Abstract
Hematopoietic growth factors have shown clinical benefits in patients undergoing chemotherapy and stem cell transplantation, but few studies have been performed to assess whether the benefits are worth the costs. We reviewed 196 patients undergoing T-cell depleted related donor bone marrow transplantation (BMT) between 1990 and 1996 to assess the effect of growth factor use on time to engraftment and costs of hospitalization. Beginning in 1994, based on encouraging results in autologous transplantation, patients (n = 81) were treated with granulocyte colony-stimulating factor (G-CSF) starting at day +1 after marrow infusion until engraftment. Between January 1, 1990 and January 1, 1994, patients (n = 115) did not receive growth factor. CD6 depletion of donor marrow was the only form of prophylaxis against graft-versus-host disease (GVHD). Despite receiving a lower stem cell dose (P = .004), the group receiving G-CSF had a decreased time to engraftment (20 days v 12 days, P < .0001) and time from marrow infusion to discharge (23 days v 17 days,P < .0001). In multivariate modeling, the use of G-CSF was the most significant factor predicting time to engraftment and discharge. Incidence of grades II-IV GVHD, early mortality, percentage of patients who engrafted, and relapse rates did not differ between the groups. Inpatient charges during the first 50 days after marrow infusion (including readmissions) were available on 110 patients and were converted to costs using departmental ratios of costs of charges. Median costs were significantly lower in the group receiving G-CSF ($80,600 v $84,000, P = .0373). Thus, use of G-CSF in this setting allows earlier hospital discharge with lower costs.
© 1998 by The American Society of Hematology.
HEMATOPOIETIC GROWTH factors have been used during stem cell transplantation in an effort to speed engraftment, decrease infectious complications, shorten length of hospitalization, and decrease costs. In autologous transplantation, growth factors are commonly administered due to favorable effects on time to engraftment and length of hospitalization.1-5 Three reports of randomized trials in autologous patients have also noted other benefits including decreased bacterial infections5and costs.4 6
In allogeneic transplantation, the efficacy of growth factors appears to depend on whether or not methotrexate is used for graft-versus-host disease (GVHD) prophylaxis. In patients who receive only cyclosporine with or without steroids or T-cell depletion, growth factors decrease time to engraftment.7-12 However, it is controversial whether earlier engraftment in allogeneic marrow recipients is associated with improvement in other clinical outcomes. One study documented decreased mucositis, infections, and duration of hospitalization,7 while another concluded that shortened duration of neutropenia did not translate into clinical benefits.8 In patients receiving methotrexate for GVHD prophylaxis, the majority of studies have not reported earlier engraftment or other benefits.9 12-14
Before January 1, 1994, granulocyte colony-stimulating factor (G-CSF) was not routinely administered at our institution. After January 1, 1994, we added G-CSF to the standard supportive regimen in our T-cell–depleted allogeneic transplant protocols based on the efficacy of growth factors after autologous bone marrow transplantation (BMT). G-CSF was administered at a dose of 5 μg/kg/d starting at day +1 after marrow infusion and continued until engraftment. No other changes were made in prophylaxis for GVHD or infection. This large cohort provided the opportunity to study effects of G-CSF on time to engraftment, length of hospitalization, and inpatient costs to help determine whether the additional cost of growth factor administration is justified.
MATERIALS AND METHODS
Patients.
Information on 196 patients undergoing matched related donor BMT between January 1990 and December 1996 for a variety of hematologic malignancies was abstracted from the clinical database of the Dana-Farber Cancer Institute. Patient-donor pairs were HLA-identical by serologic or molecular matching and tested for mixed lymphocyte reactivity. Patients were excluded from this analysis if they had previously undergone autologous or allogeneic stem cell transplantation, were conditioned with busulfan/cyclophosphamide or received grafts from syngeneic, mismatched or unrelated donors. All patients meeting the eligibility criteria were included in this analysis.
All patients received cyclophosphamide 60 mg/kg intravenously (IV) for 2 days and total body irradiation (1,400 to 1,560 cGy) before bone marrow infusion on day 0. GVHD prophylaxis consisted solely of T-cell depletion with T12, an anti-CD6 monoclonal IgM antibody, and rabbit complement resulting in a 1.5 to 2.0 log decrease in T cells.15 16 No methotrexate, cyclosporine, or corticosteroids was administered prophylactically posttransplant. Patients were treated in HEPA-filtered rooms and received oral prophylactic antibiotics and acyclovir. Before January 1, 1994, G-CSF was not given to allogeneic transplant patients. After January 1, 1994, G-CSF (5 μg/kg/d subcutaneously or IV) was administered to all patients beginning on day +1 after marrow infusion until the absolute neutrophil count was greater than 1,000 cells/μL. Patients were discharged from the hospital once engrafted, afebrile, and with adequate oral intake. Other than the institution of routine G-CSF therapy, there were no identifiable changes in use of prophylactic medications or supportive care during this time period. Patients living outside the local area were generally kept in the vicinity for approximately 50 days postmarrow infusion and admitted to the Dana-Farber Cancer Institute if hospital readmission was necessary.
