+Lin− cells was kept overnight at 37°C in the absence of cytokines and then stained with anti-CD34-FITC. The settings (PKH26 v CD34-FITC) of the FACS Calibur (Becton Dickinson Immunocytometry Systems) were determined using these cells (control D0). Short-term suspension cultures of PKH26-labeled CD34+Thy-1+Lin−cells were set up in different cytokine combinations, as described above. Cells were stained on D2, D4, and D6 with anti-CD34 (HPCA-2)-FITC and anti-Thy-1-Cy5 (GM201-Cy5 conjugated at SyStemix) and analyzed on the FACS Calibur.
RESULTS
Increase of total cell number and of CD34+cell number.
In the present study, we examined the effects of single, double, and triple cytokine combinations in 6-day suspension cultures of PKH26-labeled BM CD34+Thy-1+Lin− cells. Previous studies using PKH26 did not show any detrimental effects of PKH26 labeling on cellular function.36 38 As shown in Table 1, single cytokines did not increase the number of CD34+ or total cells. Combinations of two cytokines of TPO, KL, and FL maintained the CD34+ cell number with a slight increase (1.7-fold) in total cell number. Among those tested, the combination of three cytokines, TPO, KL, and FL, induced the highest increases of both total cell (4.7-fold) and of CD34+ cell number (3.4-fold). The three-factor combination IL-3, IL-6, and LIF did not stimulate an increase in total cell number.
Comparison of different cytokine combinations containing FL.
We examined the effect of FL alone and in combination with one or two other cytokines in three to six experiments. In Fig 1, we demonstrate how the quadrants were set on the control unstimulated cells and show dot plots from a representative experiment. When cultured in FL alone, most CD34+Thy-1+Lin− cells remained undivided (78%). Sixty-eight percent (mean 73%) of postdivision cells lost CD34 expression. The addition of KL to FL reduced by half the percentage of undivided cells (to 40%), and 55% (mean 58%) of postdivision cells lost CD34 expression. The addition of TPO to KL and FL stimulated much greater division (4% remained undivided) with loss of CD34 on only 29% (mean 27%) of postdivision cells. With other combinations containing TPO, eg, TPO and FL or TPO, IL-3, and FL, we also observed that loss of CD34 expression only occurred on about 30% of postdivision cells. We had previously shown that IL-3 induces not only division, but also differentiation (CD34 loss) of human HSC.11 The addition of TPO seems not only to contribute to greater cell division but also to overcome the effect of IL-3 to promote differentiation.
To determine whether retention of CD34hi expression postdivision correlated with retention of functional PHP, CD34/PKH26 subsets were purified postculture in three different cytokine conditions and assayed for CAFC frequency. CD34hiPKH26lo and CD34lo/− PKH26losubsets from TPO, KL, and FL cultures were also assayed for in vivo SCID-hu bone repopulating activity.
FACS sorting of cultured cell subpopulations subdivided by PKH26 fluorescence and CD34 staining.
In subdividing CD34hi cells based on cell division, we tried to exclude the CD34lo/− subpopulation, because it is known that CAFC are contained mainly within the CD34hi population,37 as confirmed in Table 2. The majority of cells in IL-3, IL-6, and LIF did not divide (mean 75%) by day 6; therefore, we sorted CD34hi PKHhi versus CD34hiPKHlo (mean 7.5%; Fig 2). The same cell populations were sorted from cultures with TPO and KL in which a mean of 53% of cells remained undivided and a mean of 23% of cells were CD34hiPKHlo. In TPO, KL, and FL, all the cells had divided; therefore, we sorted for CD34hi PKHlo (mean 71%) versus the CD34lo/− PKHlo (mean 26%) population of differentiated postdivision cells.
Analysis of CAFC frequencies and phenotype of cobblestone areas.
