In vitro studies on hematopoietic control mechanisms have been hampered by the heterogeneity of the analyzed cell populations, ie, lack of lineage specificity and developmental stage homogeneity of progenitor/precursor cells growing in culture. We developed unicellular culture systems for unilineage differentiation of purified hematopoietic progenitor cells followed by daughter cell analysis at cellular and molecular level. In the culture system reported here, (1) the growth factor (GF) stimulus induces cord blood (CB) progenitor cells to proliferate and differentiate/mature exclusively along the erythroid lineage; (2) this erythropoietic wave is characterized by less than 4% apoptotic cells; (3) asymmetric divisions are virtually absent, ie, nonresponsive hematopoietic progenitors with no erythropoietic potential are forced into apoptosis; (4) the system is cell division controlled (cdc), ie, the number of divisions performed by each cell is monitored. Single-cell reverse transcriptase-polymerase chain reaction (RT-PCR) analysis was applied to this culture system to investigate gene expression of diverse receptors, markers of differentiation, and transcription factors (EKLF, GATA-1, GATA-2, p45 NF-E2, PU.1, and SCL/Tal1) at discrete stages of erythropoietic development. Freshly isolated CD34+ cells expressed CD34, c-kit, PU.1, and GATA-2 but did not express CD36, erythropoietin receptor (EpoR), SCL/Tal1, EKLF, NF-E2, GATA-1, or glyocophorin A (GPA). In early to intermediate stages of erythroid differentiation we monitored the induction of CD36, Tal1, EKLF, NF-E2, and GATA-1 that preceeded expression of EpoR. In late stages of erythroid maturation, GPA was upregulated, whereas CD34, c-kit, PU.1, and GATA-2 were barely or not detected. In addition, competitive single-cell RT-PCR was used to assay CD34 mRNA transcripts in sibling CD34+CD38− cells differentiating in unilineage erythroid cultures: this analysis allowed us to semiquantitate the gradual downmodulation of CD34 mRNA from progenitor cells through their differentiating erythroid progeny. It is concluded that this novel culture system, coupled with single-cell RT-PCR analysis, may eliminate the ambiguities intrinsic to molecular studies on heterogeneous populations of hematopoietic progenitors/precursors growing in culture, particularly in the initial stages of development.
DIFFERENTIATION OF hematopoietic stem cells is a regulated developmental cascade that generates lineage(s)-committed progenitor cells that feed into maturing precursors and then terminal elements circulating in peripheral blood (PB). Hematopoietic stem cells possess three important properties: (1) extensive self-renewal capacity, (2) broad differentiation potential, and (3) prolonged maintenance in a noncycling state. These features gradually taper off in differentiating progenitors.1-3 Upon induction to cycling, stem cells and primitive progenitors may undergo symmetric divisions (ie, either self-replication or differentiation) and/or asymmetric divisions (ie, generation of a self-replicated and a differentiated cell).4 5
Hematopoietic stem/progenitor cell differentiation has been interpreted in terms of stochastic,6 inductive,7 or hybrid models.8 According to the stochastic hypothesis, random intrinsic molecular events are responsible for committment, whereas exogenous hematopoietic growth factors allow survival and proliferation of preprogrammed cells.9,10 According to the inductive model, hematopoietic growth factors and cell-mediated regulatory mechanisms trigger stem/progenitor cells to differentiate along a particular lineage(s).11 Alternatively, a hybrid model proposes that stochastic events prevail at early developmental stages, whereas growth factor-mediated inductive events operate thereafter.12 Indeed, purified hematopoietic progenitors express the receptors for multilineage growth factors (ie, interleukin-3 [IL-3] and granulocyte-macrophage colony-stimulating factor [GM-CSF]), but barely express or do not express the receptors for late-acting unilineage cytokines (ie, erythropoietin [Epo], and macrophage colony-stimulating factor [M-CSF]) as well as mainly late-acting unilineage cytokines (ie, thrombopoietin [TPO] and granulocyte colony-stimulating factor [G-CSF]). The latter receptors accumulate through differentiation/maturation in their specific lineage, whereas they are downmodulated in the other series. Furthermore, interaction of early hematopoietic growth factors with their receptors upmodulates distal growth factor receptors.13 Regardless of the proposed model, it is generally accepted that lineage choice is mediated via activation of differentiation gene program(s) and specifically via a network of transcription factors, which orchestrate these gene programs at the transcriptional level.14-16 Leukemia cell lines and murine hematopoietic progenitor cell lines (32D and FDCP-Mix)17,18have been extensively used for transcription factor studies; however, they reflect selected stages and lineages of normal hematopoiesis and are partially or totally independent of growth factors and other physiological control mechanisms. Studies on knock-out mice have provided basic insight into molecular mechanisms underlying hematopoiesis,16 but results may not always apply to human adult hematopoiesis due to several limitations such as gene redundancy, position effects of the disrupted gene on adjacent genes, lethal effects in early ontogenesis, lack of tissue specificity, and species differences.14,19 In vitro studies on hematopoietic control mechanisms have been hampered by (1) the extreme rarity of early hematopoietic progenitors and stem cells, (2) limitations of current protocols for purification of homogeneous stem/progenitor cell subsets, and (3) unavailability of pure progenitors and precursors at discrete developmental stages through a selected lineage. Analysis of the molecular basis of human adult hematopoiesis has been facilitated by development of unilineage progenitor cell differentiation in bulk culture.20-27 In these systems, purified early progenitor/stem cells are induced into a wave of gradual differentiation/maturation along a specific lineage(s), thus providing a tool to evaluate expression and function of developmentally regulated genes. However, unilineage bulk cultures bear two limitations: (1) hematopoietic progenitors programmed for differentiation along other lineage(s) undergo apoptosis and (2) cells analyzed at a given culture time reflect a narrow but still overlapping range of different developmental stages.
We have developed a novel strategy based on hematopoietic progenitor cell unicellular, unilineage differentiation culture, followed by daughter cell analysis at cellular and molecular levels. This requires well-defined culture conditions20-27 as well as stringently controlled reverse transcriptase-polymerase chain reaction (RT-PCR) technology, ie, the unambiguous detection of multiple genes in single progenitor/precursors undergoing unilineage differentiation/maturation. This approach was applied here to evaluate the pattern of gene expression at discrete stages of cord blood (CB) progenitor cell differentiation and maturation along the erythroid lineage by RT-PCR analysis at the single-cell level. Single-cell competitive RT-PCR was also performed in selected experiments. We show that discrete developmental stages of erythroid differentiation (defined by the number of cell divisions performed by each progenitor cell) are characterized by a specific expression pattern of genes coding for transcription factors, growth factor receptors, and differentiation markers.
MATERIALS AND METHODS
Cell Preparation
CB was obtained from healthy, full-term neonates according to institutional guidelines in 50 mL polypropylene tubes containing 20 U/mL preservative-free heparin, 1 mmol/L adenosine, and 2 mmol/L theophylline.28 Low-density cells (<1.077 g/mL) were isolated using first Ficoll (Biochrom KG, Berlin, Germany) and then a discontinuous Percoll gradient (LD/P cells) as described previously.28 The CD34+ cells were isolated by using the MiniMACS CD34 isolation system (Miltenyi, Bergisch Gladbach, Germany) following the manufacturer‘s instructions and then sorted by flow cytometry. The CD34+Lin− cells were purified by a positive/negative selection strategy using the MiniMACS CD34 Multisort kit (Miltenyi). Briefly, 2 to 3 × 108LD/P cells were incubated for 5 minutes at 12°C in 200 μL FcR blocking reagent (Miltenyi). Cells were then incubated without washing with 200 μL CD34 Multisort microbeads and 25 μL of each of the following biotinylated Lin-Moabs in a total volume of 1 mL in phosphate-buffered saline (PBS)-EDTA/0.5% bovine serum albumin (BSA): anti-CD2 (Immunotech, IM, Hamburg, Germany), anti-CD5, anti-CD10, anti-CD13, anti-CD19, anti-CD56 (Leinco, Ballwin, MO), anti-CD7 (Serotec, Oxford, UK), anti-CD11b, anti-CD11c, anti-CD14, anti-CD15 (Sigma, München, Germany), anti-CD16 (Pharmingen, Hamburg, Germany), anti-CD33, anti-CD45RA (Becton Dickinson, Heidelberg, Germany), anti-CD20, and anti-CD61 (Southern Biotechnology Associates, Birmingham, AL). Cells were incubated for 45 minutes at 4°C, centrifuged for 10 minutes at 300g, and resuspended in PBS-EDTA. Rinsed MS separation columns (Miltenyi) were placed into a magnetic support and 1 × 108 cells in 500 μL were loaded onto the column. The eluate comprising the CD34− fraction was disregarded and the column was washed 3 times with 500 μL of buffer. The CD34+ cells were isolated after removal of the column from the magnet and eluted with a total volume of 1.5 mL of buffer. This fraction containing CD34+ cells was loaded onto a second freshly prepared column and CD34+ cells isolated as described above. The microbead-labeled CD34+ cell suspension was incubated with 20 μL of Multisort Release Reagent per milliliter for 10 minutes at 12°C to release beads from cells. The cell suspension was then loaded onto a third freshly prepared MS column placed in a magnet. The eluate containing bead-free CD34+ cells was centrifuged through a cushion of PBS/10% BSA for 10 minutes at 600g. The pellet was resuspended after adding 30 μL of stop reagent and 10 μL of streptavidin-conjugated microbeads (Miltenyi) and then incubated for 30 minutes at 6°C. Cells were resuspended in 500 μL of buffer and loaded onto a freshly prepared MS column placed in a magnet. The eluate representing CD34+Lin−cells was further processed for fluorescence-activated cell sorting (FACS) analysis and cell culture.
