Abstract
Leukotrienes (LT) are mediators derived from the 5-lipoxygenase (5-LO) pathway, which play a role in host defense, and are synthesized by both monocytes (peripheral blood monocyte [PBM]) and neutrophils (PMN). Because 5-LO metabolism is reduced in alveolar macrophages and PMN from acquired immunodeficiency syndrome (AIDS) subjects, we investigated the synthesis of LT by PBM and PMN from these subjects. There was a reduction (74.2% ± 8.8% of control) in LT synthesis in PBM from human immunodeficiency virus (HIV)-infected compared with normal subjects. Expression of 5-LO (51.2% ± 8.8% of control), and 5-LO activating protein (FLAP) (48.5% ± 8.0% of control) was reduced in parallel. We hypothesized that this reduction in LT synthetic capacity in PBM and PMN was due to reduced cytokine production by CD4 T cells, such as granulocyte-macrophage colony-stimulating factor (GM-CSF). We treated 10 AIDS subjects with GM-CSF for 5 days. PBM 5-LO metabolism ex vivo was selectively increased after GM-CSF therapy and was associated with increased 5-LO and FLAP expression. PMN leukotriene B4(LTB4) synthesis was also augmented and associated with increased 5-LO, FLAP, and cytosolic phospholipase A2 expression. In conclusion, as previously demonstrated for PMN, PBM from AIDS subjects also demonstrate reduced 5-LO metabolism. GM-CSF therapy reversed this defect in both PBM and PMN. In view of the role of LT in antimicrobial function, cytokine administration in AIDS may play a role as adjunct therapy for infections.
INFECTION WITH human immunodeficiency virus (HIV) results in the development of acquired immunodeficiency syndrome (AIDS) by causing depletion and dysfunction of CD4 T lymphocytes.1 However, the dysfunction of other cell types including macrophages2,3 and neutrophils4 also occurs in AIDS. There is evidence that chemotaxis, phagocytosis, and killing of microorganisms by macrophages, monocytes (peripheral blood monocyte [PBM]),5 and neutrophils (PMN)6 is reduced with the progression of HIV disease. Furthermore, there is evidence of reduced synthesis of specific mediators associated with the reduction in CD4 count. 5-lipoxygenase (5-LO) is the enzyme, which catalyzes the formation of leukotriene (LT) from the fatty acid arachidonic acid (AA) bound to membrane phospholipids.7,8These proinflammatory mediators are involved in the pathogenesis of a number of disease states including asthma.9 Our laboratory has previously shown that LT synthesis is reduced in alveolar macrophages (AM) from HIV-infected subjects compared with healthy controls.10 Furthermore, in a recent study, we demonstrated that PMN from AIDS patients demonstrated reduced LT synthetic capacity compared with controls.11 However, there is no information about the effects of HIV on the 5-LO pathway in PBM, which are precursor cells for AM.
LT also play an important role in host defense mechanisms against microorganisms.12,13 5-LO knockout mice are more susceptible to pneumonia from Klebsiella pneumoniaethan are wild-type mice.14 LT deficiency is associated with impaired phagocytosis and killing of microorganisms.15The addition of exogenous LTs in vitro to LT-deficient cells boosts phagocytosis.14 Likewise, in vivo treatment of AIDS patients with granulocyte colony-stimulating factor (G-CSF) boosted PMN 5-LO metabolism, and this resulted in increased anticryptococcal activity.11
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a cytokine produced predominantly by activated T lymphocytes.16-18With the depletion and dysfunction of CD4 T lymphocytes in AIDS, levels of GM-CSF decline.19-21 GM-CSF has been shown to be an important constituent of lymphocyte-derived conditioned medium, which is capable of increasing 5-LO metabolism by mononuclear phagocytes.18,22 In addition, treatment of PMN with GM-CSF in vitro has resulted in increased 5-LO metabolism via increased expression of 5-LO enzyme23 and its helper protein 5-LO activating protein, termed FLAP.24 Furthermore, treatment of patients with GM-CSF results in increased urinary LTE4levels.25 26 In view of these observations, we examined 5-LO metabolism in PBM from AIDS subjects and investigated the effect of GM-CSF therapy on PBM and PMN 5-LO metabolism after treatment of these patients, as GM-CSF was expected to affect both mononuclear and granulocytic cells.
MATERIALS AND METHODS
Clinical study protocol.