The PHP activity of the sorted CD34/PKH26 subpopulations of cultured cells was estimated in vitro by use of the CAFC assay, comparing the CAFC frequencies with the starting population of CD34+Thy-1+Lin− cells. The mean frequencies of CAFC within the starting population of CD34+Thy-1+Lin− cells ranged from 1/21 to 1/46 (95% confidence limits, 1/16 to 1/52; Table 2). Because of the limited number of cells obtained from each fresh BM, only one cytokine combination could be tested per experiment, giving rise to some tissue variation. In the case of IL-3, IL-6, and LIF, the undivided CD34hi PKHhi subpopulation remained primitive, retaining the same mean CAFC frequency as the preculture CD34+Thy-1+Lin− population. However, the frequency of CAFC within the small CD34hiPKHlo subpopulation had decreased 21-fold to a mean of 1/440 (1/249 to 1/813).
In addition, we evaluated the ability of cultured cell subpopulations to give rise to both myeloid and B-lymphoid cells in long-term stromal culture. Detection of CD34+ cells in 5-week cobblestone areas suggests retention of primitiveness among the cell subpopulations assayed. Analysis of a minimum of 10 small cobblestone areas generated from this population showed that divided CD34hi cells from cultures containing IL-3, IL-6, and LIF gave rise to only CD33+ myeloid cells (Table 3).
After culture with TPO and KL, the undivided CD34hiPKHhi cells again had a similar CAFC frequency to the uncultured CD34+Thy-1+Lin−population. In these conditions, the mean frequency of CAFC in the divided CD34hi PKHlo subpopulation was reduced 2.3-fold, compared with the starting cell population (Table 2). CD34hi PKHlo cells retained the potential, at limiting dilution, to give rise to CD19+ B-lymphoid, CD33+ myeloid, and CD34+ progenitor cells after 5 weeks of culture in the CAFC assay. However, the proportion of wells containing greater than 1% CD34+ cells was reduced 22-fold compared with the starting cell population (Table 3). For each cell population, all cobblestone areas analyzed contained CD33+myeloid cells.
The mean values for six experiments with the combination of TPO, KL, and FL are shown in Table 2. The mean CAFC frequency remained the same in the CD34hi PKHlo subpopulation, compared with the starting CD34+Thy-1+Lin− cell population. These cells also, at limiting dilution, retained their ability to give rise to CD19+ B-lymphoid progenitors, CD33+ myeloid cells, and CD34+ progenitor cells. CD19+ cells were observed in a similar proportion (∼50%) of cobblestone areas examined for both the uncultured CD34+Thy-1+Lin− cells and postculture CD34hi PKHlo cells. Forty-nine percent of cobblestone areas generated from CD34hiPKHlo cells contained CD34+ cells, as compared with 67% for the uncultured CD34+Thy-1+Lin− cells (Table3). As expected, the CD34lo/− PKHlo cells had very low CAFC frequency (mean 1/3,000).
Increase in CAFC numbers among total and CD34hiPKHlocells.
We compared the increase of CAFC numbers from CD34+Thy-1+Lin− cells in different culture conditions (Fig 3). Of the three different cytokine combinations analyzed, only TPO, KL, and FL increased the mean number of total cells, CD34+ cells, and CAFC (Table 1 and Fig 3). If we compare the number of CAFC within the CD34hi PKHlo population with the original number of CAFC placed in culture, we can see that only in TPO, KL, and FL were CAFC numbers increased among cells that had divided (mean 3.2-fold), although values ranged from maintenance to a 7.6-fold increase. The CAFC number among divided CD34hi cells at day 6 was not significantly different from the number measured among CD34+Thy-1+Lin− cells at day 0 for TPO and KL cultures (n = 2, P = .19), but increased CAFC number among CD34hi PKHlo cells in TPO, KL, and FL cultures approached statistical significance (n = 6, P = .07). The number of measurable CAFC among divided CD34hicells from IL-3, IL-6, and LIF cultures had significantly decreased (n = 2, P = .03). All CAFC detectable in D6 IL-3, IL-6, and LIF cultures were derived from undivided CD34hi cells.