Clinical endpoints.
Engraftment was defined as the first day an absolute neutrophil count (ANC) greater than 500 × 106 cells/L was achieved as documented on two separate determinations. Failure to engraft was defined as an ANC less than 100 × 106 cells/L after day 28. Time to engraftment and length of stay (LOS) was measured from date of marrow infusion until engraftment and hospital discharge, respectively. Acute GVHD was graded according to standard criteria.17
Costs.
We limited the cost analysis to patients transplanted after September 1992 because information was not available electronically before this date. All inpatient charge information after September 1992 was retrieved from the institutional accounting system for the period of hospitalization through the first 50 days after marrow infusion. All charges were included for hospital readmissions before day +50 and for patients whose initial transplant hospitalization extended beyond day +50. Charges for the donor work-up, harvest, and hospitalization were excluded. Patient bills documenting individual items were not available for analysis, and thus, we relied on aggregated costs by department. Unfortunately, this did not allow examination of specific contributors to costs.
Because of the retrospective nature of this analysis, it was not possible to collect costs accrued outside the Dana-Farber Cancer Institute’s inpatient accounting system. These include costs of medications obtained from pharmacies outside the Dana-Farber Cancer Institute and outpatient charges. In addition, nonmedical costs such as caregiver time, transportation, and local housing costs could not be ascertained for this analysis. Costs were calculated from charges using departmental cost to charge ratios. All costs were inflation-adjusted to 1996 dollars using the medical consumer price index.
Statistical analysis.
Univariate analyses were performed to compare patients’ characteristics, costs, and clinical endpoints between those who did and did not receive G-CSF (Fisher’s exact test and Wilcoxon rank-sum test, two-sided). Multiple regression models were fit to determine the effect of G-CSF on the costs and the clinical endpoints adjusting for differences in the patient characteristics. A linear model was used for the hospital cost and the natural logarithms of time to engraftment (days), length of stay, and time from engraftment to discharge. A proportional hazards model was used to model time to relapse. Significance testing of covariates in the models was done at alpha = 0.05. No adjustments for multiple endpoint testing were applied to theP values.
Patient characteristics compared included patient and donor age, donor-patient gender combinations (female donor into male patientv all other combinations), disease status (early diseasev advanced disease), degree of pretreatment (heavily treatedv not heavily pretreated), cytomegalovirus (CMV) status of donor-patient (both negative v all other combinations), stem cell dose, and the dose of total body irradiation (1,400 cGy, 1,480 cGy, or 1,560 cGy). The clinical endpoints compared were failure to engraft, time to engraftment, LOS, time from engraftment to discharge, survival to 50 days post-stem cell infusion, maximum grade of acute GVHD (grades 0 to 1 v 2 to 4), and time to relapse. In the regression models, presence of grade 2 to 4 acute GVHD was included in addition to patient characteristics. To adjust for the difference in time to engraftment among patients, this variable was included as a covariate in the model of time from engraftment to discharge.
Patients were considered to have early disease if they were transplanted for acute myelogenous leukemia (AML) or acute lymphocytic leukemia (ALL) in first remission, or chronic myeloid leukemia (CML) in chronic phase. All other disease states were classified as advanced disease. Most patients were classified as heavily pretreated, as all patients with AML, ALL, multiple myeloma (MM), non-Hodgkin’s lymphoma (NHL), and chronic lymphocytic leukemia (CLL) received combination chemotherapy before transplantation. Patients with CML were considered heavily pretreated if they received chemotherapy other than hydroxyurea, busulfan, or interferon. Approximately 50% of patients transplanted for myelodysplastic syndrome received chemotherapy before transplantation and were considered heavily pretreated.
RESULTS
Patient characteristics.
Patient characteristics are shown in Table1. There were 111 men and 85 women. Several hematologic diseases were represented (50 CML, 39 MM, 38 AML, 20 ALL, 19 NHL, 18 CLL, 11 myelodysplastic syndrome [MDS], and 1 Hodgkin’s disease [HD]). There was no difference between the groups that did and did not receive G-CSF in terms of patient age, patient/donor gender combinations, disease status, and degree of pretreatment. The later cohort receiving G-CSF received a significantly lower median stem cell dose (4.8 v 5.8 × 106lymphomononuclear cells/kg patient weight, P = .004), was more likely to be CMV seronegative in both patient/donor (P= .03), and received more radiation (P < .0001) than patients treated earlier who did not receive G-CSF. Seventeen patients were missing data for one or more of the covariates and are not included in the regression models.