Immunofluorescence Staining and Flow Cytometry
For purity control, purified CD34+Lin−cells were incubated for 10 minutes at 4°C in PBS/1% BSA containing 10 μg/100 μL of purified mouse IgG (Sigma). Cells were incubated without washing for 30 minutes at 4°C with phycoerythrin (PE)-conjugated anti-CD34 (8G12; Becton Dickinson) and streptavidin-fluorescein isothiocyanate (FITC; DAKO, Hamburg, Germany), or a cocktail of FITC-labeled Lin-MoAbs was used. After 3 washes with PBS/1% BSA, cells were analyzed using a Calibur flow cytometer (Becton Dickinson). CD34+Lin− cells induced to unilineage differentiation (bulk culture) were stained accordingly using PE-conjugated anti-CD34 and FITC-labeled anti-CD36, anti–c-kit, anti-GPA (Immunotech), or anti-CD71 (Becton Dickinson). For cell sorting, column-purified CD34+ cells (0.5 to 1 × 106/100 μL) were stained with anti-CD34-FITC (8G12) and anti-CD38-PE (Becton Dickinson) and then sorted with a FACS Vantage (Becton Dickinson) equipped with an automatic cell deposition unit (ACDU) essentially as described previously.29 Single sorted cells were processed for RT-PCR or unicellular, unilineage culture as described below.
Serum-Free Liquid Suspension Culture
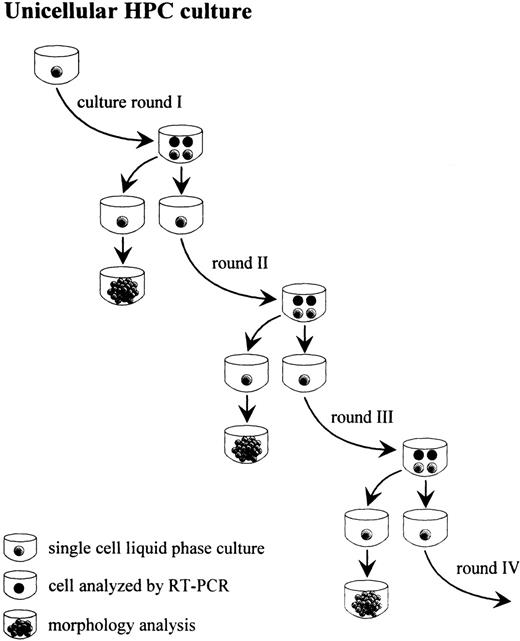
Single ACDU-sorted CD34+CD38− cells or CD34+Lin− cells were grown in individual wells of a round-bottom 96-well plate (Falcon, Heidelberg, Germany) in serum-free medium26 containing 0.1 U/mL IL-3, 0.05 U GM-CSF, and 3 U/mL Epo.22 Cells were incubated in a fully humidified atmosphere of 5% CO2/5% O2 in air. Each well was examined daily for evaluation of sibling cells and processed as shown in Fig 1. Briefly, when wells contained 4 cells (round I, 2 cell divisions), 1 cell was transferred in liquid culture and allowed to terminally differentiate/proliferate for morphologic evaluation, 1 cell was replated for serum-free liquid suspension culture, and each of the remaining 2 cells was processed separately for RT-PCR. A second, third, and fourth round of culture (4, 6, and 8 cell divisions, respectively) and RT-PCR analysis was performed for sibling cells. This culture system was designated cell division controlled (cdc) culture. For comparative experiments to examine the homogeneity of gene expression among daughter cells, 3 of 4 cells of round I, II, III, or IV from cdc cultures (Fig 1) were each subjected to RT-PCR as described below. In addition, single cells were grown in unilineage erythroid cultures until wells contained 4, 8 to 12, or 16 to 24 cells, ie, non-cell division number controlled (non-cdc) cultures, followed by single-cell RT-PCR analysis. Individual cells were isolated by using a siliconized Pasteur pipet attached to a micromanipulator. The frequency of apoptosis at the single-cell level was determined at initiation of single cell cultures, after 1 and 2 cell divisions, as shown in Fig 2, as well as after 6 cell divisions. Only wells containing a single cell at initiation of each culture as evaluated by light microscopy were monitored for 10 days. Cells were scored apoptotic if no cell division or no cell was detected during the evaluation period. For evaluation of symmetric cell divisions, 3 cells from each 4-cell stage (rounds I through III) were transferred into separate wells containing the unilineage erythroid growth factor cocktail and allowed to terminally differentiate/proliferate for morphologic analysis. The remaining fourth cell was used to initiate the subsequent culture round. For flow cytometry analysis (bulk culture experiments), 2.5 × 104CD34+Lin− cells were grown in 500 μL of the same medium used for unicellular culture and analyzed by FACS at days 0, 6, 10, and 14.
Unilineage, single-cell culture of purified CD34+Lin− cells or ACDU-sorted CD34+CD38− cells from CB: sibling analysis (clonogenic capacity and gene expression, as evaluated by single cell RT-PCR) at sequential rounds of cell division.
Unilineage, single-cell culture of purified CD34+Lin− cells or ACDU-sorted CD34+CD38− cells from CB: sibling analysis (clonogenic capacity and gene expression, as evaluated by single cell RT-PCR) at sequential rounds of cell division.
Evaluation of apoptosis in unicellular, unilineage erythroid cultures. Single cells were scored apoptotic if no cell division(s) was detectable or if no cell(s) was detectable after 10 days of culture. At inititation of single-cell culture, each well was monitored by an inverted microscope. Only wells containing 1 cell were monitored. The proportion of single apoptotic cells was evaluated for freshly sorted CD34+CD38− cells or purified CD34+Lin− cells (A), siblings thereof, ie, after the first cell division (B), and single cells after 2 cell divisions (C).
Evaluation of apoptosis in unicellular, unilineage erythroid cultures. Single cells were scored apoptotic if no cell division(s) was detectable or if no cell(s) was detectable after 10 days of culture. At inititation of single-cell culture, each well was monitored by an inverted microscope. Only wells containing 1 cell were monitored. The proportion of single apoptotic cells was evaluated for freshly sorted CD34+CD38− cells or purified CD34+Lin− cells (A), siblings thereof, ie, after the first cell division (B), and single cells after 2 cell divisions (C).
Apoptosis in Unilineage Erythroid Bulk Culture
CB LD/P cells were labeled with PKH 26 (Sigma) essentially as described by the manufacturer. Briefly, 2 × 108 LD/P cells were incubated for 3 minutes at room temperature in 10 mL of diluent C (Sigma) containing 2 × 10−6 mol/L PKH26. The labeling reaction was stopped by adding 10 mL of heat-inactivated fetal calf serum (FCS; CCPro, Neustadt, Germany). Cells were washed with PBS/0.5% BSA and then processed for isolation of CD34+Lin− cells as described above. For detection of apoptotic cells, freshly purified PKH26 labeled CD34+Lin− cells and the same cells after 2 and 6 days of unilineage erythroid bulk culture were stained with 10 μL of FITC-conjugated Annexin V in 100 μL binding buffer (Apoptosis staining kit; R&D Systems, Minneapolis, MN). Cells were analyzed by using a FACSCalibur (Becton Dickinson).
