Abstract
By stimulating the expression of murine IP-10 and Mig, CXC chemokines that inhibit neovascularization and cause damage to established tumor vasculature, human B cells immortalized with Epstein-Barr virus (EBV) can promote an effective antitumor response in athymic mice. In the present study, we examined the potential role of EBV in the induction of this antitumor response. Using a panel of EBV+ and EBV− Burkitt lymphoma (BL) cell lines, a significant correlation was detected between the expression of the EBV latency gene LMP1 and the occurrence of spontaneous tumor regression in athymic mice. Inoculation of LMP1+ and LMP1− BL cells in the same subcutaneous site resulted in tumors that completely regressed in a manner indistinguishable from that induced by EBV-immortalized B cells. EBV-converted BL30 and BL41 sublines infected with B95-8 virus expressed LMP1, generated tumors that frequently regressed spontaneously, and promoted an effective antitumor response against progressively growing tumors. In contrast, the EBV− BL30 and BL41 cell lines and the EBV-converted BL30 and BL41 infected with P3HR-1 virus did not express LMP1 protein, and generated progressively growing tumors in nude mice. When transfected with the LMP1 gene, BL41 cells produced tumors that regressed spontaneously in most cases, and could induce the regression of tumors derived from BL41 cells transfected with vector alone. Tumors induced by LMP1-expressing cells expressed murine IP-10 and Mig and displayed histological evidence of extensive tumor tissue necrosis and vascular damage. We conclude that the EBV protein LMP1 is likely responsible for the antitumor response elicited by EBV-immortalized cells in athymic mice.
WHEN INFECTED WITH Epstein-Barr virus (EBV), B cells can acquire unlimited growth potential, and can thus represent a continuous challenge to the EBV-infected individual. T-cell immunity plays a critical role in the defense against EBV infection, particularly through a potent cytotoxic T-cell response directed at B cells latently infected with the virus. A subset of latent cycle EBV antigens is preferentially targeted by EBV-immune T cells, including EBNA2A, 3B and 3C, and LMP2.1 T-cell–independent host responses also contribute to the defense against EBV disease and EBV-induced lymphoproliferative diseases are rare even in the context of severe T-cell immunodeficiency.2
Recently, we have reported on an experimental athymic mouse model that detects an array of non–T-cell–mediated host responses elicited by EBV-immortalized cells resulting in a potent antitumor response.3-6 When injected subcutaneously into irradiated athymic mice, human B cells immortalized with EBV are either nontumorigenic or produce small tumors that soon regress through tissue necrosis.3,4,7,8 When injected subcutaneously in conjunction with a variety of human tumor-derived cell lines that are malignant in this nude mouse model, the EBV-immortalized cells cause the tumors to undergo a characteristic regression process through necrosis and scarring.4 Even established subcutaneous human Burkitt tumors that reproducibly kill athymic mice can be effectively treated by local inoculation with EBV-immortalized cells.3
Studies aimed at clarifying the mechanisms of tumor regression induced by EBV-immortalized cells in athymic mice have showed that inhibition of tumor neovascularization in conjunction with damage to established tumor vasculature play a central role in this process,3 and that the CXC chemokines IP-10 (interferon-inducible protein 10) and Mig are likely mediators of these effects.5,6 IP-10 and Mig are expressed at much higher levels in lymphoblastoid-cell line–treated tumor tissues undergoing regression compared with untreated progressively growing tumors, and these chemokines can act as inhibitors of growth factor–induced neovascularization in vivo, cause damage to established tumor vasculature, and exert antitumor effects in the nude mouse.5,6,9 10 However, the mechanism by which EBV-immortalized cells elicit the host response leading to the rejection of a variety of human tumors established in the nude mouse has remained elusive.
In the course of investigations designed to study Burkitt lymphoma (BL) growth in athymic mice, we have observed that certain EBV-carrying BL cell lines undergo spontaneous tumor regression in a manner similar to that exhibited by EBV-immortalized cells.11 Based on this observation, we have now examined in detail the role of EBV as a potential inducer of the host antitumor response observed in athymic mice. We report that expression of the latent EBV protein LMP1 can account for the antitumor effects typically induced by lymphoblastoid cell treatment of Burkitt tumors.
MATERIALS AND METHODS
Cell lines.
BL cell lines were either derived from biopsies at the National Cancer Institute or obtained from the American Type Culture Collection (Rockville, MD) or the National Institute of General Medical Sciences (Camden, NJ). The PA682 cell lines were derived from a patient with acquired immunodeficiency syndrome–associated BL from different sites of tumor involvement: peripheral blood (PA682PB), bone marrow (PA682BM2), and pleural effusion (PA682PE2).12,13 All cell lines were maintained in RPMI 1640 medium (Biofluids) supplemented with 12% heat-inactivated fetal calf serum (FCS; Reheis, Armour Pharmaceuticals, Kan Ka Kee, IL), 2 mmol/L L-glutamine (GIBCO-BRL, Grand Island, NY), and 5 μg/mL gentamicin (Sigma Chemical Co, St Louis, MO). LMP1-transfected and control BL41 cells, a gift of Dr E. Kieff (Harvard Medical School, Boston, MA), were maintained in guanosine triphosphate (GTP) selection media as previously described.14 All cell lines were mycoplasma-free, and were HLA-typed for identification.
Animal studies.
Six- to eight-week-old female BALB/c nu/nu mice (National Cancer Institute) maintained in pathogen-limited conditions were used throughout the studies. Twenty-four hours before initiation of the experiments, the mice received 400-rad total body irradiation. Exponentially growing cell lines with a viability greater than 90% were injected (107 cells from each of the cell lines, in 0.2 mL RPMI 1640 medium containing 10% FCS) subcutaneously into the right abdominal quadrant through a 25-gauge needle on plastic 1-mL syringes. All animals were observed weekly and tumor size estimated in cm2 as the product of two-dimensional caliper measurements (longest perpendicular length and width).
Western blot analysis.