We performed a prospective trial of GM-CSF therapy in 10 consecutively enrolled nonsmoking HIV-infected subjects with low (<100 cm2) CD4 counts without evidence of opportunistic infection and cytokine therapy as part of their medical regimen. The experimental protocol was approved by the University of Michigan Institutional Review Board for Approval of Research Involving Human Subjects. Full informed consent was obtained from all subjects before the study. All subjects were on their regular antiretroviral therapy. Subjects were treated with GM-CSF at a dose of 250 μg/m2 subcutaneously on each of days 1 to 5. PMN and PBM were isolated on day 1 before GM-CSF therapy, day 4 during therapy, and on day 8, 3 days after stopping GM-CSF. Total white cell and absolute PMN (ANC) and absolute PBM counts (AMC) were performed on day 1, day 4, and day 8. In addition, HIV load, as measured by bDNA,27 was determined before and after the drug study. Subjects were carefully monitored for adverse events during the study. PMN and PBM 5-LO metabolism in vitro were also determined (see below). HIV-negative healthy control subjects without evidence of systemic infection were also studied. These subjects were volunteers, both male and female, nonsmokers on no medication, who were recruited from the employees at University Hospital.
Cell isolation.
PMN were isolated from venous blood drawn from HIV-infected subjects with low CD4 counts and healthy controls. A volume of 5 mL of heparinized whole blood was layered over 3.5 mL of a mixture of sodium metrizoate and Ficoll (1-Step Polymorphs; Accurate Chemical & Scientific Corp, Westbury, NY) in a 15-mL centrifuge tube. The sample was centrifuged at 450g for 30 minutes in a swing-out rotor at 22°C. After centrifugation, the distinct lower PMN-containing band was aspirated and diluted in 0.45% NaCl solution to restore normal osmolality. The cells were washed twice in 0.5 N saline and resuspended in Iscove’s medium. This resulted in less than 5% contamination with erythrocytes and greater than 95% PMN, as determined by Diff-Quik staining. Human PBM were isolated from HIV-infected subjects with low CD4 counts and healthy controls. They were purified by centrifugation through Ficoll-Paque (Pharmacia LKB, Uppsala, Sweden), followed by adherence of the mononuclear layer on plastic for 1 hour at 37°C at 5% CO2. After vigorously washing 3 times, the remaining cells were greater than 90% pure as determined by nonspecific esterase testing. There was no difference in the purity of PMN and PBM obtained from healthy control subjects and HIV-infected subjects. Viability of PMN and PBM was greater than 90% as determined by trypan blue exclusion.
Quantitation of maximal LT synthetic capacity.
Freshly isolated PMN were suspended in Iscove’s medium at 1 × 106 /mL in eppendorf tubes, and incubated for 30 minutes at 37°C with 10 μmol/L calcium ionophore A23187 (Calbiochem, La Jolla, CA) or vehicle (0.5% dimethyl sulfoxide [DMSO]) alone. The cells were then centrifuged and the supernatant was frozen at −70°C for subsequent LTB4 analysis by enzyme-linked immunoassay (EIA) (Cayman Chemical, Ann Arbor, MI). Isolated PBM were adhered for 1 hour, washed, and then stimulated with A23187 (10 μmol/L) and exogenous AA (50 μmol/L) for 30 minutes. There was no difference in adherence properties between PBM from healthy controls and HIV-infected subjects. We have shown that the combination of A23187 and high dose exogenous AA is the maximal stimulus for LT synthesis in PBM (data not shown). To directly compare EIA results from controls and HIV-infected subjects, the sample from each HIV-infected subject was analyzed in parallel with that of a healthy control subject studied on the same day. For each sample, the average of duplicate determinations was calculated. Data are expressed as pg product per 1 × 106 cells.
Antiretroviral agent preparation in vitro.
The nucleoside inhibitor azidothymidine (AZT) (Retrovir) was prepared by dissolving a 100-mg capsule in 40 mL water and plated onto cells at a final concentration (Cmax clinical dose) of 0.63 μg/mL. 3TC (Epivir), also a nucleoside inhibitor, was prepared by dissolving a 150-mg tablet in 40 mL water, and plated onto cells at a final concentration (Cmax clinical dose) of 1.5 μg/mL. The protease inhibitor Indinovir (Crixivan) was prepared by dissolving a 400-mg capsule in 40 mL water and plated onto cells at a final concentration (Cmax clinical dose) of 8.9 mg/L. The GM-CSF dose used in vitro was 250 U/mL.
Analysis of [3H]AA release and metabolism.