Increase of postdivision CAFC numbers during 6 days of culture in TPO, KL, and FL. Increased numbers of CAFC (CD34hi PKHhi and PKHlo) were determined by dividing the number at day 6 by the number placed in culture at day 0 (left-hand columns). On the right, columns show the fold increase in numbers of CAFC within the CD34hiPKHlo (postdivision) population, compared with the number within the CD34+Thy-1+Lin−population placed in culture at D0. There was a mean 3.2-fold increase (range 1- to 7.6-fold) in postdivision CAFC in TPO, KL, and FL cultures. Data shown for IL-3, IL-6, and LIF as well as TPO and KL are the means of two experiments. Data for TPO, KL, and FL are the means of six experiments (4 normal and 2 multi-organ donor BM). Error bars show the SEM and P values indicate the significance of the change in CAFC number from D0 to D6. (▤) IL-3, IL-6, and LIF; (▩) TPO and KL; (▪) TPO, KL, and FL.
Increase of postdivision CAFC numbers during 6 days of culture in TPO, KL, and FL. Increased numbers of CAFC (CD34hi PKHhi and PKHlo) were determined by dividing the number at day 6 by the number placed in culture at day 0 (left-hand columns). On the right, columns show the fold increase in numbers of CAFC within the CD34hiPKHlo (postdivision) population, compared with the number within the CD34+Thy-1+Lin−population placed in culture at D0. There was a mean 3.2-fold increase (range 1- to 7.6-fold) in postdivision CAFC in TPO, KL, and FL cultures. Data shown for IL-3, IL-6, and LIF as well as TPO and KL are the means of two experiments. Data for TPO, KL, and FL are the means of six experiments (4 normal and 2 multi-organ donor BM). Error bars show the SEM and P values indicate the significance of the change in CAFC number from D0 to D6. (▤) IL-3, IL-6, and LIF; (▩) TPO and KL; (▪) TPO, KL, and FL.
Dose of uncultured CD34+Thy-1+Lin−cells that gives reconstitution of 100% of SCID-hu bone grafts.34,39
The percentage of grafts showing donor reconstitution at each CD34+Thy-1+Lin− cell dose tested is shown in Fig 4. Using Poisson distribution analysis, the frequency of SCID-hu bone repopulating cells was 1 per 2,000 CD34+Thy-1+Lin− cells. At five times this limit dose or 10,000 cells, donor reconstitution was observed in 100% of grafts.
Titration of BM CD34+Thy-1+Lin− cells in the SCID-hu bone model. Bone grafts were injected with a range of doses of CD34+Thy-1+ Lin− cells (1,000 to 30,000) per graft. Data are the mean of four separate experiments from 4 different BM donors. Donor reconstitution means that greater than 1% of hematopoietic cells were positive for donor HLA antigen.
Titration of BM CD34+Thy-1+Lin− cells in the SCID-hu bone model. Bone grafts were injected with a range of doses of CD34+Thy-1+ Lin− cells (1,000 to 30,000) per graft. Data are the mean of four separate experiments from 4 different BM donors. Donor reconstitution means that greater than 1% of hematopoietic cells were positive for donor HLA antigen.
Engraftment of CD34hiPKHlocells from 6-day culture in TPO, KL, and FL in SCID-hu bone.
CD34hi PKHlo cells from 6-day cultures of CD34+Thy-1+ Lin− cells in TPO, KL, and FL clearly contained increased numbers of CAFC. In addition, we asked whether the same cell population retained its ability to repopulate human bone in vivo, using the SCID-hu bone assay.34 39 To obtain sufficient cells, we purified CD34+Thy-1+Lin− cells from cryopreserved BM CD34+ cells isolated from multiorgan donors. Uncultured CD34+Thy-1+Lin− cells and CD34hi PKHlo as well as CD34lo/− PKHlo cells from D6 TPO, KL, and FL cultures were injected into the fetal human bone grafts. Ten thousand cells were injected per graft, because this cell dose provides consistent engraftment of uncultured BM CD34+Thy-1+Lin− cells (Fig4).
Cultured CD34hi PKHlo cells engrafted to a similar level as the uncultured population of CD34+Thy-1+Lin− cells (4 of 4 grafts; Fig 5 and Table4). In experiment A (Table 4), the mean percentage of donor cells was 34.3% ± 22.3% for CD34hiPKHlo cells, comparable with 25.0% ± 13.5% for uncultured CD34+Thy-1+Lin−cells. FACScan analysis shows that multilineage engraftment occurred in both cases, because the cells isolated from the bones after 8 weeks included donor B-lymphoid (CD19+), myeloid (CD33+), and progenitor cells (CD34+; Fig 5). Cells of the CD34lo/− PKHlo subpopulation engrafted in 1 of 4 bones and did not give rise to cobblestone areas in vitro. This single engraftment (3.5% ± 5.3% donor) could have been due to a low level of contamination (6%, seen in reanalysis) of the CD34lo/− population with CD34hicells.