Protein extracts from cells were solubilized in sodium dodecyl sulfate (SDS)-sample buffer, boiled for 10 minutes, subjected to electrophoresis on a 1% SDS/8.0% polyacrylamide gel (2 × 105 cell equivalents per lane) and transferred onto Immobilon-P filters (Millipore Corp, Bedford, MA). Loading accuracy and efficiency of transfer were evaluated by staining the gels with Coomassie blue (Sigma) and the membranes with Ponceau S (Sigma). Membranes were incubated for 1 hour with a mouse monoclonal antibody (MoAb) to EBNA1 (AB-1 mouse IgG1; Oncogene Science, Cambridge, MA), EBNA2 (PE2, mouse IgG1), or LMP1 (S12, mouse IgG2a; or CS1-4, mouse IgG1). Antibody bound to antigen was detected with a horseradish peroxidase-labeled goat anti-mouse antibody and a chemiluminescence detection system, following the manufacturer's recommendations (Amersham Life Science Inc, Arlington Heights, IL).
RNA preparation and reverse transcriptase-polymerase chain reaction (RT-PCR) analysis.
Total RNA was extracted from cells by the phenol/guanidinium thiocyanate method (RNAzol; GIBCO-BRL). The first cDNA strand was synthesized from 5 μg of total RNA using the SuperScript preamplification system (GIBCO-BRL). The reaction mixtures containing oligo (dT)12-18 were incubated for 50 minutes at 42°C. For PCR analysis of EBV genes primer pairs specific for EBNA1 outer (GTAACTTAGGAAGCGTTTCT and CAATGCAACTTGGACGTTTT), EBNA1 inner (AGCTTCCCTGGGATGAGCGT and TCTTCCCCGTCCTCGTCCAT), EBNA2 outer (ACTTTGAGCCACCCACAGTAACCA and TGGAGTGTCTGACAGTTGTTCCTG), and EBNA2 inner (CATAGAAGAAGAAGAGGATGAAGA and GTAGGGATTCGAGGGAATTACTGA) were selected.15 PCR amplifications were performed in 50 μL of reaction mixture containing 5 μL of cDNA template, 20 mmol/L Tris-HCl (pH 8.4), 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.2 mmol/L dNTP mixture, 0.4 μmol/L for each outer primer pair, and 2.5 U of Taq DNA polymerase (GIBCO-BRL). For the nested PCR, 2.5 μL of the first amplified product was used as a template with the inner primers. An initial denaturation step of 3 minutes at 95°C was followed by 30 cycles of denaturation at 95°C for 1 minute, annealing at 57°C to 62°C (depending on the primers used) for 1 minute, and extension at 72°C for 2 minutes on a RoboCycler (Stratagene, La Jolla, CA). Amplified product was electrophoresed through a 2% agarose gel prestained with 1 μg/mL of ethidium bromide and was visualized under UV light.
For PCR analysis of chemokine expression, 80 ng of cDNA was amplified using radiolabeled dNTP by a semiquantitative PCR.16 The number of amplification cycles was selected for each primer pair to provide a signal within the linear portion of a product versus template amplification curve.16 Aliquots from each amplification reaction were electrophoresed on 6% acrylamide, Tris-borate EDTA gels (Long Ranger; AT Biochem, Malvern, PA) followed by autoradiography and quantitation by phosphorimage analysis. Nucleotide sequences for murine 5′ and 3′ primers, respectively (followed in parentheses by annealing temperature in degrees celsius), were as follows: IP-10, ACCATGAACCCAAGTGCTGCCGTC and GCTTCACTCCAGTTAAGGAGCCCT (62); Mig, ACTCAGCTCTGCCATGAAGTCCGC and AAAGGCTGCTCTGCCAGGGAAGGC (60); G3PDH, GCCACCCGAAAGACTGTGGATGGC and CATGTAGGCCATGAGGTCCACCAC (57). The number of amplification cycles used were 21 for G3PDH, 24 for IP-10, and 28 for Mig. Primers were designed to discriminate between genomic and cDNA by spanning at least one intron.
Histology.
Tumors were removed in toto, fixed in 10% neutral buffered formalin solution (Sigma), embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin.
RESULTS
Spontaneous regression of EBV+ BL in athymic mice.
To examine potential correlations between the presence of EBV in BL cells and the spontaneous regression of certain Burkitt tumors, we injected subcutaneously into athymic mice 17 tumorigenic11EBV+ and EBV− BL cell lines, and looked for the occurrence of spontaneous tumor regression. Eight of these BL cell lines are EBV+ and nine are EBV−, as determined by RT-PCR analysis of EBNA-1 mRNA (Table 1). A dose of 107exponentially growing viable BL cells was selected for injection because previous experiments had shown that this cell dose generally produces the highest tumor take achievable with BL cells.11The occurrence of a tumor was defined as the appearance of a subcutaneous mass at the site of injection, measuring at least 0.25 cm2 in surface area. Tumors were considered as undergoing regression when three consecutive tumor measurements separated by 1 week showed a size reduction of at least 20%, or when, in the absence of measurable tumor size increase, tumor tissue necrosis and/or fibrosis was found to involve at least 25% of tumor tissue sections through the maximum tumor diameter.5 As shown in Table 1, neither the frequency with which these BL cell lines formed tumors nor the time to tumor appearance differed significantly among EBV+ and EBV− BL cell lines. In contrast, the frequency of spontaneous tumor regression differed substantially among EBV+ and EBV− cell lines. Although six of eight EBV+ lines formed tumors that spontaneously regressed at least 50% of the time, only one of nine EBV− cell lines formed tumors that spontaneously regressed at least 50% of the time. This difference (6 of 8 versus 1 of 9) of spontaneous regression among tumors induced by EBV+ and EBV− cell lines was significant (P = .015, Fisher's Exact test), as assessed by chi square analysis of a 2 × 2 contingency table. Two of the eight EBV+ cell lines, Namalwa and Daudi, produced tumors that either never or infrequently (1 of 14 tumors) regressed spontaneously. Seven of the nine EBV− cell lines produced tumors that did not regress spontaneously, and one (Louckes) produced tumors that regressed infrequently (1 of 6 tumors). Spontaneous regression occurring in tumors induced by the EBV− BL cell lines (BML 895 and Louckes) occurred later than those noted in tumors induced by the EBV+ cell lines (94 ± 27v 44 ± 20 days after tumor appearance), raising the possibility that different mechanisms of tumor regression might be operative. Together, these experiments showed a significant correlation between EBV status of a BL cell line and the line's ability to produce tumors that spontaneously regress in athymic mice.