Isolated PMN from HIV-infected subjects were incubated overnight. Release and metabolism of AA was assessed in PMN whose lipids were prelabeled by incubation with [3H]AA. This was accomplished by including 0.5 μCi [3H]AA (specific activity, 60 to 100 Ci/mmol) (Dupont-New England Nuclear, Boston, MA) in the medium during culture for 1.5 hours. Cells were then centrifuged, washed, and AA metabolism was determined by incubation in Iscoves medium for 30 minutes with 10 μmol/L A23187. To assess total AA release, cells were stimulated with A23187 in the presence of 0.1% bovine serum albumin (BSA) and radioactivity in the medium determined. PBM were prelabeled for 16 hours with 0.5 μCi [3H]AA in the medium. Cells were then washed and stimulated with A23187. Cell supernatants were extracted using C18 Sep-Paks, and radiolabeled eicosanoids were separated by reverse-phase high-performance liquid chromatography (HPLC),28 identified by coelution with authentic standards (Cayman Chemicals), and quantitated using an on-line radioactivity detector (Radiomatic Model 515; Packard, Downers Grove, IL). Radiolabeled products released were expressed as a percentage of radioactivity incorporated into cellular membrane phospholipids.
Immunoblot analysis of total cellular 5-LO, FLAP, and cytosolic phospholipase A2.
Steady-state quantities of 5-LO, FLAP, and cytosolic phospholipase A2 (cPLA2) proteins were assessed by immunoblot analysis using a modification of methods described previously.29 Briefly, equal amounts of protein (5 to 20 μg) were separated on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels by the method of Laemmli.30 High- and low-molecular-weight rainbow markers (Amersham, Arlington Heights, IL) were also loaded on each gel. After overnight transfer to nitrocellulose membranes (Bio-Rad Laboratories, Richmond, CA), blots were blocked by incubating for 1 hour with 10% nonfat dried milk in Tris-buffered saline (TBS), washed in TBS containing 0.1% Tween (TBS-T), and incubated at room temperature for 1 hour with rabbit polyclonal antisera raised against either human leukocyte 5-LO (1:3,000 dilution), amino acid residues 41-52 of the human FLAP sequence (1:5,000 dilution), or recombinant human cPLA2 (1:1,000 dilution). Antisera against 5-LO and FLAP were provided by Dr J. Evans (Merck Frosst, Pointe Claire-Dorval, Quebec, Canada); anti-cPLA2 antiserum was provided by Dr J. Clark (Genetics Institute, Cambridge, MA). After washing, blots were incubated for 1 hour with horseradish peroxidase-conjugated goat anti-rabbit IgG (Amersham) at a dilution of 1:5,000 in TBS-T. Membranes were then washed and developed using the ECL chemiluminescent Western blotting system (Amersham). Multiple exposures of each blot were obtained. Densities of luminescent bands were quantitated in appropriately exposed autoradiographs by video densitometry using NIH Image software (Scion Corp, Frederick, MD). Samples obtained from healthy controls and HIV-infected subjects were loaded on the same gel and underwent immunoblot analysis in parallel.
Data analysis.
Where indicated, data were expressed as the mean ± standard error of mean (SEM). Differences between the mean values of HIV-infected and healthy control groups were analyzed by analysis of variance (ANOVA), with statistical significance assessed by the Scheffe test. A P value <.05 was considered significant.
RESULTS
AA metabolism in PBM from AIDS patients.
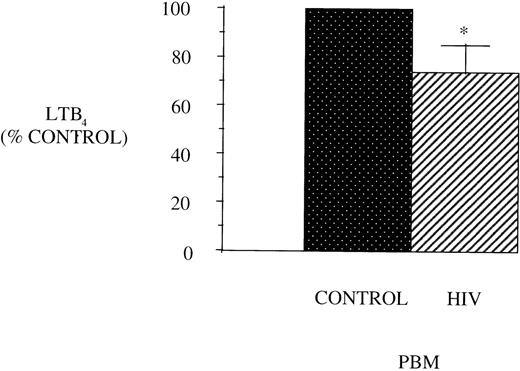
Figure 1 shows the maximal LT synthetic capacity of PBM obtained from healthy control and AIDS patients, assessed by quantitating immunoreactive LTB4 synthesis after activation with A23187 and exogenous AA. LTB4synthesis was significantly reduced in PBM from HIV-infected subjects compared with healthy control subjects.
LTB4 synthesis in PBM from healthy controls and AIDS subjects. PBM were isolated as described in Materials and Methods and plated at 1 × 106/mL in Dulbecco’s modified Eagle’s medium (DMEM). Maximal LTB4 release was determined after stimulation with A23187 (10 μmol/L) and AA (50 μmol/L) for 30 minutes at 37°C. Medium was analyzed for LTB4 by EIA and expressed as pg/106 cells, n = 10, *P < .001. Healthy control PBM LTB4 levels were 4,797.2 ± 884.5 pg/106 cells.
LTB4 synthesis in PBM from healthy controls and AIDS subjects. PBM were isolated as described in Materials and Methods and plated at 1 × 106/mL in Dulbecco’s modified Eagle’s medium (DMEM). Maximal LTB4 release was determined after stimulation with A23187 (10 μmol/L) and AA (50 μmol/L) for 30 minutes at 37°C. Medium was analyzed for LTB4 by EIA and expressed as pg/106 cells, n = 10, *P < .001. Healthy control PBM LTB4 levels were 4,797.2 ± 884.5 pg/106 cells.