CD34+ cells that have divided during 6 days of culture in TPO, KL, and FL retain their capacity for in vivo marrow repopulation in the SCID-hu bone assay. Uncultured CD34+Thy-1+Lin− BM cells and CD34hi PKHlo and CD34lo/−PKHlo cells from D6 TPO, KL, and FL cultures were injected into SCID-hu bone grafts (10,000 cells per graft). FACS analysis at 8 weeks showed multilineage marrow repopulation by both the uncultured cells and the CD34hi cells postdivision in culture (PKHlo). The x-axis shows staining for donor HLA allotype. The y-axis shows staining for total human cells (W6/32, antihuman class I MHC) or lineage markers.
CD34+ cells that have divided during 6 days of culture in TPO, KL, and FL retain their capacity for in vivo marrow repopulation in the SCID-hu bone assay. Uncultured CD34+Thy-1+Lin− BM cells and CD34hi PKHlo and CD34lo/−PKHlo cells from D6 TPO, KL, and FL cultures were injected into SCID-hu bone grafts (10,000 cells per graft). FACS analysis at 8 weeks showed multilineage marrow repopulation by both the uncultured cells and the CD34hi cells postdivision in culture (PKHlo). The x-axis shows staining for donor HLA allotype. The y-axis shows staining for total human cells (W6/32, antihuman class I MHC) or lineage markers.
In a second experiment (B), in which the contamination of CD34lo/− with CD34hi cells was less than 2%, we could show that 0 of 4 bones injected with the CD34lo/− PKHlo subset engrafted, but 4 of 4 grafts injected with CD34hi PKHlo cells from D6 TPO, KL, and FL cultures again showed multilineage engraftment, with a mean of 59.0% ± 12.0% donor cells (Table 4). This confirms that, after 6 days of culture, the in vivo marrow repopulating capacity of CD34+Thy-1+Lin− cells is retained within the CD34hi population postdivision in TPO, KL, and FL.
Comparison of the kinetics of cell division in TPO, KL, and FL and IL-3, IL-6, and LIF.
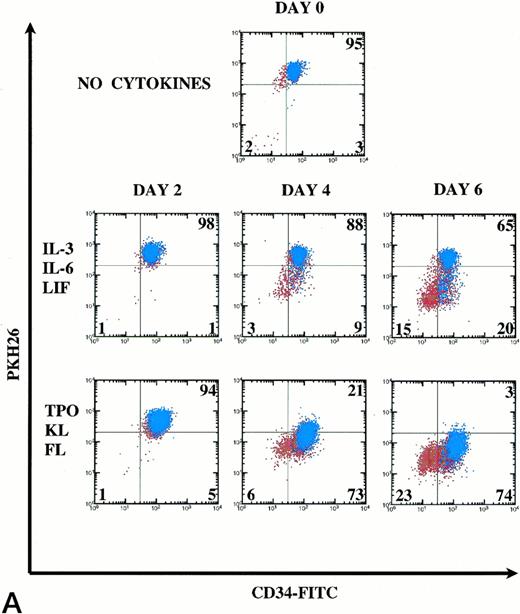
For retroviral gene transduction of PHP and HSC, it will be important to know the timepoint where division is maximal, but differentiation is minimal. We, therefore, examined CD34 retention (primitiveness) and PKH26 loss (division) by CD34+Thy-1+Lin− cells at D2, D4, and D6. In addition, we stained the cells to follow Thy-1 expression as a marker of PHP (blue in Fig6A). We chose to compare the cytokine combination of IL-3, IL-6, and LIF with TPO, KL, and FL, which in our study stimulated greater division of PHP with retention of primitive phenotype. A representative experiment (of 3 experiments) is shown in Fig 6.