Regression of EBV− BL tumors induced by EBV+ BL cells.
When injected subcutaneously into athymic mice, EBV-immortalized B cells either do not give rise to tumors or give rise to small tumors that spontaneously regress.3 In addition, we found that EBV-immortalized cells can promote the regression of a variety of human tumors, including BL tumors, that would otherwise grow progressively in the subcutaneous tissue of athymic mice.4 We examined whether EBV+ BL cells could exert a similar antitumor effect. To this end, we inoculated athymic mice subcutaneously with the EBV− BL cell line CA46 (that regularly gives rise to progressively growing tumors in this model) either alone or in conjunction with one of three EBV+ BL cell lines (PA682 BM2, PA682 PB, and PA682 PE213) that frequently undergo spontaneous regression in this animal model. As a control, we coinjected the EBV− CA46 cell line with one of two EBV− BL cell lines, EW36 and BL41. EW36 is nontumorigenic11; BL41 gives rise to progressively growing subcutaneous tumors in athymic mice (Table 1). As shown in Table2, when injected alone in nude mice, CA46 formed large progressively growing subcutaneous tumors. In contrast, when injected in conjunction with one of the EBV+ BL cell lines (PA682 PB, PA682 PE2, or PA682 BM2), CA46 gave rise to tumors that spontaneously regressed in 50%, 66%, and 100% of cases, respectively. The remaining tumors were considered as growing progressively, despite reduced tumor growth compared with the controls, and some evidence of tumor tissue necrosis (not shown). It should be noted that all of the evaluable tumors (three mice were killed during regression for in vitro studies) considered as spontaneously regressing disappeared completely, and the animals were cured. The pattern of tumor regression was indistinguishable morphologically from that of tumors induced by the inoculation of CA46 cells in conjunction with EBV-immortalized lymphoblastoid cells. No regression was observed when CA46 cells were coinjected with the EBV− BL cell lines EW36 or BL41 (Table 2). These results show that certain EBV+ BL cells can give rise to subcutaneous tumors that spontaneously regress in athymic mice, and promote the regression of subcutaneous BL tumors that usually grow progressively in this model.
BL cell expression of the EBV-encoded LMP1 protein correlates with tumor regression.
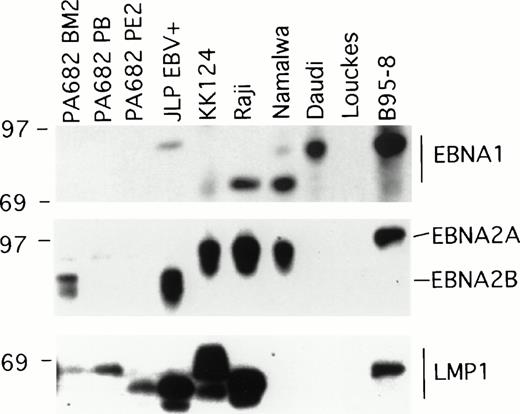
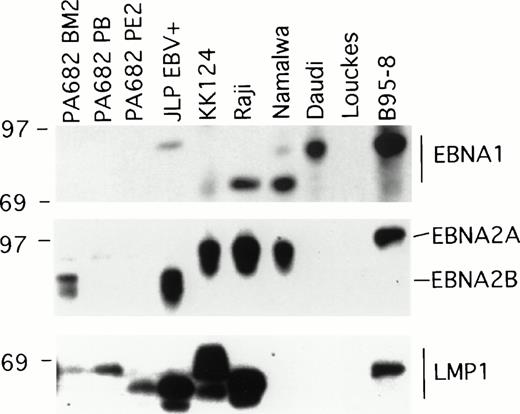
Two of the EBV+ BL cell lines, Namalwa and Daudi, generally produced subcutaneous tumors that grew progressively in athymic mice (Table 1), and thus differed from the other six EBV+ cell lines that generally produced tumors that spontaneously regressed. EBV+ BL cell lines are known to differ in the patterns of EBV gene expression and this determines certain phenotypic differences with respect to the expression of cell activation and adhesion molecules, and growth characteristics of the infected cells.17 We examined the patterns of EBV gene expression in the panel of EBV+ BL cell lines. The EBV+control B95-8 cell line expressed the EBNA1, EBNA2, and LMP1 gene by immunoblotting with the MoAbs AB-1, PE2, and S12 that specifically recognize these proteins, respectively, whereas the EBV−control cell line Louckes did not (Fig 1). EBNA1 was detected in all EBV+ BL cell lines, with the exception of the PA682 BM2, PA682 PB, and PA682 PE2 cell lines (Fig 1).These cell lines express a mutant EBNA1 protein12 that is not recognized by the anti-EBNA1 MoAb AB-1 used here. The differences in the apparent molecular weight of EBNA1 detected in these BL cell lines (Fig 1) is likely attributable to variations in the internal repeat region 3 within the EBNA1 coding region.18
Western blot analysis of EBV gene expression in BL cell lines. Cell extracts from 2 × 105 cell equivalents were electrophoresed through 8% Tris-glycine gels, transferred onto nylon membranes, probed with specific MoAb antibodies that recognize EBNA1 (AB-1), EBNA2 (PE2), and LMP1 (S12), and developed.
Western blot analysis of EBV gene expression in BL cell lines. Cell extracts from 2 × 105 cell equivalents were electrophoresed through 8% Tris-glycine gels, transferred onto nylon membranes, probed with specific MoAb antibodies that recognize EBNA1 (AB-1), EBNA2 (PE2), and LMP1 (S12), and developed.