Neutrophil engraftment.
Clinical outcomes are shown in Table 2. Only one patient in each group failed to engraft (1%). There was a statistically significant difference between median day of neutrophil engraftment for patients who received and did not receive G-CSF (12 days v 20 days, P < .0001). Patients receiving G-CSF engrafted faster despite receiving a lower stem cell dose. In multivariate analysis (based on 177 patients with complete covariate and time to engraftment data), heavy pretreatment (P = .0002), acute GVHD grade II to IV (P = .03) and use of G-CSF (P< .0001) predicted decreased logarithm of time to engraftment. No other variables examined were predictive.
Acute GVHD, early mortality, and relapse.
Incidence of grades II to IV acute GVHD (18% v.19%, P= .99) and death within the first 50 days after stem cell infusion (3%v 5%, P = .72) were low in both cohorts and not significantly different. Relapse rates at 2 years were identical among the groups (35% v 35%, P = .99). In multivariate modeling, the predictors of longer time to relapse were early disease (P = .005) and whether a male patient had a female donor (P = .04).
Length of hospitalization.
Length of transplant hospitalization (measured from the time of marrow infusion) was significantly less for the patients receiving G-CSF than for those not receiving G-CSF (17 days v 23 days, P < .0001). Multivariate analysis (based on 179 patients with complete data) controlling for other factors showed that decreased length of hospitalization was associated with use of G-CSF (P = .0001) and whether patients were heavily pretreated (P = .02). In the subset of patients used for the cost analysis (n = 110), readmission rates did not differ significantly between patients who did and did not receive G-CSF (13.2% v 9.5%, P = .768) and median total inpatient days (including readmissions) within 50 days of marrow infusion (17 days v 24 days, P = .0001) remained significantly different.
We were concerned that better outpatient supportive care in recent years may have allowed earlier discharge after engraftment accounting for the decreased length of hospitalization. We did not find evidence for this. Table 3 shows the median time to engraftment, time from engraftment to discharge, and LOS by year of transplant. There was an abrupt decrease in median time to engraftment and median length of hospitalization in 1994, the year G-CSF was instituted. Median time from engraftment to discharge was consistently 3 days except in 1994 when it was 5 days. In univariate comparisons, the time from engraftment to discharge was longer for patients receiving G-CSF (3 v 4 days, P = .002). This difference, however, was not significant after adjusting for other covariates including time to engraftment (P = .0001) in a multivariate model. Other significant predictors of longer time from engraftment to discharge were older patient age (P = .005) and negative donor-patient CMV status (P = .02).
Costs.
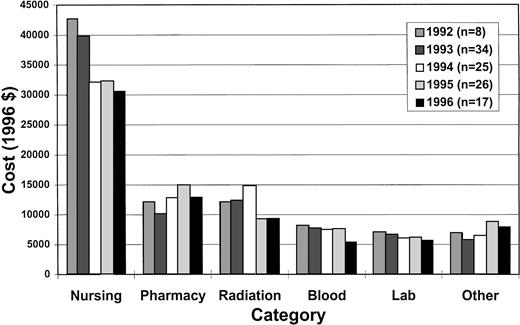
Table 4 and Fig 1 show the results of the cost analysis. Overall costs, the five highest cost categories (nursing, pharmacy, radiation therapy, blood bank, and laboratory) and miscellaneous other costs are shown. Room and board costs are included in the nursing category. The median overall costs were significantly different between those who did and did not receive G-CSF ($80,600v $84,000, P = .0373). As expected, there were higher median pharmacy costs ($13,100 v $10,600, P = .0055) and lower nursing costs ($32,000 v $39,800,P = .0001) in the G-CSF group. However, radiation therapy costs were significantly higher in the group that did not receive G-CSF ($12,300 v $9,300, P = .0008) despite lower radiation doses, attributable to ancillary charges (predominantly ambulance transportation charges). When radiation therapy costs were excluded from the analysis, median total costs fell ($67,100 v $71,200,P = .0710). In multivariate analysis of overall costs (n = 99), the only variable associated with lower costs was heavy pretreatment (P = .006). This may be due to earlier engraftment in the heavily pretreated, more immunosuppressed patients, as this association disappeared when LOS was added as a covariate to the model.
Cost categories. The median costs of different categories is shown by year of transplant.
Cost categories. The median costs of different categories is shown by year of transplant.