Isolation of mRNA From Single Cells/Small Numbers of Cells and Sequence Specific (SSP) RT-PCR
The poly(A)+ mRNA was isolated by using the PolyATract system 1000 (Promega, Heidelberg, Germany). For single cells or small numbers of cells, ie, 100 cells, the system was scaled down to allow isolation of mRNAs.30,31 The mRNA isolation was performed with slight modifications of the manufacturer’s instructions. Briefly, cells were transferred to 25 μL guanidine thiocyanate (GuSCN) lysis buffer containing 2% β-mercaptoethanol (β-ME) in a 1.5 mL polypropylene tube and immediately vortexed thoroughly. Fifty microliters of dilution buffer preheated to 70°C containing 1% β-ME and 5 pmol biotinylated oligo(dT) were added. The homogenate was incubated at 70°C for 5 minutes and then centrifuged at 12.000g for 15 minutes at room temperature. The supernatant containing poly(A)+ mRNA hybridized to biotinylated oligo(dT) was transferred to a fresh tube containing washed streptavidin-conjugated paramagnetic particles (SA-PMP) and incubated for 5 minutes at room temperature. The SA-PMP-mRNA conjugates were then isolated by magnetic separation. To dissociate bound mRNAs from SA-PMPs, washed SA-PMP-mRNA hybrids were resuspended in RNase-free dH2O for 2 minutes. After magnetic separation, the supernatant containing dissociated mRNAs was transferred to a fresh tube, ethanol precipitated, and further processed for cDNA synthesis. The mRNA was dissolved in 10 μL reverse transcriptase buffer (50 mmol/L Tris-HCl, pH 8.3, 75 mmol/L KCl, 3 mmol/L MgCl2) containing 100 U Moloney murine leukemia virus (MMLV) reverse transcriptase (Superscript; BRL/LTI, Eggenstein, Germany), 0.2 μg oligo(dT), 15 U Rnasin (Promega), 10 mmol/L dithiothreitol (DTT), and 0.5 mmol/L of each dNTP and was reverse transcribed for 1 hour at 42°C. For RT-PCR analysis, the cDNA from individual cells was divided in up to 4 aliquots. Genbank locus and primer sequence were reported previously28 29 or were CD34 (Table 1), EKLF (HSU65404) positions 726-745 and 1051-1032; GATA-1 (HSERYF1), 1081-1100 and 1401-1381; GATA-2 (HUMGATA2A), 1007-1026 and 1493-1474; p45 NF-E2 (HUMNFE2A), 781-801 and 1214-1194; SCL/Tal1 (HUMSCLA), 2817-2838 and 3136-3115; PU.1 (HSSPI1), 213-232 and 758-739; β2-microglobulin (HUMB2M), 118-137 and 341-321; β-globin (HSBGL1), 377-396 and 531-510; EpoR (HUMERYTH), 887-897 and 1204-1185; CD13 (HUMAMIPEP), 2691-2710 and 2995-2976.
Each 50 μL of PCR reaction contained 1.25 U AmpliTaq DNA polymerase (Perkin Elmer Cetus, Überlingen, Germany), 200 μmol/L of each dNTP, and 0.5 μmol/L of each oligonucleotide primer in PCR buffer (10 mmol/L Tris-HCl, pH 8.3 to 9.0, 50 mmol/L KCl, 1.5 to 3.5 mmol/L MgCl2, and 0.001% gelatin). Reactions were amplified in a DNA thermal cycler (PCR 9600 system; Perkin Elmer Cetus) for 40 to 60 cycles using predetermined optimal cycling parameters for each primer pair. The sensitivity of the RT-PCR was determined by serial dilution of cDNA obtained from sorted cells that were CD34+, CD34+CD36+, CD34+c-kit+, GPA+CD36+, CD7+, CD13+, CD14+, CD16+, CD19+ cells or from single erythroblasts isolated from erythroid colonies (burst-forming unit-erythroid [BFU-E]), as described previously.28 Cell lines K562 or HL-60 were used for sensitivity testing for GATA-1, GATA-2, p45 NF-E2, EKLF, SCL/Tal1, and PU.1. The RT-PCR products were electrophoresed through a 2% SEAKEM (FMC BioProducts, Biozym, Hess. Oldendorf, Germany) agarose gel, stained, transferred onto Genescreen Plus membranes (DuPont, Bad Homburg, Germany), and hybridized with corresponding 32P-labeled 30-mer oligonucleotide probes using standard techniques.
Sequence-Independent (SIP) RT-PCR
SIP-RT-PCR was performed on mRNA, which was reverse transcribed with slight modifications as described for SSP-RT-PCR.32Briefly, the mRNA was dissolved in 4 μL reverse transcriptase buffer (see above) containing 100 U MMLV reverse transcriptase (BRL/LTI) and 2 U of AMV RTase (Promega), 0.1 μg oligo(dT)24, 15 U RNasin, 10 mmol/L DTT, and 2 μmol/L of each dNTP and reverse transcribed for 15 minutes at 37°C. Samples were heat-inactivated at 65°C for 10 minutes and then stored on ice. The cDNA was polyadenylate-tailed at 37°C for 30 minutes in an 8 μL reaction containing 200 μmol/L dATP and 10 U terminal transferase by using a terminal transferase kit (Boehringer Mannheim, Mannheim, Germany). PCR was performed in a volume of 50 μL using 5 μmol/L oligo(dT)24X primer.32 Ten microliters of SIP-RT-PCR product was electrophoresed through 2% agarose and hybridized using 32P-labeled 35-mer probes (sequences not shown) for the detection of the CD34, CD36, glycophorin A, epoR, c-kit, GATA-1, GATA-2, NF-E2, SCL/Tal1, EKLF, PU.1, or β2-microglobulin PCR product. In addition, 2.5 to 5 μL of SIP-RT-PCR product was reamplified using sequence-specific primers as well as intron spanning primers for detection of the β2-microglobulin gene.
Competitive RT-PCR
The cRNA deletion construct (dc) for the semiquantitation of CD34 mRNA was synthesized by a non–plasmid-based technique. Briefly, the RT-PCR product corresponding to wild-type (wt) CD34 was isolated from the agarose gel and reamplified with upstream primer DelCD34 (Table 1) and the downstream primer used for amplification of wt CD34. The resulting construct was separated by agarose gel electrophoresis, isolated, and then reamplified with wt upstream primer T7DelCD34 containing the T7 RNA polymerase promoter sequence at the 5′-end (Table 1) and the wt downstream primer pTCD34 containing a 5′-poly(T)24tail. The resulting PCR product was purified from the agarose gel and 1 μg was processed for in vitro mRNA transcription with the T7-Megascript kit following the manufacturer’s instructions (Ambion, Austin, TX). The DNase I-treated cRNA CD34 deletion construct was phenol/chloroform extracted, precipitated, and then quantitated by UV spectrophotometry. RT-PCR of the cRNA dc results in a 156-bp amplification product, whereas a 229-bp product is detected after RT-PCR of wt CD34 mRNA. To evaluate expression levels of CD34 mRNA, sorted CD34+CD38− cells were used for the competitive RT-PCR. A dilution series containing known amounts of cRNA dc, ie, 10, 100, 200, 400, 800, 1,600, or 3,200 transcripts, was added to each cell lysate derived from 10 CD34+CD38− cells and coisolated with the unknown amounts of wt CD34 mRNA by using the PolyATract System 1000 (data not shown). After reverse transcription of both dc cRNA and wt mRNA in a one-tube reaction, 40 cycles of competitive PCR in the presence of 50 μCi/mL α-32P-dATP was performed. Quantitation of mRNA was performed essentially as described previously.33 Briefly, RT-PCR products corresponding to wt CD34 and dc CD34 were excised from the agarose gel and the radioactive counts in each determined. To correct for lower α-32P-dATP incorporation into the smaller dc PCR product, cpm from dc bands were multiplied by 229/156. To determine the point of equivalence,33 values were plotted as log of input dc concentration (X-axis) versus the ratio of the corrected dc cpm to the wt cpm (Y-axis). Based on two experiments, 10 CD34+CD38− cells contained approximately 100 CD34 mRNA transcripts.
A simplified competitive RT-PCR protocol was performed for the semiquantitation of CD34 mRNA transcripts in individual cells. Thus, titration was omitted and the 8/10 of the mRNA isolated from a single cell was processed for RT-PCR in the presence of 10 CD34 cRNA dc transcripts. Based on prior titrations (see above), the number of cRNA dc transcripts corresponded to the approximate number of wt CD34 transcripts per CD34+CD38− cell, ie, approximate point of equivalence.
Immunohistochemistry
Immunohistochemistry was performed for CD34+Lin− cells induced to unilineage erythroid differentiation/proliferation (bulk culture) by immunogold staining with silver enhancement. Freshly isolated CD34+Lin− cells as well as cells after 6 and 12 days of culture were cytospun onto poly-L-lysine–coated slides, fixed for 20 minutes at 4°C with cold PBS/10% formaldehyde, and washed 2 times with PBS. Cytospins were treated for 3.5 minutes with PBS/0.125% Triton-X 100, washed with PBS, and then incubated for 30 minutes at room temperature with PBS/5% BSA/5% goat serum/0.5% cold water fish gelatin (blocking buffer). Slides were washed twice for 5 minutes with PBS and once with PBS/0.1% Aurion BSA-c/20 mmol/L NaN3 (incubation buffer; Biotrend, Köln, Germany) for 5 minutes and then incubated with rat anti–GATA-1 MoAb, mouse anti–GATA-2 MoAb, or rabbit anti–NF-E2 antibody (Santa Cruz Biotechnology, Heidelberg, Germany) for 45 minutes at room temperature. Slides were washed thoroughly twice for 10 minutes each with PBS and for 10 minutes with incubation buffer and then incubated with the appropriate secondary gold-conjugated antibody (ultrasmall gold-conjugated goat-antirat IgG, goat-antimouse IgG, goat-antirabbit IgG; Biotrend) for 2 hours at room temperature. Slides were washed thoroughly, postfixed for 5 minutes with PBS/2% glutaraldehyde, and then washed with PBS and 4 times for 5 minutes with dH2O. Slides were then incubated for 20 to 25 minutes at room temperature with silverenhancement reagent (Biotrend) and monitored by light microscopy for color development. After three 5-minute washes with dH2O, cells were counterstained with May-Grünwald-Giemsa.