EBNA2A or EBNA2B, distinguished by differences in the U2 region encoding EBNA2,19 were detected by immunoblotting with the PE2 MoAb in five of the eight EBV+ BL cell lines. RT-PCR analysis failed to detect EBNA2 specific transcripts in PA682 PB, PA682 PE2, and Daudi cell lines, indicating that our failure to detect EBNA2 expression in these BL cell lines was not attributable to low level expression of EBNA2 or to expression of a mutant EBNA2 protein not recognized by the antibody used (data not shown). This finding is consistent with the observation that Daudi cells contain a deletion of EBNA2 coding sequences.20
Expression of LMP1 protein was detected by Western blotting with the S12 MoAb in all EBV+ BL lines, with the exception of Namalwa and Daudi (Fig 1). The observed variations in the relative molecular weight of LMP1-related bands (Fig 1) has been previously attributed to variation in the carboxyl terminal domain of LMP1.18 Expression of the LMP1 protein was also undetectable in the BL cell lines Namalwa and Daudi by Western blotting with a pool of MoAbs to LMP1 (CS1-4) that recognize distinct LMP1 epitopes (not shown), indicating that the failure to detect LMP1 expression in Namalwa and Daudi was not attributable to the presence of LMP1 variants. These results show a direct correlation between the expression of LMP, but not EBNA1 or EBNA2, and tumor regression associated with EBV+ BL cell lines.
Effects of EBV infection on BL tumor outcome.
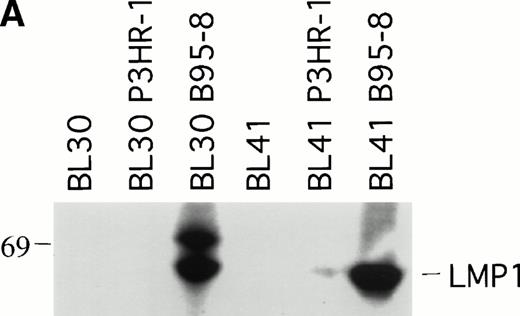
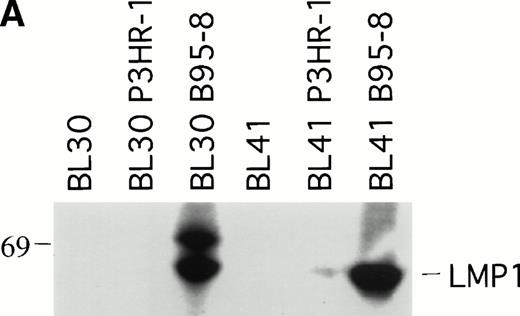
To explore further the relationship between LMP1 expression and the occurrence of spontaneous BL tumor regression in athymic mice, we examined whether EBV infection of EBV− BL cells might affect the pattern of progressive tumor growth exhibited by EBV− BL cells. To this end, the EBV− BL30 and BL41 cell lines and their EBV-converted sublines BL30-B95-8, BL30-P3HR-1, BL41-B95-8, and BL41-P3HR-1 were tested in nude mice. P3HR-1 virus is a defective EBV that is unable to initiate growth transformation and is deleted for a DNA fragment that includes some of the IR1 copies, all of U2 and IR2, and a portion of U3 regions coding for EBNA2 and EBNALP.19 Cells infected with P3HR-1 generally do not express LMP1 because of a loss of the transactivating function of EBNA2.21 In contrast, B95-8 virus can induce the immortalization of B cells that express nine EBV latent viral gene products, EBNA1, EBNA2, EBNA3A, EBNA3B, EBNALP, LMP1, and LMP2.19 We confirmed that the P3HR-1 and B95-8–converted BL cell lines BL30 and BL41 are EBV infected because they expressed EBNA1 by RT-PCR analysis (data not shown). As expected, the B95-8 but not the P3HR-1–infected BL30 and BL41 cell lines expressed LMP1 by immunoblotting with S12 MoAb (Fig2A).
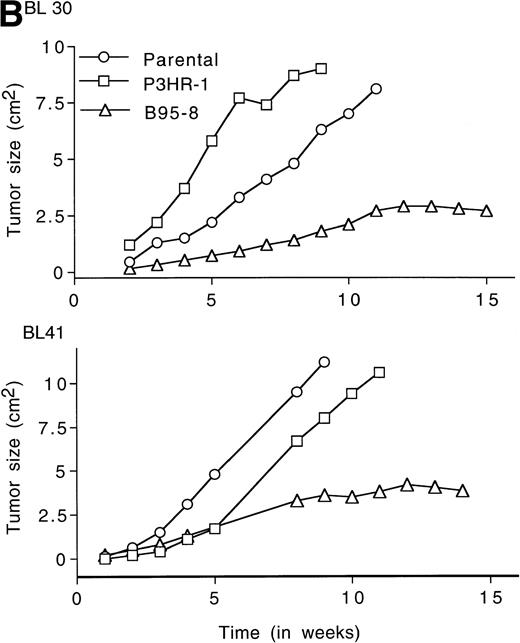
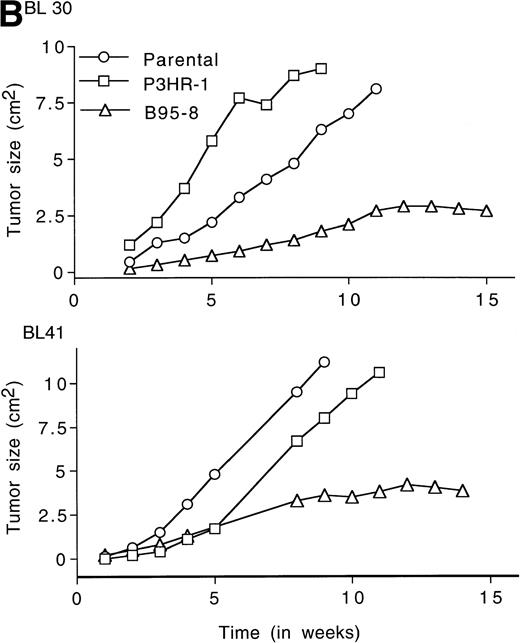
LMP1 expression and tumorigenicity of BL30 and BL41 cell lines. (A) Western blot analysis of LMP1 expression in the BL30 and BL41 cell lines infected with P3HR-1 or B95-8 EBV. Cell extracts from 2 × 105 cell equivalents were electrophoresed through 8% Tris-glycine gels, transferred onto nylon membranes, and probed with a murine MoAb to LMP1 (S12). (B) Growth curves of tumors induced by subcutaneous inoculation of the EBV− and EBV-converted BL30 and BL41 cell lines. Each data point represents the mean tumor size of all mice in each group (5 to 10 mice per group). Mice injected with parental or P3HR1-converted cell lines were killed when the tumors had reached large sizes and impaired animal mobility. (○), Parental; (□), P3HR-1; (▵), B95-8.