One possible explanation for the reduction in 5-LO metabolic capacity of PBM from AIDS subjects could be a decrease in PLA2-mediated availability of AA. Therefore, we radiolabeled endogenous AA in PBM and stimulated the cells with the agonist, A23187. We found that this decrease in LT synthesis not due to a decrease in capacity for AA release (AIDS patients, 6.5% ± 2.7% incorporated AA; healthy controls, 5.4% ± 1.5% incorporated AA, n = 4, P = .8). Further evidence that the LT synthetic defect was not explained by reduced AA release is that other metabolites of AA, the prostaglandins (PG), were increased in PBM from AIDS subjects (153.9% ± 13.2% of control, n = 9, P < .05) compared with cells from healthy control subjects. This is in keeping with evidence of increased cyclooxygenase (COX) metabolism in PBM and AM obtained from HIV-infected subjects.10,31 32
5-LO and FLAP expression in PBM from HIV-infected subjects.
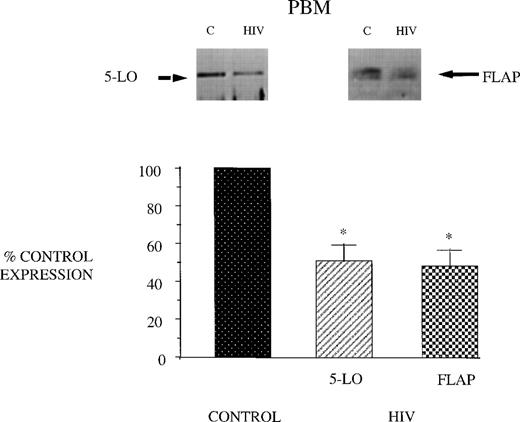
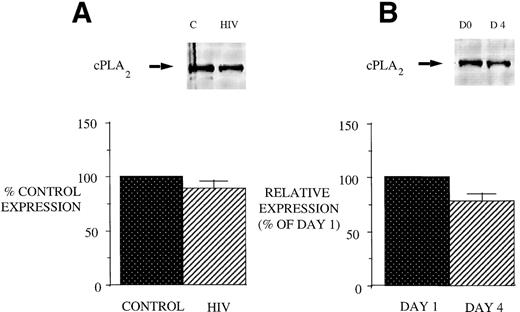
Reduced 5-LO metabolic capacity in PBM from HIV-infected subjects might reflect altered expression of 5-LO and/or FLAP proteins in cells. Therefore, we performed Western blot analysis for 5-LO and FLAP on crude lysates of PBM from healthy controls and HIV-infected subjects (Fig 2). Eight subjects were studied because of inadequate cellular protein from 2 of the enrolled subjects to perform Western blot analysis. Cells from AIDS subjects exhibited a reduction in both 5-LO and FLAP expression relative to healthy control subjects (taken as 100%). The expression of cPLA2 protein was unchanged in PBM from AIDS subjects compared with those obtained from healthy control subjects (Fig 3). This is in keeping with the similar magnitude of AA release in these 2 groups and emphasizes the selectivity of the defect involving the 5-LO pathway proteins in AIDS.
5-LO and FLAP expression in PBM from control and AIDS subjects. Equal amounts (20 μg of protein) of crude cellular lysate from PBM were subjected to immunoblot analysis for 5-LO and FLAP as described in Materials and Methods. Top panel, representative autoradiograph of a Western blot demonstrating the amount of 5-LO (left) and FLAP (right) in PBM from healthy control and HIV-infected subjects. Lower panel, relative expression of 5-LO and FLAP in PBM from healthy control and HIV-infected subjects, as assessed by densitometry and expressed as a percent of values derived from cells from healthy control subjects. Data represent the mean ± SEM from n = 8 subjects. *P < .001.