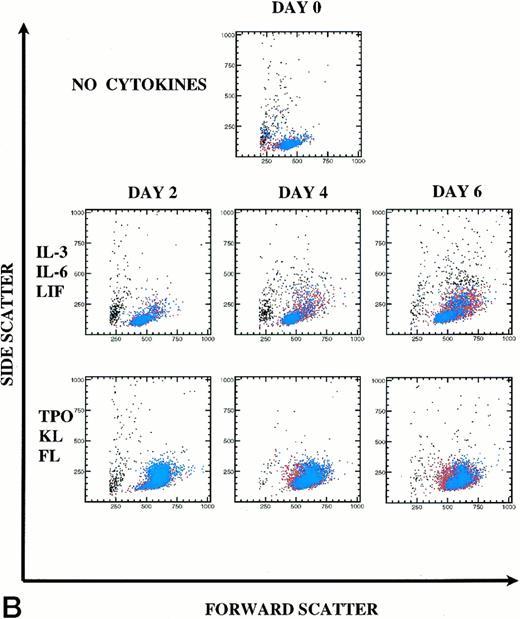
Kinetics of CD34+Thy-1+Lin− cell division and differentiation during 6 days of culture. Comparison between IL-3, IL-6, and LIF and TPO, KL, and FL. (A) PKH26 versus CD34 fluorescence. Live Thy-1+ cells are shown in blue and live Thy-1− cells are shown in red. Percentages of PKHhi and CD34hi PKHlo and CD34lo PKHlo/− cell subsets are shown for this representative experiment (of 3 experiments). (B) forward versus side scatter. Live Thy-1+ cells are shown in blue, and live Thy-1− cells are shown in red.
Kinetics of CD34+Thy-1+Lin− cell division and differentiation during 6 days of culture. Comparison between IL-3, IL-6, and LIF and TPO, KL, and FL. (A) PKH26 versus CD34 fluorescence. Live Thy-1+ cells are shown in blue and live Thy-1− cells are shown in red. Percentages of PKHhi and CD34hi PKHlo and CD34lo PKHlo/− cell subsets are shown for this representative experiment (of 3 experiments). (B) forward versus side scatter. Live Thy-1+ cells are shown in blue, and live Thy-1− cells are shown in red.
In IL-3, IL-6, and LIF, 65% (mean 80%) of cells remained undivided at D6. Forty-three percent of postdivision cells lost expression of CD34 and also appeared to lose Thy-1 expression (Fig 6A). Only 12% (mean 7%) of cells had divided by D4. In TPO, KL, and FL, most cells underwent the first cell division between D2 and D4, because only 5% (mean 7%) of cells lost PKH26 fluorescence by D2, yet already by D4, 73% (mean 75%) of the cells lost PKH26 fluorescence. CD34hi expression was retained on 92% of postdivision cells. By D6, 97% of the cells had divided with retention of CD34hi expression on 76% of these cells. Clearly, Thy-1 expression (blue) was retained on the CD34hiPKHlo cells from TPO, KL, and FL D6 cultures, which is consistent with the retention of PHP and in vivo engraftment activity demonstrated within this cell population. In Fig 6B, the early myelopoietic effects of IL-3 could be detected as an increase in side scatter of the cultured cells. In contrast, in TPO, KL, and FL there was less cell death and the scatter profiles indicate a predominance of blast morphology during the 6 days of suspension culture, consistent with less differentiation. The increase in size (FSC) of the Thy-1+ cell subset in TPO, KL, and FL is shown in blue (Fig6B).
DISCUSSION
TPO synergizes with KL to promote multilineage proliferation of both human10,11,29,30 and mouse HSC.26-28,35 FL is a ligand for the flk2/flt3 tyrosine kinase receptor18,19 that seems to have a unique expanding effect on human peripheral blood long-term culture-initiating cells (LTC-IC).24 TPO has been shown to synergize with both KL and FL to enhance both the number and size of clones formed by murine Sca1+Lin− progenitor cells.35 FL appears to partially replace the requirement for stroma to maintain the human long-term repopulating HSC during gene transduction,13whereas IL-3, IL-6, and KL were insufficient.4
We have previously described the ability of TPO25 to increase the number of human CD34+ cells detectable after long-term culture.11 We also showed that TPO and KL could synergize to drive division of primitive human BM CD34+Lin−Rhodamine123locells with retention of CD34 expression.11 The question remained whether the CD34+ cells that had undergone division (PKHlo) retained primitive functional characteristics. In the present study, culture with TPO, KL, and FL stimulated virtually all CD34+Thy-1+Lin− cells to divide by day 6, with a 3.4-fold increase in numbers of CD34+ cells.