LMP1 expression and tumorigenicity of BL30 and BL41 cell lines. (A) Western blot analysis of LMP1 expression in the BL30 and BL41 cell lines infected with P3HR-1 or B95-8 EBV. Cell extracts from 2 × 105 cell equivalents were electrophoresed through 8% Tris-glycine gels, transferred onto nylon membranes, and probed with a murine MoAb to LMP1 (S12). (B) Growth curves of tumors induced by subcutaneous inoculation of the EBV− and EBV-converted BL30 and BL41 cell lines. Each data point represents the mean tumor size of all mice in each group (5 to 10 mice per group). Mice injected with parental or P3HR1-converted cell lines were killed when the tumors had reached large sizes and impaired animal mobility. (○), Parental; (□), P3HR-1; (▵), B95-8.
Tumors developed in all mice injected with EBV− and EBV-converted BL30 and BL41 cell lines, except for one mouse injected with BL41-B95-8 (Fig 2B and Table 3).Tumors induced by the EBV− and the P3HR-1–converted BL30 and BL41 cell lines exhibited similar growth patterns, forming large progressively growing tumors with no signs of tumor regression (Fig2B). As a group, tumors induced by the B95-8–converted BL30 and BL41 cell lines appeared somewhat later, grew at a diminished rate, and reached a maximum size after approximately 11 weeks without exhibiting significant size change over the next 3 to 4 weeks (Table 3, and Fig2B). All 5 tumors induced by BL30-B95-8 and 7 of 8 tumors induced by BL41-B95 were considered as undergoing spontaneous regression because they either exhibited progressive size reductions (at least 20% during 3 weeks observation) or displayed extensive tumor tissue necrosis and/or fibrosis (at least 25% of tumor sections through the longest diameter). Among the tumors derived from the BL41-B95-8 cell line, 4 out of 8 displayed complete tumor regression. Only 1 tumor out of 13 induced by B95-8–converted BL cell lines grew progressively. Tissue excised from this tumor as well as from other representative tumors induced by B95-8–converted BL cell lines was found to have maintained the expression of LMP1 protein (not shown).
In parallel experiments, we examined whether the B95-8–converted BL30 and BL41 cell lines could induce the regression of autologous EBV− cell lines. Coinjection of the cell lines BL30 with BL30-B95-8, and BL41 with BL41-B95-8 into the same subcutaneous site produced tumors that underwent spontaneous regression in 4 out of 5 cases and 2 out of 5 cases, respectively (Table 3). Immunoblotting of tissue extracts from representative tumors derived from coinjection of EBV− and EBV+ BL cell lines, including tumors that failed to spontaneously regress, detected the presence of LMP1 expression. Thus, infection of EBV− BL cells with B95-8 EBV changed the outcome of tumors derived from the cell line.
LMP1-transfected EBV− BL cells give rise to tumors that spontaneously regress.
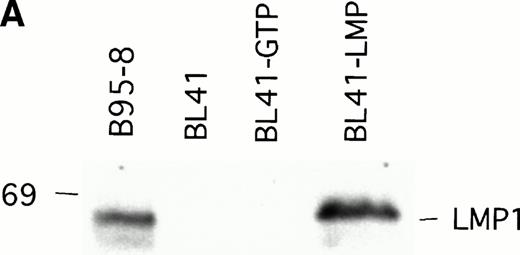
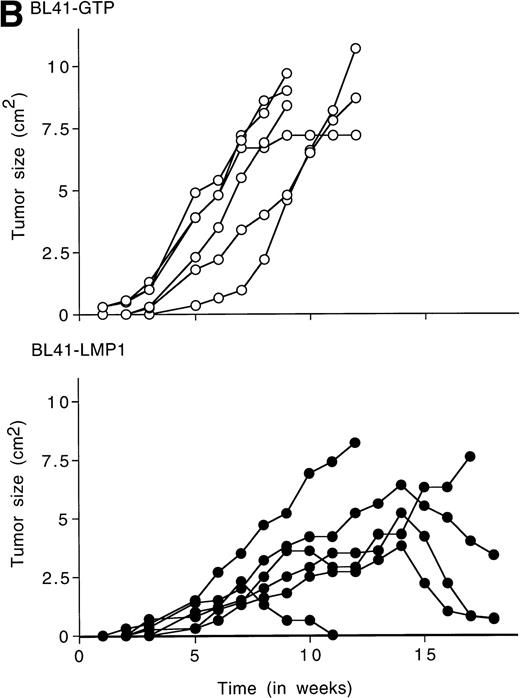
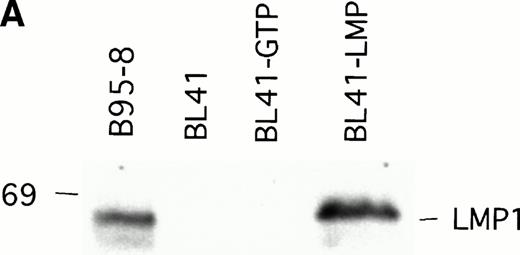
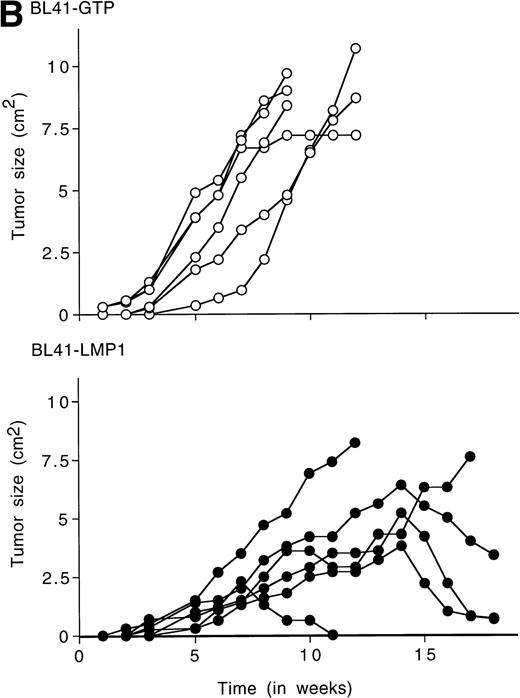
To examine whether LMP1 expression alone is sufficient to confer subcutaneous BL tumors an ability to spontaneously regress in nude mice, we examined the outcome of tumors induced by an LMP1-transfected BL41 cell line, designated LMP1. A 63-Kd (kilodalton) band, corresponding to LMP1 expressed in B95-8 cells, was detected in the LMP1 but not the GTP-transfected and untransfected parental BL41 cells by immunoblotting with the S12 MoAb (Fig3A). As expected, all tumors induced by inoculation of the parental EBV− BL41 cell line and five out of six tumors induced by inoculation of the GTP vector control BL41 cell line grew rapidly and gave rise to progressively growing tumors (Table 4 and Fig 3B, top). In addition, inoculation of vector-transfected BL41 cells (GTP cell line) in conjunction with LMP1-transfected BL41 cells (LMP1 cell line) produced subcutaneous tumors that regressed spontaneously in three out of six cases (Table 4). These results indicate that expression of LMP1 alone confers EBV− BL cells an ability to generate tumors that spontaneously regress in athymic mice.