Clonogenic Progenitor Cell Assay
One hundred CD34+Lin− cells/mL were plated in 35-mm plastic tissue culture dishes containing a 1 mL mixture of IMDM (Sigma), 1% methylcellulose (Sigma), 40% of pretested FCS (CCPro), 1% BSA (Sigma), 2 × 10−4 mol/L α-thioglycerol (Sigma), and 400 μg/mL fully iron-saturated human transferrin (Sigma). Cultures were supplemented with 500 U/mL IL-3 (Genzyme, Rüsselsheim, Germany), 100 U/mL GM-CSF (Genzyme), 100 ng/mL human stem cell factor (hSCF; kindly provided by Amgen, Thousand Oaks, CA), 80 ng flt-3 ligand (CCPro), 50 ng G-CSF (R&D Systems), 50 ng M-CSF (R&D Systems), and 3 U/mL Epo (kindly provided by Boehringer Mannheim). Cultures were incubated at 37°C in a 100% humidified atmosphere of 5% CO2/5% O2 in air. BFU-E, colony-forming units–granulocyte-macrophage (CFU-GM), and CFU-Mix comprising granulocytes and/or monocytes and erythroid cells were scored on day 14 using an inverted microscope.
RESULTS
Column Purified CD34+Lin− Cells
The CD34+Lin− cells isolated by positive/negative selection comprised 99.1% ± 0.8% (mean ± SD; n = 8) pure CD34+ cells by flow cytometry analysis (Fig 3, day 0). Less than 2% of purified CD34+Lin− cells expressed lineage markers (not shown). The CD34+Lin− population comprised 87.5% ± 4% (n = 6) clonogenic progenitors (55% ± 9% BFU-E, 35% ± 7% CFU-GM, and 10% ± 2% CFU-Mix), from which the majority (>70%) gave rise to macroscopic visible colonies after 14 days of culture.
Flow cytometry analysis of purified CB CD34+Lin− cells induced to unilineage erythroid differentiation in bulk culture. CD34+Lin− cells were seeded at 2.5 × 104 cells/500 μL in serum-free medium containing 0.1 U/mL IL-3, 0.05 U/mL GM-CSF, and 3 U/mL Epo.
Flow cytometry analysis of purified CB CD34+Lin− cells induced to unilineage erythroid differentiation in bulk culture. CD34+Lin− cells were seeded at 2.5 × 104 cells/500 μL in serum-free medium containing 0.1 U/mL IL-3, 0.05 U/mL GM-CSF, and 3 U/mL Epo.
Expression of Progenitor Cell Surface Antigens and Transcription Factors in Unilineage Erythroid Bulk Culture
Freshly purified CD34+Lin− cells were forced to unilineage erythroid differentiation in serum-free liquid culture as previously described.20 Surface antigens linked to erythroid differentiation were analyzed by FACS until day 14. Figure3 shows that, at day 0, CD34+Lin− cells are GPA−, predominantly c-kit+/low, CD71low/−, and largely CD36−. The proportion of GPA+ cells gradually increased in culture, and at day 14 virtually all cells expressed GPA. In contrast, expression of the CD34 antigen gradually decreased from day 0 to 14. A distinct pattern of expression was noted for CD36, CD71, and c-kit. The c-kit antigen initially expressed at low levels was first upregulated at day 6 and then progressively decreased until day 14. The majority of cells became strongly positive for CD71 at day 10 and were weakly to strongly CD71+ at day 14. Similarly, the vast majority of cells expressed CD36 at day 10 and then became weakly to strongly positive for CD36 after 14 days of culture. After 12 to 14 days of culture, almost all cells were CD34−c-kit−CD36+CD71+GPA+and were erythroblasts by morphology analysis (Fig 4, day 12).
Expression of transcription factors GATA-1, GATA-2, and p45 NF-E2 in CB CD34+Lin− cells induced to unilineage erythroid differentiation. Transcription factor expression was evaluated by immunohistochemistry (immunogold staining with silverenhancement method) using specific MoAbs (original magnification × 400 and × 1,000). Arrows indicate nuclear staining of selected cells. Control staining was performed with irrelevant, isotype-matched rat or mouse MoAbs as well as with irrelevant rabit antibody.
Expression of transcription factors GATA-1, GATA-2, and p45 NF-E2 in CB CD34+Lin− cells induced to unilineage erythroid differentiation. Transcription factor expression was evaluated by immunohistochemistry (immunogold staining with silverenhancement method) using specific MoAbs (original magnification × 400 and × 1,000). Arrows indicate nuclear staining of selected cells. Control staining was performed with irrelevant, isotype-matched rat or mouse MoAbs as well as with irrelevant rabit antibody.
Immunohistochemistry was applied to investigate expression of transcription factors in CD34+Lin− cells induced to unilineage erythroid differentiation in bulk culture. Virtually all of the freshly purified CD34+Lin− cells expressed GATA-2 at day 0 (Fig 4, upper panel). Day 0 cells did not stain with anti–GATA-1 or anti-p45 NF-E2 (Fig 4, upper panel). At days 6 and 12 of culture, virtually all cells stained positive with anti–GATA-1, anti–GATA-2, and anti-p45 NF-E2. All transcription factor antibodies intensely immunolabeled cells and immunoreactivity was predominantly confined to the nuclei (Fig 4).
Apoptosis in Unilineage Erythroid Culture
Apoptosis in erythroid bulk culture.
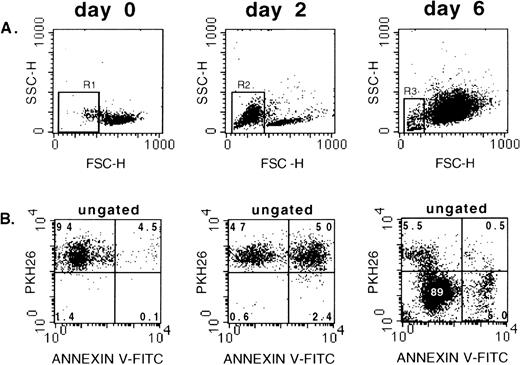
Apoptosis was evaluated in unilineage erythroid bulk culture of PKH26-labeled CD34+Lin− cells using Annexin-V-FITC. We used the red fluorescent membrane dye PKH26 (half-life of elution >100 days),34 which partitions in daughter cells after cell division. Thus, the PKH26/Annexin V staining allowed determination of the proportion of apoptotic cells in nondividing and proliferating populations. Figure 5A demonstrates FSC/SSC properties of freshly purified and cultured cells. Figure 5B shows that virtually all day 0 CD34+Lin− cells were brightly stained with PKH26, and approximately 4.5% of those scored apoptotic as determined by Annexin V staining. Backgating on Annexin V+ cells showed that almost all apoptotic day 0 cells were found in the low FSC gate (gate R1; Fig 5A). At day 2 of culture, almost all cells were still PKH26+ (Fig 5B; day 2). However, approximately one half of the PKH26+ cells were positive for Annexin V. Again, backgating of Annexin V+cells showed that virtually all apoptotic day-2 cells were characterized by low FSC (gate R2; Fig 5A) but stained bright for PKH26. After 6 days of culture, the vast majority of cells had proliferated and showed low to barely detectable PKH26 fluorescence (Fig 5B). Notably, less than 6% of day 6 PKH26−/lowcells were apoptotic. Again, the vast majority of day-6 Annexin V+ cells were characterized by low FSC as determined by backgating (R3; Fig 5A). Interestingly, after 6 days of culture (Fig5B), a minority of cells remained PKH26+ and were not stained with Annexin V (Fig 5B, day 6, upper left quadrant). However, this population was not further characterized.
Analysis of apoptosis and cell division in unilineage erythroid bulk culture. Freshly purified PKH26 labeled CD34+Lin− cells were stained with Annexin V-FITC (day 0) or transferred to unilineage erythroid culture and stained with Annexin V at days 2 and 6. (A) FSC/SSC of day-0, -2, and -6 cells. (B) Ungated cells stained with PKH26 and Annexin V-FITC. Day 0: note that only a minority of freshly isolated CD34+Lin− cells scored apoptotic when stained with Annexin V. Day 2: virtually all cells are still PKH26+ (nondividing cells), but more than 50% stain with Annexin V. Day 6: the majority of cells are proliferating because they stain barely or do not stain with PKH26. Some of these cells are Annexin V+, ie, they are apoptotic. Gates R1, R2, and R3: dead, apoptotic cells with low FSC.