Expansion of LTC-IC (mean, 7.5-fold) has previously been described from whole BM mononuclear cells using 14-day continuous perfusion culture bioreactors containing a stromal layer.41 In addition, Petzer et al42 have shown 30-fold expansion of LTC-IC within 10 days, starting with a highly purified HSC population and using a combination of 6 cytokines, including KL and FL. In our study, we have used static cultures containing only 3 cytokines (TPO, KL, and FL) and observed a mean 3.2-fold increase of CAFC numbers within 6 days within a population that has been shown to be postdivision, based on loss of PKH26 fluorescence. The minor population of CD34hiPKHlo postdivision cells from cultures with IL-3, IL-6, and LIF were found to contain very few CAFC, in contrast to undivided CD34hi PKHhi cells, which retained CAFC at high frequency.38 Only by using PKH26 to separate undivided and divided CD34hi cells could we show that CAFC in cultures containing IL-3, IL-6, and LIF represent undivided cells, whereas CAFC from cultures with TPO, KL, and FL had all been generated de novo by cell division. In the study by Petzer et al,42 only TPO and FL when used alone stimulated a net increase of LTC-IC from CD34+CD38− cells within 10 days. TPO and FL have also been shown to induce extensive renewal with little differentiation of cord blood LTC-IC ex vivo.31 In our system, in TPO and KL or in TPO, KL, and FL, CD34hiPKHlo cells showed retention of the ability to give rise to B-lymphoid as well as early myeloid cells in 5-week stromal cultures.
Long-term bone repopulating cells are likely to be more primitive than the majority of those that read out in the 5-week CAFC assay. Our demonstration that CD34hi PKHlo cells from 6-day cultures with TPO, KL, and FL retained the ability to give a high level of engraftment (both B-lymphoid and myeloid) at 8 weeks in our SCID-hu bone transplant model indicates that the multipotency and engraftment potential of CD34+Thy-1+Lin− cells was preserved during cell division in vitro. Ex vivo expansion of HSC that retains the ability to engraft may allow reduction of periods of cytopenia when numbers of such cells are limiting for autologous transplantation, as well as production of sufficient numbers to overcome allogeneic transplant barriers.
Levels of gene transfer into pluripotent HSC remain low, potentially due to the failure to induce division of the majority of primitive HSC within the short transduction period. TPO has been proposed to shorten the G0 period of dormant murine progenitor cells.27 We suggest that the rapid division of PHP stimulated by TPO, KL, and FL may be due to this combination of factors driving quiescent PHP to exit G0, as well as shortening the G1 phase of the cell cycle.43 44 Based on these premises, an optimal time to achieve integration of retroviral vectors into dividing HSC would, therefore, be between day 2 and day 4, using TPO, KL, and FL. Further studies will be necessary to determine whether achieving maximal division of HSC will be sufficient to overcome the barrier to transducing pluripotent long-term engrafting stem cells.
ACKNOWLEDGMENT
The authors are grateful to all in the cell processing lab at SyStemix for providing us with preselected BM CD34+ cells and to Kwok Yu for conjugation of antibodies. We gratefully acknowledge members of the SyStemix FACS Department, Brenda Lee, Jennine Lunetta, and especially Mike Reitsma, for their support and helpful discussions during cell sorting and analysis and Shirley Chen and Gun Hansteen for SCID-hu bone assays. Thanks to Kathy Wright and the SyStemix protein expression group for providing us with KL and FL, to Linda Osborne for the Sys-1 cultures and help with CAFC frequency analysis, and to Chris Gerard for consulting on statistical analysis. We also thank Dr Tim Austin for critical review of the manuscript and Dr M. Abi Abitorabi for valuable discussions.
Address reprint requests to Lesley J. Murray, PhD, SyStemix, 3155 Porter Dr, Palo Alto, CA 94304.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.