5-LO and FLAP expression in PBM from control and AIDS subjects. Equal amounts (20 μg of protein) of crude cellular lysate from PBM were subjected to immunoblot analysis for 5-LO and FLAP as described in Materials and Methods. Top panel, representative autoradiograph of a Western blot demonstrating the amount of 5-LO (left) and FLAP (right) in PBM from healthy control and HIV-infected subjects. Lower panel, relative expression of 5-LO and FLAP in PBM from healthy control and HIV-infected subjects, as assessed by densitometry and expressed as a percent of values derived from cells from healthy control subjects. Data represent the mean ± SEM from n = 8 subjects. *P < .001.
cPLA2 expression in PBM from healthy control and AIDS subjects and after GM-CSF therapy of AIDS patients. Equal amounts (20 μg of protein) of crude cellular lysate from PBM were subjected to immunoblot analysis for 5-LO and FLAP as described in Materials and Methods. Top panel, a representative Western blot and lower panel, mean densitometric data. (A) cPLA2 expression in PBM from healthy control and HIV-infected subjects expressed as a percent of values obtained from healthy control subjects. (B) cPLA2 expression in PBM from HIV-infected subjects treated with GM-CSF therapy, as assessed by densitometry and expressed as a percent of values derived from day 1 PBM. n = 9, P = not significant (NS).
cPLA2 expression in PBM from healthy control and AIDS subjects and after GM-CSF therapy of AIDS patients. Equal amounts (20 μg of protein) of crude cellular lysate from PBM were subjected to immunoblot analysis for 5-LO and FLAP as described in Materials and Methods. Top panel, a representative Western blot and lower panel, mean densitometric data. (A) cPLA2 expression in PBM from healthy control and HIV-infected subjects expressed as a percent of values obtained from healthy control subjects. (B) cPLA2 expression in PBM from HIV-infected subjects treated with GM-CSF therapy, as assessed by densitometry and expressed as a percent of values derived from day 1 PBM. n = 9, P = not significant (NS).
Effect of GM-CSF on AIDS patients.
The HIV group consisted of 8 males and 2 females, with a mean age of 44.9 ± 2.5 years. One subject withdrew from the study on day 4 because of side effects (syncope) of GM-CSF therapy. The mean CD4 count was 59 ± 10.8 cm2. All patients were receiving antiviral therapy before and during the study; 100% were receiving 2 nucleoside agents and 90% were receiving protease inhibitor therapy. The hematologic characteristics of these subjects are shown in Table 1. There was a significant increase in white cell count and ANC after GM-CSF treatment. The AMC was also increased during GM-CSF therapy: (day 1, 0.4 ± 0.06 × 103/mL; day 4, 0.6 ± 0.08 × 103/mL; and day 8, 0.5 ± 007 × 103/mL, P= .035 comparing day 1 and day 4, n = 9). This increase in PBM counts peaked at day 4 and returned to control levels on day 8. The plasma HIV RNA was not significantly changed after GM-CSF therapy (pretherapy, 3.04 ± 1.7 (range, <0.4 to 5.33) 104 particles bDNA/mL, n = 10; posttherapy 3.88 ± 1.83 (range, <0.4 to 5.54) 104 particles bDNA/mL, P = ns comparing pre and posttherapy bDNA, n = 9). The side effect profile of GM-CSF therapy included syncope (n = 1), myalgia/bone pain (n = 2), headache (n = 2), and vomiting (n = 1).
Effect of GM-CSF treatment on AA metabolism of PBM from HIV-infected subjects.
Because PBM from AIDS subjects also demonstrate a defect in 5-LO metabolism, we next examined the effect of GM-CSF therapy on PBM AA metabolism. Figure 4 shows that GM-CSF treatment increases LT synthetic capacity at day 4, which returns to baseline levels by day 8. This increase in 5-LO metabolism could not be accounted for by an increase in AA release, as shown in the representative HPLC profile (Fig 5). In additional support of the selective increase in 5-LO metabolism, there was no increase in PBM COX metabolites after GM-CSF therapy (data not shown).
Effect of in vivo GM-CSF therapy on PBM LT synthesis in AIDS subjects. PBM were isolated on day 1, day 4, and day 8 of the study protocol as described in Materials and Methods and plated at 1 × 106/mL in DMEM. Maximal LTB4release was determined after stimulation with A23187 (10 μmol/L) and exogenous AA (50 μmol/L) for 30 minutes at 37°C. Medium was analyzed for LTB4 by EIA and the value obtained for HIV-infected subjects was expressed as a percent of the LTB4 values observed for AIDS subjects on day 1 (2,851.9 ± 448.7 pg/106 cells). *P = .05, n = 8.
Effect of in vivo GM-CSF therapy on PBM LT synthesis in AIDS subjects. PBM were isolated on day 1, day 4, and day 8 of the study protocol as described in Materials and Methods and plated at 1 × 106/mL in DMEM. Maximal LTB4release was determined after stimulation with A23187 (10 μmol/L) and exogenous AA (50 μmol/L) for 30 minutes at 37°C. Medium was analyzed for LTB4 by EIA and the value obtained for HIV-infected subjects was expressed as a percent of the LTB4 values observed for AIDS subjects on day 1 (2,851.9 ± 448.7 pg/106 cells). *P = .05, n = 8.