Analysis of apoptosis and cell division in unilineage erythroid bulk culture. Freshly purified PKH26 labeled CD34+Lin− cells were stained with Annexin V-FITC (day 0) or transferred to unilineage erythroid culture and stained with Annexin V at days 2 and 6. (A) FSC/SSC of day-0, -2, and -6 cells. (B) Ungated cells stained with PKH26 and Annexin V-FITC. Day 0: note that only a minority of freshly isolated CD34+Lin− cells scored apoptotic when stained with Annexin V. Day 2: virtually all cells are still PKH26+ (nondividing cells), but more than 50% stain with Annexin V. Day 6: the majority of cells are proliferating because they stain barely or do not stain with PKH26. Some of these cells are Annexin V+, ie, they are apoptotic. Gates R1, R2, and R3: dead, apoptotic cells with low FSC.
Unicellular cultures.
In the described unicellular, unilineage culture system (Fig 1), apoptosis of individual cells may occur. In this case, the surviving cell(s) must perfom one or more cell divisions to generate 4 siblings. Because proliferation/cell division is usually associated with differentiation, apoptotic events in the described culture system would hinder the accurate analysis of developmentally regulated genes. We therefore investigated the frequency of apoptotic cell death in unicellular, unilineage erythroid cultures of CD34+Lin− cells and CD34+CD38− cells as outlined in Fig 2. Table 2 summarizes the proportion of apoptotic cells from both populations that had evolved after 1, 2, or 6 cell divisions, ie, cycling cells. Fifty-four percent ± 16% (mean ± SD; n = 4 independent experiments) and 97.5% ± 1.5% (n = 4 experiments) of freshly purified CD34+Lin− cells and sorted CD34+CD38− cells, respectively, underwent apoptosis in unilineage erythroid culture. The proportion of apoptotic cells among cycling cells from both CD34+ populations ranged from 0.7% to 4% (Table 2). The proportion of apoptotic cells among cycling cells from both CD34+ popluations was not different between either tested group (P for all >.3; Student’s t-test).
Unicellular-Unilineage Erythroid Cultures and Single-Cell RT-PCR
Figure 1 outlines processing of single cells grown in the unilineage, unicellular culture system. To investigate whether symmetric divisions occur in unilineage culture, individual cells from cultures containing 4 siblings at culture rounds I, II, and III were transferred to a separate culture and allowed to generate terminally differentiated progeny for morphology analysis. Single cells from round IV usually did not generate a sufficient number of progeny for cytospin preparations. By morphology analysis, all cells (>150 cells were processed) from round I to III forced to erythroid differentiation in hematopoietic progenitor cell liquid phase culture pertained to the erythroid lineage. Figure 6A shows a freshly seeded single CD34+Lin− cell that generated 4 siblings (round I; Fig 6B). Figure 6C shows a representative May-Grünwald-Giemsa–stained cytospin preparation of erythroblasts generated from an individual round I cell. SSP-RT-PCR allowed the analysis of up to 4 genes per single cell in separate reactions and was reproducible in approximately 90% of the experiments when mRNA was divided into 2 or 3 aliquots and processed for SSP-RT-PCR in separate reactions. Notably, using intron-spanning primers for β-actin (not shown) or β2-microglobulin for SSP-RT-PCR on mRNA isolated from single cells (eg, diverse subsets of CD34+cells as well as colony-derived macrophages, erythroblasts, or megakaryocytes), coamplification of genomic DNA sequences was not detected in more than 400 SSP-RT-PCR reactions. Therefore, genomic DNA is not copurified along with mRNA using the described mRNA isolation methodology.
Morphology of CD34+Lin− cells from unicellular, unilineage erythroid cultures. (A) A freshly seeded single cell is shown that had generated 4 siblings after approximately 50 hours of culture (B) (original magnification for [A] and [B] was 100-fold). One of the siblings was transferred to a separate well containing unilineage erythroid culture medium and allowed to further differentiate/proliferate (C) (a representative May-Grünwald-Giemsa–stained cytospin preparation is shown; original magnification × 1,000).
Morphology of CD34+Lin− cells from unicellular, unilineage erythroid cultures. (A) A freshly seeded single cell is shown that had generated 4 siblings after approximately 50 hours of culture (B) (original magnification for [A] and [B] was 100-fold). One of the siblings was transferred to a separate well containing unilineage erythroid culture medium and allowed to further differentiate/proliferate (C) (a representative May-Grünwald-Giemsa–stained cytospin preparation is shown; original magnification × 1,000).
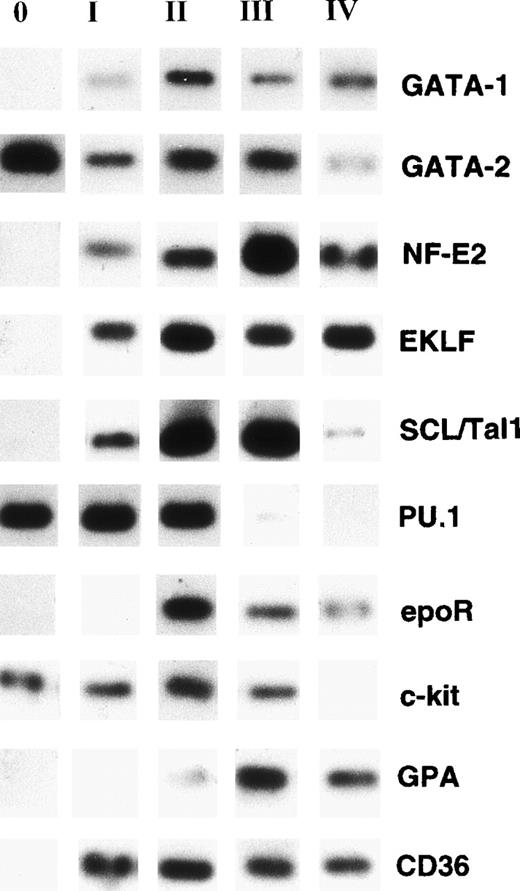
Individual CD34+Lin− cells processed for RT-PCR expressed GATA-2, PU.1, and c-kit but lacked expression of GATA-1, p45 NF-E2, SCL/Tal-1, EKLF, EpoR, CD36, and GPA (round 0; Fig 7). At round I of sibling analysis, individual cells became additionally positive at the transcriptional level for GATA-1, NF-E2, SCL/Tal1, EKLF, and CD36, whereas mRNA transcripts for EpoR and GPA were still undetectable (Fig 7). Round II cells were characterized by additional expression of EpoR and barely detectable expression of GPA. The mRNA phenotype of round III cells was similar to cells analyzed from round II (Fig 7). Late stages of erythroid differentiation (round IV) were characterized by the absence of or barely detectable levels of PU.1, GATA-2, and c-kit expression (Fig 7).
Nonquantitative single-cell RT-PCR of mRNA derived from individual cells. Data from representative experiments are shown. Single CD34+Lin− cells were induced to unilineage erythroid differentiation as described in Materials and Methods. When the 4-cell stage was reached (at round I, II, III, and IV), 2 individual daughter cells were removed from culture and processed separately for RT-PCR. mRNA was isolated from each cell and reverse transcribed into cDNA as described in Materials and Methods. The cDNA was divided into 3 aliquots that were processed in separate PCR reactions. Lane 0: Gene expression pattern of freshly isolated single CD34+Lin− cells. Lane I: single daughter cell (first round). Lane II: single daughter cell (second round). Lane III: single daughter cell (third round). Lane IV: single daughter cell (fourth round). Note that only GATA-2, PU.1, and c-kit are expressed in freshly isolated highly purified CD34+Lin− cells. Copurification of genomic DNA with mRNA from single cells was not detected by RT-PCR using intron-spanning primers for β-2 microglobulin (not shown).
Nonquantitative single-cell RT-PCR of mRNA derived from individual cells. Data from representative experiments are shown. Single CD34+Lin− cells were induced to unilineage erythroid differentiation as described in Materials and Methods. When the 4-cell stage was reached (at round I, II, III, and IV), 2 individual daughter cells were removed from culture and processed separately for RT-PCR. mRNA was isolated from each cell and reverse transcribed into cDNA as described in Materials and Methods. The cDNA was divided into 3 aliquots that were processed in separate PCR reactions. Lane 0: Gene expression pattern of freshly isolated single CD34+Lin− cells. Lane I: single daughter cell (first round). Lane II: single daughter cell (second round). Lane III: single daughter cell (third round). Lane IV: single daughter cell (fourth round). Note that only GATA-2, PU.1, and c-kit are expressed in freshly isolated highly purified CD34+Lin− cells. Copurification of genomic DNA with mRNA from single cells was not detected by RT-PCR using intron-spanning primers for β-2 microglobulin (not shown).