LMP1 expression and tumorigenicity of LMP1-transfected BL41 cells. (A) Western blot analysis of LMP1 expression in parental, B95-8-EBV–converted, and LMP1-transfected BL41 cells. Cell extracts from 2 × 105 cell equivalents were electrophoresed through 8% Tris-glycine gels, transferred onto nylon membranes, and probed with a murine MoAb to LMP1 (S12). (B) Growth curves of tumors induced by subcutaneous inoculation of LMP1 or vector-transfected BL41 cells. Growth curves of tumors from vector-transfected BL41 cells and LMP1-transfected BL41 cells are depicted for individual mice.
LMP1 expression and tumorigenicity of LMP1-transfected BL41 cells. (A) Western blot analysis of LMP1 expression in parental, B95-8-EBV–converted, and LMP1-transfected BL41 cells. Cell extracts from 2 × 105 cell equivalents were electrophoresed through 8% Tris-glycine gels, transferred onto nylon membranes, and probed with a murine MoAb to LMP1 (S12). (B) Growth curves of tumors induced by subcutaneous inoculation of LMP1 or vector-transfected BL41 cells. Growth curves of tumors from vector-transfected BL41 cells and LMP1-transfected BL41 cells are depicted for individual mice.
Histology and chemokine expression in EBV− BL tumors expressing LMP1.
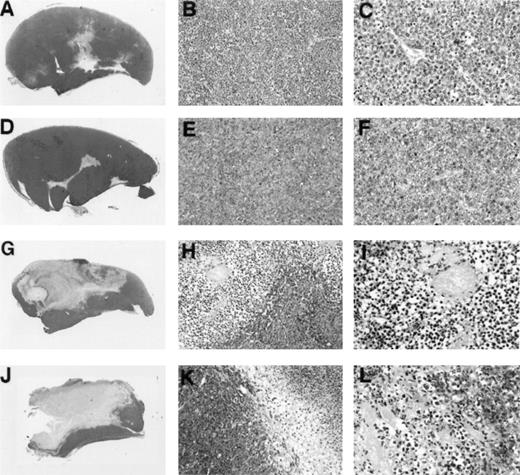
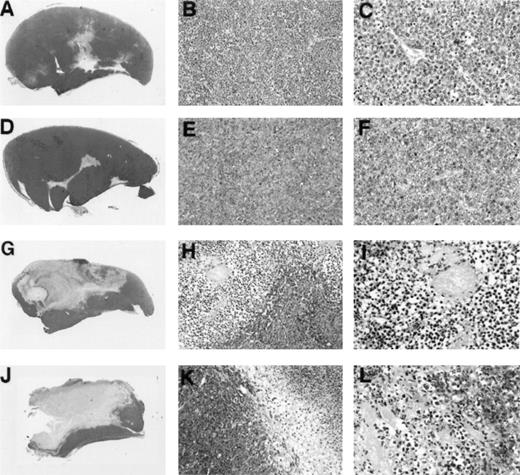
To assess further similarities between Burkitt tumor regression induced by LMP1-expressing EBV− BL cell lines and tumor regression induced by EBV-immortalized lymphoblastoid cells, we examined tumor tissue histology and chemokine expression. In earlier studies, extensive characterization of Burkitt tumor regression induced by lymphoblastoid cells had showed that tumor tissue necrosis associated with diffuse vascular damage and expression of the CXC chemokines IP-10 and Mig are hallmarks of these regressing tumors. Control progressively growing Burkitt tumors induced by inoculation of BL41 cells (Fig 4A, B, and C) or EBV-P3HR1–converted BL41 cells not expressing LMP1 (Fig 4D, E, and F)displayed little tumor necrosis and little evidence of vascular damage. By contrast, regressing tumors induced by inoculation of EBV-B95-8–converted BL41 cells expressing LMP1 (Fig 4G, H, and I) and LMP1-transfected BL41 cells (Fig 4J, K, and L) generally displayed central and homogeneous central necrosis involving large portions of the tumor mass and extending from the epidermis to the deeper portions of the tumor (Fig 4G and H). The tumor vasculature both proximal and distal to the site of tissue necrosis displayed evidence of intravascular thrombosis and intimal thickening (Fig 4I and L). The interface between viable and necrotic tumor tissue was infiltrated with cells identified morphologically as monocytes (Fig 4H and K). Inflammatory infiltrates with lymphocytes and neutrophils were unremarkable. This macroscopic and microscopic morphology is indistinguishable from that exhibited by regressing tumors induced by the coinjection of EBV− Burkitt cells and lymphoblastoid cells.3-5
Gross and microscopic morphology of LMP1-expressing and nonexpressing BL tumors. Female BALB/c nu/nu mice were injected subcutaneously with 1 × 107 cells from exponentially growing BL41 EBV− cell line (A, B, and C); EBV-P3HR-1–converted BL41 cell line (D, E, and F); EBV-B95-8–converted BL41 cell line (G, H, and I); or LMP1-transfected (J, K, and L) BL41 cell line. Tumors were removed in toto 8 to 14 weeks after the initial inoculation and processed for histology. (A, D, G, and J) Gross morphology of Burkitt's tumors removed in toto with abstract epidermis and dermis showing in (A) and (D) mostly viable-looking tumor tissue with small areas of central necrosis, and in (G) and (J) tumors with extensive necrosis and little viable tumor (no magnification). (B, E, H, and K) Microscopic morphology (original magnification × 10) of Burkitt's tumors showing in (B) and (E) viable-looking tumor tissue, and in (H) and (I) the abrupt interface between necrotic and viable tumor tissue. (C, F, I, and L) Higher power magnification (original magnification × 40) of viable tumor tissue with patent capillaries containing red blood cells (C) and (F), and capillaries occluded with thrombi at various stages of organization (I and L).