[3H]Eicosanoid profile of stimulated PBM from AIDS subjects before and during therapy with GM-CSF. Cells prelabeled overnight were incubated with 1 μmol/L A23187 for 30 minutes. HPLC elution profiles are displayed for [3H]AA metabolites synthesized by A23187-stimulated PBM from a representative HIV-infected subject on day 1 (A) and on day 4 (B). Peaks were identified by coelution with authentic standards, and the products were expressed as a percentage of incorporated radioactivity. This HPLC profile is representative of 6 subjects whose cells were radiolabeled.
[3H]Eicosanoid profile of stimulated PBM from AIDS subjects before and during therapy with GM-CSF. Cells prelabeled overnight were incubated with 1 μmol/L A23187 for 30 minutes. HPLC elution profiles are displayed for [3H]AA metabolites synthesized by A23187-stimulated PBM from a representative HIV-infected subject on day 1 (A) and on day 4 (B). Peaks were identified by coelution with authentic standards, and the products were expressed as a percentage of incorporated radioactivity. This HPLC profile is representative of 6 subjects whose cells were radiolabeled.
Effect of GM-CSF treatment on 5-LO, FLAP, and cPLA2expression in PBM from HIV-infected subjects.
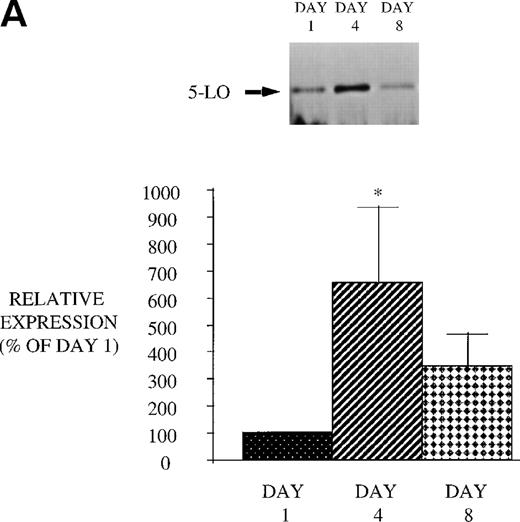
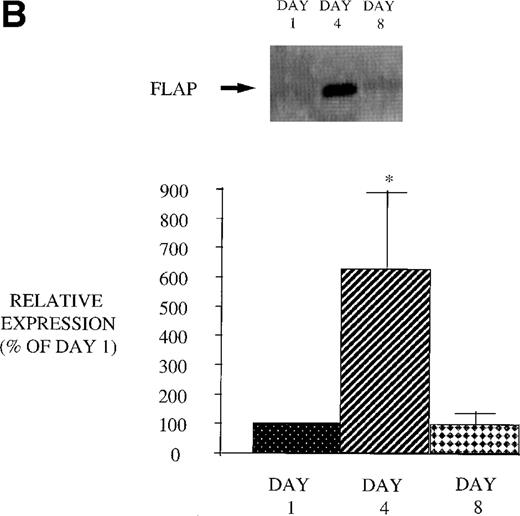
We next examined the effect of GM-CSF on 5-LO and FLAP expression. There was an increase in the expression of both 5-LO and FLAP in PBM after treatment of AIDS subjects with GM-CSF (Fig 6). This increase in protein expression peaked at day 4 and had returned to control levels by day 8. In contrast to the increase in expression of 5-LO and FLAP in PBM and increase in cPLA2 expression in PMN after GM-CSF therapy, there was no change in cPLA2 expression in PBM (Fig 3).
Effect of in vivo GM-CSF therapy on PBM 5-LO and FLAP expression in AIDS subjects. PBM were isolated on day 1, day 4, and day 8 of the study protocol, as described in Materials and Methods. Equal amounts (20 μg of protein) of crude cellular lysate from PBM were subjected to immunoblot analysis for 5-LO and FLAP. Top panel, representative autoradiograph of a Western blot demonstrating the amount of 5-LO (A) and FLAP (B) in PBM from HIV-infected subjects on protocol day 1, 4, and 8. Lower panel, relative expression of 5-LO (A) and FLAP (B) in PBM from HIV-infected subjects treated with GM-CSF, as assessed by densitometry and expressed as a percent of values derived from day 1 PBM. n = 9, *P = .05.
Effect of in vivo GM-CSF therapy on PBM 5-LO and FLAP expression in AIDS subjects. PBM were isolated on day 1, day 4, and day 8 of the study protocol, as described in Materials and Methods. Equal amounts (20 μg of protein) of crude cellular lysate from PBM were subjected to immunoblot analysis for 5-LO and FLAP. Top panel, representative autoradiograph of a Western blot demonstrating the amount of 5-LO (A) and FLAP (B) in PBM from HIV-infected subjects on protocol day 1, 4, and 8. Lower panel, relative expression of 5-LO (A) and FLAP (B) in PBM from HIV-infected subjects treated with GM-CSF, as assessed by densitometry and expressed as a percent of values derived from day 1 PBM. n = 9, *P = .05.