Because the number of genes that can be detected in single cells by SSP-RT-PCR is limited, we applied SIP-RT-PCR methodology to generate abundant quantities of representative cDNA species from a single cell for further analysis. Genomic DNA is not necessarily removed by whole cell lysate RNA extraction protocols32,35 and may bias the RT-PCR reactions. In our hands, single cells processed for SIP-RT-PCR by using the whole cell lysate (w.c.l.) method32 35resulted in coamplification of genomic DNA in approximately 30% of the experiments (Fig 8B; outermost right lane). Therefore, we used mRNA for SIP-RT-PCR. In addition to hybridizing SIP-RT-PCR amplification products with radiolabeled target gene-specific probes, we reamplified SIP-RT-PCR products by PCR using target gene-specific primers and limited our experiments to single cells isolated from round III (Fig1) of unicellular, unilineage erythroid culture. Figure 8 shows a representative experiment using an individual cell. SIP-RT-PCR (Fig 8) provided the same gene expression pattern as detected by SSP-RT-PCR (Fig 7). Moreover, single-cell RT-PCR data are supported by analysis at protein level of CD34, CD36, GPA, and c-kit as well as GATA-1, GATA-2, and NF-E2, using flow cytometry (Fig3) and immunohistochemistry (Fig 4), respectively. Notably, in some experiments, sequence-specific reamplification of SIP-RT-PCR product resulted in only barely detectable PCR signals, although strong signals were observed after direct hybridization of corresponding SIP-RT-PCR products.
Nonquantitative, sequence-independent RT-PCR (SIP-RT-PCR) of single daughter cells induced to unilineage erythroid differentiation. A representative experiment is shown. (Upper panel) The mRNA isolated from a single round III cell was processed for SIP-RT-PCR or SIP-RT-PCR was performed on whole cell lysate (w.c.l.; upper panel right lane). PCR products were then hybridized with32P-labeled probes (upper panel). Autoradiographs are shown. (Lower panel) Autoradiographs of corresponding sequence-specific PCR products from SIP-RT-PCR products shown in the upper panel. SIP-RT-PCR product (2.5 μL) was reamplified by PCR using sequence-specific pairs of primers (SSP-RT-PCR) recognizing the respective target gene. Note that the whole cell lysate (w.c.l.) SIP-RT-PCR results in coamplification of genomic DNA (outermost right lane) by using intron-spanning primers for β-microglobulin. No PCR products corresponding to genomic β2-microglobulin DNA sequences were detected when SIP-RT-PCR product from mRNA was reamplified by the same β2-microglobulin specific primers (lower panel, second lane from the right).
Nonquantitative, sequence-independent RT-PCR (SIP-RT-PCR) of single daughter cells induced to unilineage erythroid differentiation. A representative experiment is shown. (Upper panel) The mRNA isolated from a single round III cell was processed for SIP-RT-PCR or SIP-RT-PCR was performed on whole cell lysate (w.c.l.; upper panel right lane). PCR products were then hybridized with32P-labeled probes (upper panel). Autoradiographs are shown. (Lower panel) Autoradiographs of corresponding sequence-specific PCR products from SIP-RT-PCR products shown in the upper panel. SIP-RT-PCR product (2.5 μL) was reamplified by PCR using sequence-specific pairs of primers (SSP-RT-PCR) recognizing the respective target gene. Note that the whole cell lysate (w.c.l.) SIP-RT-PCR results in coamplification of genomic DNA (outermost right lane) by using intron-spanning primers for β-microglobulin. No PCR products corresponding to genomic β2-microglobulin DNA sequences were detected when SIP-RT-PCR product from mRNA was reamplified by the same β2-microglobulin specific primers (lower panel, second lane from the right).
To investigate whether heterogeneity existed among single cells from each culture round in cdc cultures, 3 individual cells from round I, II, III, or IV were separately processed for SSP-RT-PCR and analyzed for the expression of stage-specific genes CD34, CD36, GPA, EpoR, and β-globin. In addition, heterogeneity analysis was performed by analysis of single cells from non-cdc cultures that contained 4, 8 to 12, or 16 to 24 cells. In cdc cultures, each culture round comprised cells that were either all positive or all negative for a specific transcript (Fig 9). Moreover, no differences in culture round specific gene expression patterns between independent experiments were observed. Thus, single cells from specific rounds of cdc cultures displayed homogeneity with respect to the pattern of gene expression. In sharp contrast, cells derived from non-cdc cultures were heterogeneous at mRNA level in cultures containing 8 to 12 and 16 to 24 cells (Table 3).
Heterogeneity analysis by SSP-RT-PCR in cdc unicellular erythroid cultures of CD34+Lin− cells. At each culture round, 3 cells were processed separately for RT-PCR as described in Materials and Methods and 1 cell was transferred to the next culture round, and so forth. Note the absence of heterogeneity of individual cells from each culture round. An ethidium bromide-stained gel is shown.
Heterogeneity analysis by SSP-RT-PCR in cdc unicellular erythroid cultures of CD34+Lin− cells. At each culture round, 3 cells were processed separately for RT-PCR as described in Materials and Methods and 1 cell was transferred to the next culture round, and so forth. Note the absence of heterogeneity of individual cells from each culture round. An ethidium bromide-stained gel is shown.
Expression of Nonerythroid Genes in Unilineage Erythroid Bulk Culture
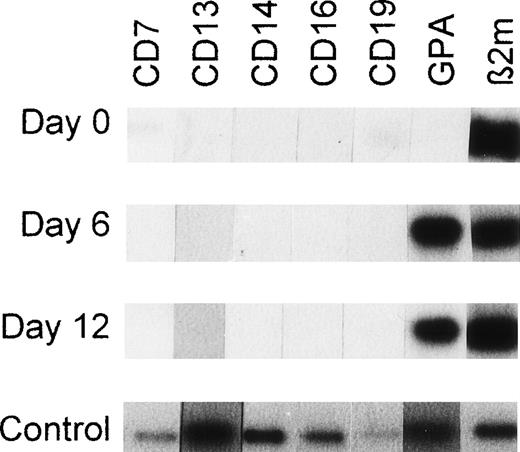
Freshly purified CD34+Lin− cells and the same cells isolated from unilineage erythroid bulk culture at day 6 or 12 were analyzed by RT-PCR using primers recognizing nonerythroid markers CD7, CD13, CD14, CD16, and CD19 as well as erythroid marker GPA. Freshly purified CD34+Lin− cells did not express CD13, CD14, CD16, or GPA, whereas CD7 and CD19 were barely detectable (Fig 10, day 0). After 6 or 12 days of unilineage erythroid culture, cells were RT-PCR–negative for CD7, CD13, CD14, CD16, and CD19 but did express GPA (Fig 10).
RT-PCR products from CD34+Lin− cells, unilineage erythroid differentiating cells and single sorted cells using primers recognizing lineage-specific markers linked to differentiation. Autoradiographs are shown. At days 0, 6, and 12, the mRNA from 100 cells was isolated and processed as described in Materials and Methods. The cDNA derived from 100 cells was divided into 7 aliquots and each aliquot was processed for RT-PCR using primers recognizing CD7 (pre-T/T cells), CD13 (myelomonocytic cells), CD14 (monocytic cells), CD16 (NK cells), CD19 (pre-B/B cells), GPA (erythroid cells), or β2-microglobulin (β2m). Day 0: freshly purified CD34+Lin− cells isolated by positive/negative selection (MiniMACS). Day 6: CD34+Lin− cells from unilineage erythroid cultures as described in Materials and Methods. Day 12: CD34+Lin− cells induced to unilineage erythroid differentiation. Control: RT-PCR sensitivity testing. Each hybridization signal represents RT-PCR products from mRNA corresponding to 0.5 cell equivalents of flow cytometry sorted cells that expressed CD7, CD13, CD14, CD16, CD19, or GPA. The outermost right band represents the β2-microglobulin RT-PCR product of a single sorted CD34+CD38− cell. Note the absence of nonerythroid CD7, CD13, CD14, CD16, and CD19 transcripts in erythroid differentiating cells at days 6 and 12 of unilineage erythroid culture.
RT-PCR products from CD34+Lin− cells, unilineage erythroid differentiating cells and single sorted cells using primers recognizing lineage-specific markers linked to differentiation. Autoradiographs are shown. At days 0, 6, and 12, the mRNA from 100 cells was isolated and processed as described in Materials and Methods. The cDNA derived from 100 cells was divided into 7 aliquots and each aliquot was processed for RT-PCR using primers recognizing CD7 (pre-T/T cells), CD13 (myelomonocytic cells), CD14 (monocytic cells), CD16 (NK cells), CD19 (pre-B/B cells), GPA (erythroid cells), or β2-microglobulin (β2m). Day 0: freshly purified CD34+Lin− cells isolated by positive/negative selection (MiniMACS). Day 6: CD34+Lin− cells from unilineage erythroid cultures as described in Materials and Methods. Day 12: CD34+Lin− cells induced to unilineage erythroid differentiation. Control: RT-PCR sensitivity testing. Each hybridization signal represents RT-PCR products from mRNA corresponding to 0.5 cell equivalents of flow cytometry sorted cells that expressed CD7, CD13, CD14, CD16, CD19, or GPA. The outermost right band represents the β2-microglobulin RT-PCR product of a single sorted CD34+CD38− cell. Note the absence of nonerythroid CD7, CD13, CD14, CD16, and CD19 transcripts in erythroid differentiating cells at days 6 and 12 of unilineage erythroid culture.