Gross and microscopic morphology of LMP1-expressing and nonexpressing BL tumors. Female BALB/c nu/nu mice were injected subcutaneously with 1 × 107 cells from exponentially growing BL41 EBV− cell line (A, B, and C); EBV-P3HR-1–converted BL41 cell line (D, E, and F); EBV-B95-8–converted BL41 cell line (G, H, and I); or LMP1-transfected (J, K, and L) BL41 cell line. Tumors were removed in toto 8 to 14 weeks after the initial inoculation and processed for histology. (A, D, G, and J) Gross morphology of Burkitt's tumors removed in toto with abstract epidermis and dermis showing in (A) and (D) mostly viable-looking tumor tissue with small areas of central necrosis, and in (G) and (J) tumors with extensive necrosis and little viable tumor (no magnification). (B, E, H, and K) Microscopic morphology (original magnification × 10) of Burkitt's tumors showing in (B) and (E) viable-looking tumor tissue, and in (H) and (I) the abrupt interface between necrotic and viable tumor tissue. (C, F, I, and L) Higher power magnification (original magnification × 40) of viable tumor tissue with patent capillaries containing red blood cells (C) and (F), and capillaries occluded with thrombi at various stages of organization (I and L).
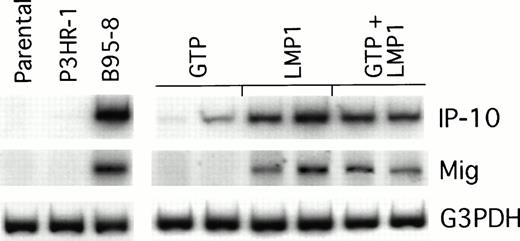
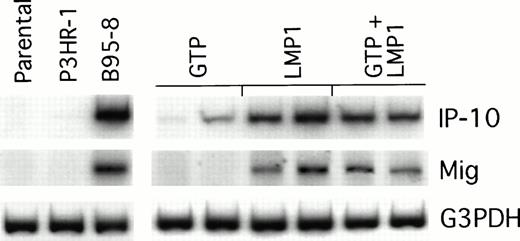
We looked for IP-10 and Mig gene expression in LMP1-expressing Burkitt tumors exhibiting macroscopic evidence of tissue necrosis, and compared it with that of control tumors growing progressively. The results of a semiquantitative RT-PCR analysis from representative regressing and progressively growing tumors are shown in Fig5. We found the RT-PCR products of murine IP-10 and Mig to be more abundant in regressing tumors induced by either B95-8–converted or LMP1-transfected BL41 cells than in the progressively growing tumors induced by either P3HR1-converted or vector control–transfected BL41 cells. Thus, the pattern of IP-10 and Mig expression in LMP1-expressing Burkitt tumors is indistinguishable from that displayed by EBV− Burkitt tumors treated with lymphoblastoid cells.5 6
Chemokine mRNA expression in LMP1-expressing and LMP1-nonexpressing Burkitt's tumors shown by semiquantitative RT-PCR analysis. Tumor tissue was obtained from Burkitt's tumors established in BALB/c nu/nu mice by subcutaneous inoculation of 107cells from LMP1-nonexpressing (parental BL41, P3HR-1–converted BL41, or GTP-transfected BL41) or LMP1-expressing BL cells (B95-8–converted BL41, LMP1-transfected BL41, or mixtures of GTP and LMP1-transfected BL41 cells). Tumors were obtained either when tumors were greater than 8 cm2 or when they developed typical tumor necrosis and scarring lesions that are indicative of tumor regression.
Chemokine mRNA expression in LMP1-expressing and LMP1-nonexpressing Burkitt's tumors shown by semiquantitative RT-PCR analysis. Tumor tissue was obtained from Burkitt's tumors established in BALB/c nu/nu mice by subcutaneous inoculation of 107cells from LMP1-nonexpressing (parental BL41, P3HR-1–converted BL41, or GTP-transfected BL41) or LMP1-expressing BL cells (B95-8–converted BL41, LMP1-transfected BL41, or mixtures of GTP and LMP1-transfected BL41 cells). Tumors were obtained either when tumors were greater than 8 cm2 or when they developed typical tumor necrosis and scarring lesions that are indicative of tumor regression.