Effect of GM-CSF treatment on AA metabolism of PMN from HIV-infected subjects.
In view of the reduced CD4 count and the associated reduced capacity to elaborate GM-CSF, which could account for their reduced LT synthetic capacity, we hypothesized that if we treated these patients with GM-CSF, we could reverse the defect in 5-LO metabolism. We studied PMN before, during, and after 5 days of treatment with 250 μg/m2 GM-CSF administered subcutaneously daily. On day 4 of treatment with GM-CSF in vivo, there was a dramatic increase in LT synthesis detected in vitro (Fig 7), as compared with the markedly reduced baseline levels. This increase in 5-LO metabolism had waned by day 8 and returned to control levels. Another mechanism by which LT synthesis can be increased by GM-CSF is by augmenting AA release. This was indeed the case, as day 4 PMN prelabeled with [3H]AA demonstrated increased total release of radioactivity. Figure 8 shows a HPLC profile, which exhibits increased quantities of radiolabeled free AA and 5-LO products synthesized by PMN after in vivo GM-CSF treatment. There was also a trend towards an increase (221% ± 21.5% of control PMN, n = 8, P = .06) in PGE2 synthesis by PMN after GM-CSF therapy.
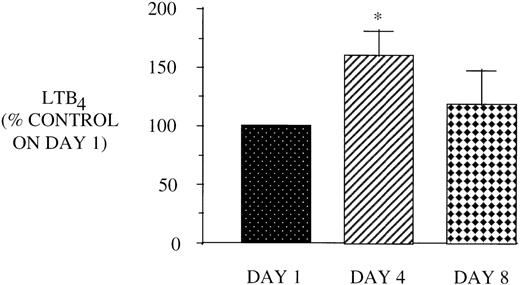
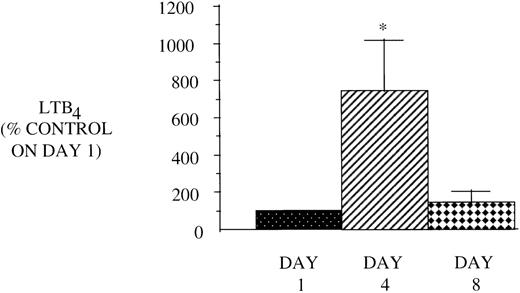
Effect of in vivo GM-CSF therapy on PMN LT synthesis in AIDS subjects. PMN were isolated on day 1, day 4, and day 8 of the study protocol as described in Materials and Methods and plated at 1 × 106/mL in DMEM. Maximal LTB4release was determined after stimulation with A23187 (10 μmol/L) for 30 minutes at 37°C. Medium was analyzed for LTB4 by EIA, and the value obtained for HIV-infected subjects was expressed as a percent of the value observed for AIDS subjects (LTB490.6 ± 14.4, pg/106 cells) on day 1. n = 9, *P= .05.
Effect of in vivo GM-CSF therapy on PMN LT synthesis in AIDS subjects. PMN were isolated on day 1, day 4, and day 8 of the study protocol as described in Materials and Methods and plated at 1 × 106/mL in DMEM. Maximal LTB4release was determined after stimulation with A23187 (10 μmol/L) for 30 minutes at 37°C. Medium was analyzed for LTB4 by EIA, and the value obtained for HIV-infected subjects was expressed as a percent of the value observed for AIDS subjects (LTB490.6 ± 14.4, pg/106 cells) on day 1. n = 9, *P= .05.
[3H]Eicosanoid profile of stimulated PMN from AIDS subjects before and during therapy with GM-CSF. Prelabeled cells were incubated with 1 μmol/L A23187 for 15 minutes. HPLC elution profiles are displayed for [3H]AA metabolites synthesized by A23187-stimulated PMN from a representative HIV-infected subject on day 1 (A) and on day 4 (B). Peaks were identified by coelution with authentic standards, and the products were expressed as a percentage of incorporated radioactivity. This HPLC profile is representative of 6 subjects whose cells were radiolabeled.
[3H]Eicosanoid profile of stimulated PMN from AIDS subjects before and during therapy with GM-CSF. Prelabeled cells were incubated with 1 μmol/L A23187 for 15 minutes. HPLC elution profiles are displayed for [3H]AA metabolites synthesized by A23187-stimulated PMN from a representative HIV-infected subject on day 1 (A) and on day 4 (B). Peaks were identified by coelution with authentic standards, and the products were expressed as a percentage of incorporated radioactivity. This HPLC profile is representative of 6 subjects whose cells were radiolabeled.