Competitive RT-PCR of Single Sibling Cells
A polyadenylated CD34 cRNA deletion construct was used for the semiquantitation of CD34 mRNA expression in single CD34+CD38− cells by competitive RT-PCR that allowed control of each variable RT-PCR step. Figure 11 shows a representative competitive RT-PCR experiment from which all daughter cells (Fig 1) transferred to unilineage erythroid culture medium generated pure erythroblasts and illustrates gradual downregulation of CD34 mRNA in single sibling cells from culture round I to IV.
Competitive single-cell RT-PCR of sibling cells induced to unilineage erythroid differentiation. A representative experiment is shown. Single CD34+CD38− cells were induced to unilineage erythroid differentiation as described in Materials and Methods. When the 4-cell stage was reached (at round I, II, III, and IV), a single daughter cell was isolated and the RNA corresponding to 0.8 cell equivalents processed for competitive RT-PCR to semiquantitate the CD34 mRNA. Polyadenylated cRNA CD34 deletion construct corresponding to 10 transcripts (based on prior titration) was added to each single-cell lysate and coprocessed with the wt mRNA. Ethidiumbromide-stained RT-PCR products were separated over a 2.5% agarose gel (Metaphor; Biozym) and photographed. Each band was excised from the gel and the radioactive counts in each were determined. Based on Cerenkov counts of excised bands, round I, II, III, and IV daughter cells contain 60%, 20%, 3%, and 0.01% of the CD34-mRNA transcripts present in a freshly sorted CD34+CD38−cell, respectively. Lane M.W.: size marker. Lane 0: single sorted CD34+CD38− cell before culture. Lane I: single daughter cell (first round). Lane II: single daughter cell (second round). Lane III: single daughter cell (third round). wt CD34 RT-PCR product is only barely detectable. Lane IV: single daughter cell (fourth round). Note that the wt CD34 mRNA message is gradually downregulated from round I to IV. Products corresponding to β2m-mRNA-transcripts but not to genomic sequences were detected when the RNA corresponding to 0.2 cell equivalents from each daughter cell was processed for RT-PCR using β2m primers (not shown).
Competitive single-cell RT-PCR of sibling cells induced to unilineage erythroid differentiation. A representative experiment is shown. Single CD34+CD38− cells were induced to unilineage erythroid differentiation as described in Materials and Methods. When the 4-cell stage was reached (at round I, II, III, and IV), a single daughter cell was isolated and the RNA corresponding to 0.8 cell equivalents processed for competitive RT-PCR to semiquantitate the CD34 mRNA. Polyadenylated cRNA CD34 deletion construct corresponding to 10 transcripts (based on prior titration) was added to each single-cell lysate and coprocessed with the wt mRNA. Ethidiumbromide-stained RT-PCR products were separated over a 2.5% agarose gel (Metaphor; Biozym) and photographed. Each band was excised from the gel and the radioactive counts in each were determined. Based on Cerenkov counts of excised bands, round I, II, III, and IV daughter cells contain 60%, 20%, 3%, and 0.01% of the CD34-mRNA transcripts present in a freshly sorted CD34+CD38−cell, respectively. Lane M.W.: size marker. Lane 0: single sorted CD34+CD38− cell before culture. Lane I: single daughter cell (first round). Lane II: single daughter cell (second round). Lane III: single daughter cell (third round). wt CD34 RT-PCR product is only barely detectable. Lane IV: single daughter cell (fourth round). Note that the wt CD34 mRNA message is gradually downregulated from round I to IV. Products corresponding to β2m-mRNA-transcripts but not to genomic sequences were detected when the RNA corresponding to 0.2 cell equivalents from each daughter cell was processed for RT-PCR using β2m primers (not shown).
DISCUSSION
To investigate expression of multiple genes at discrete stages of erythropoietic development, we applied a novel strategy consisting of hematopoietic progenitor/stem cell unicellular-unilineage culture followed by daughter cell analysis at cellular and molecular levels. The cultures were seeded with greater than 98% purified CD34+Lin− cells or sorted CD34+CD38− CB cells. Both cell populations are heterogeneous, in that they comprise putative hematopoietic stem cells (eg, long-term culture-initiating cells [LTC-ICs]) and more committed progenitors (eg, CFUs). To offset the limitations of this heterogeneity, we have developed single progenitor cell unilineage cultures, combined with single-cell RT-PCR analysis.
In the standard protocol, (1) a single progenitor cell generates 4 siblings in unilineage erythroid culture; (2) two siblings are analyzed by RT-PCR, whereas another sibling is grown to generate control unilineage progenies for morphologic evaluation; (3) the fourth sibling generates a second round of 4 sibling cells; and so forth. This approach may be successfully applied if the following experimental conditions are met: (1) asymmetric, (2) asynchronous cell divisions, and (3) apoptotic cell death are largely absent, whereas (4) the single-cell RT-PCR technology is well controlled; furthermore, (5) competitive RT-PCR analysis may add to this approach.
These experimental conditions were met in the present study. Thus, (1) asymmetric divisions did not occur; (2) in these cdc cultures, the impact of asynchronous cell divisions was negligible, ie, in each culture round the initiating cell performs 2 cell divisions generating 4 siblings; (3) the frequency of apoptotic cells was ≤4% in cycling progenitors and their progeny; finally, (4/5) RT-PCR methodology allowed unambiguous detection of multiple mRNA transcripts as well as mRNA semiquantitation at the single-cell level.
(1) The absence of asymmetric divisions is indicated by morphological analysis of mature progeny cells generated by individual siblings from each culture round. At the transcriptional level, single cells derived from culture round I to IV consistently displayed an erythroid-specific pattern of gene expression (eg, GPA, EpoR, and CD36), whereas transcripts of nonerythroid genes, ie, CD7, CD13, CD14, CD16, and CD19, were not detected by RT-PCR in erythroid differentiating cells.
(2) In these cdc cultures, the individual cells from each culture round (4 cells/round) displayed an identical mRNA expression profile: these data strongly suggest that in each round all cells pertained to an identical stage of erythroid development. In non-cdc unilineage cultures, a single progenitor cell is allowed to terminally differentiate/proliferate without repetetive replating of individual cells for initiation of subsequent culture rounds: in these culture conditions, we observed heterogeneity of the mRNA profile of the progeny after 3 cell divisions, ie, greater than 8 to 12 cells/well. This observation suggests that cells generated after 3 or more mitoses are increasingly heterogeneous in terms of developmental stage, due to asynchronuous divisions, ie, wells containing greater than 8 to 12 cells comprise cells generated by a different number of mitoses, due to different cycling times.
Conversely, asynchronuous cell divisions do not affect the unicellular-unilineage cdc culture approach. The number of mitoses is under strict control, in that after every second division a single cell is replated to initiate the next culture round.
(3) Under the described unilineage erythroid culture conditions, cells may undergo apoptosis due to growth factor starvation, as reported for hematopoietic progenitor cell lines (FDCP and 32D)36 and human erythroleukemia cells37 when deprived of IL-3 and Epo, respectively. Evaluation of apoptosis in unilineage-unicellular culture systems represented a crucial issue. Indeed, genes involved in apoptosis may interact with other ones (eg, transcription factors) and thus possibly interfere with the developmentally regulated program of gene expression. In addition, one or more sibling(s) may undergo apoptosis, whereas nonapoptotic cell(s) further proliferate. In this case, a nonapoptotic cell(s) must perform more cell division(s) to feed into the next 4-cell stage and thereby reflect more advanced stages of differentiation. Approximately 54% of individually seeded CD34+Lin− cells did not clone at the initiation of culture and were scored apoptotic. However, upon cell cycling, ie, after the first and subsequent cell divisions, apoptosis affected only 0.7% to 4% of progeny cells. These data demonstrate that apoptosis occurs frequently at the start of unilineage-unicellular cultures but represents a rare event among cycling cells. Notably, the initial 54% apoptosis frequency in unicellular cultures is in line with the results from unilineage erythroid bulk culture of CD34+Lin− cells (the ratio of apoptotic/viable cells both comprising predominantly nonproliferating PKH26+ cells peaked to ∼50% at day 2 of culture). Similar to the observed low apoptosis frequencies among cycling cells in unicellular cultures, proliferating, ie, PKH26−/low, cells at day 6 of bulk culture comprised only a low proportion, ie, ≤6%, of Annexin V+cells. In the bulk culture system, the initial apoptosis frequency could be considerably reduced by the addition of saturating doses of multilineage (IL-3/GM-CSF) or early acting (c-kit and flt-3 ligand) hematopoietic growth factors; however, this resulted in loss of unilineage erythroid growth (data not shown).