DISCUSSION
In this study, we have found evidence supporting a pivotal role for the EBV latency protein LMP1 as an inducer of T-cell independent antitumor responses elicited by EBV-immortalized cells. When injected subcutaneously into athymic mice, EBV− BL cell lines and EBV+ BL cell lines that do not express LMP1 generally gave rise to progressively growing subcutaneous tumors. In contrast, EBV+ BL cell lines expressing LMP1 produced subcutaneous tumors that spontaneously regressed. When LMP1-expressing BL cell lines were injected in conjunction with EBV− BL cell lines in the same subcutaneous site, the resultant tumors often regressed spontaneously, indicating a bystander effect. The importance of LMP1 expression was confirmed in experiments with the EBV− BL cell lines BL30 and BL41, and the EBV+ converted BL30 and BL41 cell lines. When the infecting virus was derived from the B95-8 cell line and induced the expression of LMP1 protein, the BL30 and BL41 cell lines produced tumors that spontaneously regressed in most nude mice. However, when the infecting virus was derived from the P3HR-1 cell line and, as expected, did not induce LMP1 expression, the BL30 and BL41 cell lines always produced progressively growing tumors in this model. Concordantly, tumors derived from coinjection of autologous EBV− and LMP1-expressing BL30 or BL41 cell lines spontaneously regressed in most cases. Furthermore, the EBV− BL41 cell line transfected with the LMP1 gene alone gave rise to tumors that spontaneously regressed in many cases. Histologically, regressing Burkitt tumors induced by the inoculation of LMP1-expressing EBV− Burkitt cell lines were indistinguishable from the regressing Burkitt tumors induced by the inoculation of EBV− Burkitt cell lines in conjunction with EBV+ lymphoblastoid cell lines.3 In addition, Burkitt tumors regressing because of their expression of LMP1 expressed the murine chemokines IP-10 and Mig, indicative of the induction of a host response similar to that induced by lymphoblastoid cells.5 6
The effects of LMP1 expression on the tumorigenicity of the EBV− BL41 cell line had previously been examined in transiently immunosuppressed and SCID mice,14 two murine models that differ substantially from the nude mouse model used here. In spite of these experimental differences, these models support the view that expression of LMP1 reduced the ability of BL cells to grow as subcutaneous tumors in vivo. The investigators have explained these earlier results on the basis of LMP1 activities on cell growth, and this was supported by in vitro results documenting that LMP1 expression impaired BL cells' growth in suspension and reduced their clonability in agar.14 Other experiments have confirmed that LMP1 can be toxic or growth inhibitory to cells when expressed at high levels.22-24 However, LMP1 was also reported to increase B-cell survival, possibly through induction of the bcl-2 oncogene.25 26
A direct effect of LMP1 on BL cell growth or survival is unlikely to explain the frequent occurrence of spontaneous tumor regressions associated with LMP1 expression. Here, tumor regressions occurred 7 weeks (±3 weeks) after tumor appearance, suggesting that regression of LMP1-expressing BL cells is a consequence of T-cell independent host responses elicited by LMP1. This would explain why EBV−tumors of various lineages can also be induced to regress by LMP1-expressing cells through a bystander effect.4 Previous experiments have identified several steps in the murine response to human BL cells that seem critical to tumor regression, including the expression of the murine chemokines IP-10 and Mig, mediators that can inhibit tumor angiogenesis and cause vascular damage.5 6However, the mechanism by which LMP1 can initiate this host response is unclear.
LMP1, an integral membrane protein consisting of an amino terminal cytoplasmic domain 23 amino acids long, six hydrophobic transmembrane domains, and a carboxy terminal cytoplasmic domain 200 amino acids long, is emerging as a multifunctional protein that plays a critical role in EBV transformation, and is responsible for many of the phenotypic changes characteristic of EBV-immortalized cells. Using EBV recombinants, the cytoplasmic C-terminal 200 amino acids were found to be required for efficient B-cell immortalization.27 Within the first 45 C-terminal amino acids (amino acids 187-231), a domain was identified that enables LMP1 to associate with tumor necrosis factor receptor associated factors (TRAFs),28-30 components of a signal transduction pathway used by members of the tumor necrosis factor receptor family. Because the transmembrane domains enable LMP1 to aggregate in the plasma membrane, LMP1 may mimic the aggregation of tumor necrosis factor receptor-ligand complexes that leads to signaling.31-34 Recently, the LMP1 TRAF binding domain was found to be critical to transformation of B lymphocytes,35and to mediate activation of NF-κB transcription factor.36,37 An additional and more potent NF-κB activation domain was identified within the LMP1 C-terminal amino acids 352-386, acting independently of the TRAF binding domain.30,36,37 Because of NF-κB's ability to stimulate transcription of a variety of genes that contain κB elements,38,39 NF-κB might mediate many of the phenotypic changes induced by LMP1.17,36,37,40 41
Based on these structure-function relationships, LMP1 could induce an effective murine response through activation of NF-κB transcription factor, and secondary secretion of cytokines, such as interleukin-6 (IL-6), tumor necrosis factor–α (TNFα), and IL-12. In previous experiments, human IL-6 and TNFα failed to reproduce the antitumor effects exhibited by EBV-immortalized cells in the nude mouse.3 Human IL-12, produced by EBV-immortalized cells, does not stimulate a chemokine response in the mouse because of its strict species-specificity. However, other NF-κB–induced gene products, alone or in combinations, might serve as inducers of an effective antitumor response in the mouse. An alternative possibility, also involving NF-κB transcription factor activation, is that expression of certain adhesion molecules induced by LMP1, such as LFA1 and ICAM,41,40 might be critical to engagement and activation of the host cells. Previous experiments have identified macrophages and endothelial cells as the murine cells primarily responsible for the secretion of IP-10 and Mig in BL tumors that regress.5,6 These cells constitutively express certain integrins that could serve as receptors for ligands such as LFA1 and ICAM1.42 Occupation of integrin receptors by their ligands can result in signaling and cell activation.43
Among EBV latency genes, LMP1 is essential to B-cell immortalization,35 and is the only EBV gene that can transform nonlymphoid cells.44 Expression of LMP1 in a variety of human malignancies, including nasopharyngeal carcinoma, Hodgkin's disease, nasal and nasal type T/natural killer (NK) cell lymphomas and lymphoid granulomatosis, is considered relevant to tumorigenicity in humans.2 Our observations indicate that in addition to its role in growth transformation, LMP1 also plays a role as an inducer of antitumor responses. Biopsy specimens from cases of nasal or nasal-type T/NK cell lymphomas and lymphoid granulomatosis usually express LMP1 and display histological evidence of tumor tissue necrosis and vascular damage that are similar to those of BL tumors treated with lymphoblastoid cells in the mouse.45 We have detected high level expression of the chemokines IP-10 and Mig in the tissue specimens from cases of T/NK cell lymphomas and lymphoid granulomatosis.46 Thus, chemokine expression, vascular damage, and tumor tissue necrosis might represent evidence of LMP1-induced responses.
Elucidation of the mechanisms by which LMP1 mediates antitumor responses will be important to our understanding of the complexities of this protein and might lead to novel therapeutic approaches to cancer treatment.
ACKNOWLEDGMENT
The authors thank Dr G. Gupta for performing statistical analysis, Dr J. Teruya-Feldstein for histopathologic analysis, and Dr K. Bhatia for helpful discussions.
Address reprint requests to Barry W. Cherney, PhD, Center for Biologics Evaluation and Research, Bldg 29A, Room 2D06, 1401 Rockville Pike (HFM535), Rockville, MD 20852-1448.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.