DISCUSSION
This study confirms that G-CSF decreases time to engraftment (20 daysv 12 days, P < .0001) and length of hospitalization (23 days v 17 days, P < .0001) in patients undergoing T-cell–depleted allogeneic transplantation. In addition, total hospital costs were lower when G-CSF was used ($84,000 v$80,600, P = .0373). No study of either autologous or allogeneic transplantation, including ours, has ever detected a difference in rates of GVHD, relapse, or overall survival. Thus, our study suggests that G-CSF results in a shorter length of hospitalization and cost savings when used as an adjunct in T-cell–depleted marrow transplantation.
Although our analysis was retrospective and used sequential cohorts, comparison of patient characteristics suggests that the two groups were fairly similar, and the characteristics, which did differ between groups, were not shown to be predictive of time to engraftment, length of hospitalization, and costs in multivariate analysis. We were concerned that the changing nature of supportive care may have accounted for improved outcomes in later years after institution of G-CSF. However, examination of Table 3 shows there was an abrupt decrease in time to engraftment and length of hospitalization after G-CSF was added, which was maintained in years 1994 to 1996. In addition, if expanded home care and outpatient options were responsible for decreased length of hospitalization, we might expect to see a decreasing time from engraftment to discharge. We did not find evidence for this. In fact, early engraftment was negatively correlated with time from engraftment to discharge, perhaps because other organ toxicity then became the limiting factor. The aberrantly long time from engraftment to discharge in 1994 might be explained as a period of adjustment as clinicians adapted their practice to faster engraftment times, as this interval fell to the baseline value of 3 days after 1994.
The difference in costs was not as striking as might have been expected given the highly statistically significant difference in time to engraftment and LOS. This may be due to several reasons. First, although costs of nursing (which includes room and board) declined as LOS decreased with G-CSF, they were somewhat offset by increased pharmacy costs. Second, as clinicians know, outcomes of transplantation, and thus costs, are often unpredictable. There was substantial variability in the underlying distributions of costs in both groups, making it more difficult to show statistically significant differences even if the median values of the groups appeared different. In addition, due to limitations of the accounting system, we were only able to obtain costs on 42 patients in the non–G-CSF group, which limited the power of the analysis. Finally, this study compared sequential cohorts of patients, and hospital policies and cost-accounting methods do change over time. Lower radiation therapy costs in the group receiving G-CSF contributed to the statistically significant difference in overall costs, although the decrease was attributable to a change in hospital policy, which allowed transport of patients by hospital personnel instead of by ambulance, and not to use of G-CSF. These influences on costs highlight the fact that cost determinants may be very different than traditional clinical outcomes, and that studies must be powered accordingly. Although a randomized trial would be a preferable study design for costs, the size of the trial would need to be significantly greater for a cost endpoint than a clinical endpoint, and is currently prohibitive.
We found that heavy pretreatment was the most predictive of lower total hospital costs. We hypothesize that these patients are more immunosuppressed when they enter transplant allowing faster engraftment. Indeed, heavy pretreatment was found to be predictive of time to engraftment, and when LOS was added as a covariate in the cost model, heavy pretreatment was no longer statistically significant.
Several changes in clinical practice could reduce the total dose of growth factors administered and perhaps increase cost savings. It may be possible to delay the start of growth factor therapy or give lower doses without compromising efficacy. In a study of patients receiving autologous marrow, a dose response effect of postinfusion G-CSF was noted, but even the lowest dose of 2 μg/kg/d resulted in significantly decreased time to engraftment.1 A study of 38 patients undergoing chemotherapy/G-CSF primed autologous peripheral blood transplantation randomized patients to either low dose G-CSF (50 μg/m2/d) or placebo post-stem cell infusion. This study found that lower than conventional doses of growth factor still resulted in decreased duration of neutropenia and length of hospitalization.4 The only dose finding study in the allogeneic setting was performed by Nemunaitis et al evaluating GM-CSF in matched sibling marrow transplantation. Their study did not document a dose response because engraftment was accelerated equally by 30 μg/m2 and 250 μg/m2.9 Nevertheless, the higher dose has been chosen for clinical practice.
Thus, our study suggests that use of G-CSF in T-cell–depleted related donor marrow transplantation resulted in faster engraftment, earlier hospital discharge, and lower inpatient costs without compromising clinical outcomes. This study supports the use of G-CSF in this setting from a clinical and economic perspective.
ACKNOWLEDGMENT
The authors thank our colleagues at the Dana-Farber Cancer Institute for the care of these patients and Maryanne Mullen for helping with the cost data.
Supported by Grants No. CA75267-01 and AI29530 from the National Institutes of Health, Bethesda, MD.
Address reprint requests to Robert J. Soiffer, MD, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.