A high proportion, ie, 97.5%, of CD34+CD38− cells initially underwent apoptosis in unicellular-unilineage culture. This finding suggests that this subset predominantly comprises cells that are more primitive than those CD34+Lin−. Indeed, primitive CD34+CD38− cells express c-kit, FLT-3, and only barely detectable transcripts for IL3 receptor α-chain but no Epo receptor, whereas addition of saturating doses of IL-3 and early acting growth factors (c-kit and flt-3 ligands) considerably reduced apoptosis frequency in unicellular culture to approximately 35%; this resulted in loss of unilineage growth (data not shown).
Altogether, our observations suggest that the unilineage erythroid culture conditions select for progenitor cells potentially responsive to the Epo stimulus. In line with the model of cascade transactivation of hematopoietic growth factor receptors,13 the purified quiescent progenitor cells express IL-3R and GM-CSFR,12 and in unilineage erythroid culture are triggered into cycling by IL-3/GM-CSF. These growth factors also induce upmodulation of unilineage hematopoietic growth factor receptors, particularly EpoR on progenitor cells with erythroid differentiation potential.13 In the presence of saturating levels of Epo (and the absence of other unilineage growth factors), these progenitor cells are induced to increasing expression of EpoR and hence differentiation and then maturation along the erythroid lineage.12 On the other hand, progenitor cells with no erythroid differentiation potential do not express EpoR and undergo apoptosis.
(4) The symmetry and synchrony of cell divisions, coupled with low frequency of apoptosis for dividing cells, enabled us to investigate the pattern of gene expression at discrete stages of erythroid differentiation by single-cell RT-PCR. RT-PCR analysis of RNA from single cells32,35,38 is easily obscured by the copurified genomic DNA. To address this issue, we isolated mRNA from individual cells and then performed RT-PCR for detection of multiple mRNA species. Furthermore, we developed single-cell competitive RT-PCR methodology33 and applied it to semiquantitate CD34 mRNA transcripts in single progenitor cells induced to erythroid maturation. The number of CD34 transcripts gradually declined from freshly sorted CD34+CD38− erythroid-induced cells from round I through IV, thus indicating that single-cell RT-PCR can be rendered semiquantitative in the unicellular-unilineage culture system. Our results demonstrate that discrete stages of erythoid development are characterized by a specific pattern of gene expression. In unicellular erythroid culture, expression of the early transcription factors GATA-2 and PU.1 is monitored in the initial hematopoietic progenitor cells and gradually tapers off in erythropoietic differentiation; inversely, expression of the late transcription factors GATA-1, p45 NF-E2, and EKLF is absent in freshly purified progenitors, whereas it is gradually induced and sustainedly expressed in erythroid development. A similar expression pattern is observed for the receptors of early acting hematopoietic growth factors (eg, c-kit) as compared with lineage-specific late growth factor receptor EpoR, ie, they are expressed as early and late hematopoietic growth factor receptors, respectively. Although GATA-2 and PU.1 were detected in individual freshly purified CB CD34+Lin−cells or CB CD34+CD38− cells, the expression/function of these transcription factors may not be strictly limited to early hematopoietic development.16,22 39-41
In line with the present and previous bulk culture studies using progenitor cells undergoing unilineage erythroid or granulopoietic differentiation (previous reports20,22,25,42 and this report), the single-cell RT-PCR data on GATA-1 and p45 NF-E2 demonstrate that both transcription factors are not expressed in freshly purified CD34+Lin− cells but are detectable during erythroid differentiation. Accordingly, GATA-1 mRNA is expressed in more differentiated CD34+CD38++but not in early PB CD34++CD38−cells.43 No data about expression of EKLF at discrete stages of adult erythroid differentiation have been reported. Our results show that expression of EKLF during erythroid development parallels expression of GATA-1 and NF-E2. Thus, EKLF not expressed in early CD34+Lin− cells was found upregulated before its late expressed target gene β-globin.44 These expression results are in line with functional studies indicating that (1) GATA-1−embryonic stem cells fail to contribute to mature erythroid cells,45 whereas other hematopoietic lineages are not affected46; (2) targeted disruption of NF-E2 or EKLF does not affect the early steps of hematopoiesis47-49; and (3) treatment of adult hematopoietic progenitor cells with antisense oligomers targeting GATA-1 or NF-E2 mRNA selectively inhibits erythroid but not myeloid colony formation.22
SCL/Tal1 is barely or not expressed in adult24 and CB (present finding) early progenitors, but is induced upon erythroid unilineage differentiation through terminal maturation, as observed for late erythroid transcription factors. Similar observations were previously reported.43 The lack of expression of SCL/Tal1 in early progenitor cells is possibly due to the biphasic expression/function profile of this transcription factor,50which may peak in early ontogeny at hematopoietic stem cell level and in perinatal/postnatal life during hematopoietic progenitor cell erythroid differentiation.
A few studies on transcription factor expression in hematopoiesis are apparently in contrast with our results. Expression of GATA-1 and SCL/Tal1 was demonstrated in murine multipotential FDCPmix cells.51 Furthermore, GATA-1, NF-E2, and SCL/Tal1 transcripts were monitored in human BM CD34+CD38− or quiescent G0phase cells by a modified SIP-RT-PCR method, which also allowed semiquantitation of gene expression at the single-cell level.38 These findings may be reconciled with the present results22,24 43 in view of (1) differences between primary cells and cell lines as well as species specificity; (2) differences of analyzed progenitor/stem cell populations and purification methods; and (3) possible lack of SIP-RT-PCR specificity (eg, coamplification of genomic DNA and/or hybridization of specific probes to coamplified sequences related to the target gene), as indicated by our own observations (see Results).
Our study shows that RT-PCR analysis can be applied to assay multiple RNA species in single hematopoietic progenitor cells and daughter cells at discrete, sequential stages of unilineage erythroid differentiation/maturation. The described unilineage-unicellular culture system together with single-cell RT-PCR, preferably of competitive type, has the potential to functionally investigate relevant genes (eg, cell cycle genes, transcription factors, and growth factor receptor genes) through gene inhibition or overexpression strategies (eg, oligomer antisense or retroviral sense/antisense treatment) of purified progenitors to shed light on the cross-talk between the downmodulated gene(s) and other genes.
In conclusion, the described novel approach may eliminate ambiguities deriving from molecular analysis of heterogeneous populations of hematopoietic progenitors/ precursors growing in culture, particularly in the initial stages of development. This approach will enable studies on hematopoietic stem/progenitor cells aimed to analyze both markers linked to differentiation and expression of genes regulating their proliferation and differentiation in normal or malignant hematopoiesis; particularly, the single-cell RT-PCR approach will be used for gene expression studies in highly purified progenitors undergoing unilineage erythroid,25 granulocytic,23monocytic,27 or megakaryocytic26differentiation in liquid suspension culture.
Supported by Grant No. E/B 41G/T0347/T5920 from the BMVg, Grant No. FI4P-CT95-0029 from the European Communities, the Arthur N. Saydman Trust Fund, and a Research Fellowship from the Lucille P. Markey Charitable Trust.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Benedikt L. Ziegler, MD, University of Tübingen, Department of Medicine, Division of Hematology & Oncology, Otfried-Müller-Str. 10, D-72076 Tübingen, Germany; e-mail: benedikt.ziegler@uni-tuebingen.de.




![Fig. 6. Morphology of CD34+Lin− cells from unicellular, unilineage erythroid cultures. (A) A freshly seeded single cell is shown that had generated 4 siblings after approximately 50 hours of culture (B) (original magnification for [A] and [B] was 100-fold). One of the siblings was transferred to a separate well containing unilineage erythroid culture medium and allowed to further differentiate/proliferate (C) (a representative May-Grünwald-Giemsa–stained cytospin preparation is shown; original magnification × 1,000).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/10/10.1182_blood.v93.10.3355.410k30_3355_3368/5/m_blod41030006y.jpeg?Expires=1763885043&Signature=IUcpfcbKzNHXdPdUp1Wt~XF~rl9ZWPecVCOqAnKVL~1xzDAeJB5L4~Agmb4jvmDksOLOIRXqUrMcq-u8BSao2GQlIitTdUBtHwEDk26CG-abT5on9thKxJRCYVKNxIecWs~FAADZgQwZdMcYaQ0Fo38sTA~KkXrj4-okpm9cLvxIXM0Llrrwefdu8cSVWGNe27li-EaGZhX17532XHQWvsnjBxQq2csSOb654v6zV1qdwUiS1RAQfmmMU-OLNZuL-rC8448J8sT~1zivpPWg1VoYpIj6Q2yM~K9-7cJZ7JdOM3pHeAbiieEGSoL2NnCsqSq55AKloURtBX6NAdI-JQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)