Effect of GM-CSF treatment on 5-LO, FLAP, and cPLA2expression in PMN from HIV-infected subjects.
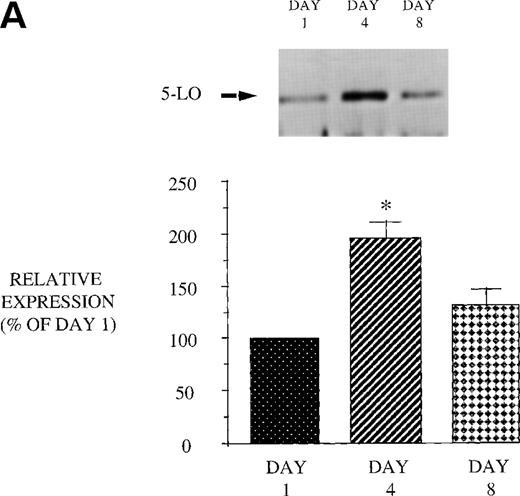
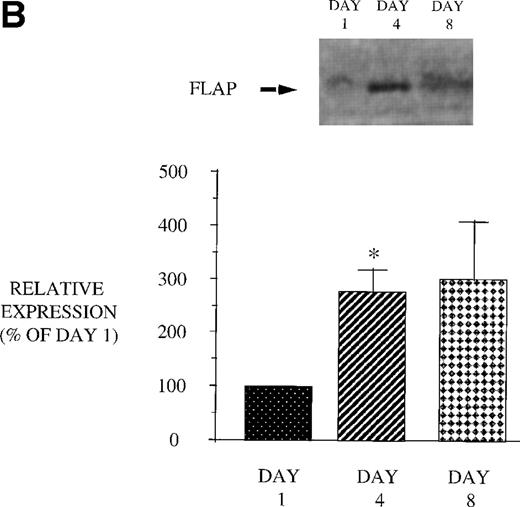
We next examined the effect of GM-CSF treatment on PMN expression of the 2 proteins, 5-LO and FLAP, essential for LT synthesis. Both 5-LO and FLAP expression were increased on day 4 compared with day 1 (Fig 9). The increase in protein expression returned to control levels 3 days after GM-CSF therapy was discontinued (day 8). In addition, we examined the expression of cPLA2. GM-CSF also increased cPLA2 expression (day 4: 343.8% ± 86.1% of day 1, P = .02 n = 8; day 8: 138.9% ± 45.6% of day 1). This increase in cPLA2 expression correlated with the increase in AA release. These observations suggest that increased expression of 5-LO, FLAP, and cPLA2 likely account for the increase in LT synthetic capacity after GM-CSF therapy.
Effect of in vivo GM-CSF therapy on PMN 5-LO and FLAP expression in AIDS subjects. PMN were isolated on day 1, day 4, and day 8 of the study protocol, as described in Materials and Methods. Equal amounts (20 μg of protein) of crude cellular lysate from PMN were subjected to immunoblot analysis for 5-LO and FLAP. Top panel, representative autoradiograph of a Western blot demonstrating the amount of 5-LO (A) and FLAP (B) in PMN from HIV-infected subjects on protocol day 1, 4, and 8. Lower panel, relative expression of 5-LO (A) and FLAP (B) in PMN from HIV-infected subjects treated with GM-CSF, as assessed by densitometry and expressed as a percent of values derived from day 1 PMN. n = 9, * P = .05.
Effect of in vivo GM-CSF therapy on PMN 5-LO and FLAP expression in AIDS subjects. PMN were isolated on day 1, day 4, and day 8 of the study protocol, as described in Materials and Methods. Equal amounts (20 μg of protein) of crude cellular lysate from PMN were subjected to immunoblot analysis for 5-LO and FLAP. Top panel, representative autoradiograph of a Western blot demonstrating the amount of 5-LO (A) and FLAP (B) in PMN from HIV-infected subjects on protocol day 1, 4, and 8. Lower panel, relative expression of 5-LO (A) and FLAP (B) in PMN from HIV-infected subjects treated with GM-CSF, as assessed by densitometry and expressed as a percent of values derived from day 1 PMN. n = 9, * P = .05.
Effect of antiretroviral agents and GM-CSF on PBM and PMN 5-LO metabolism in vitro.
All of the AIDS patients were on antiretroviral therapy, including nucleoside and protease inhibitors. Because there is an alteration in lipid metabolism in patients receiving protease inhibitors, we considered that this defect in 5-LO metabolism could be a reflection of AIDS therapy. Furthermore, GM-CSF therapy is known to alter drug metabolism within myeloid cells, particularly nucleosides. However, incubation of PBM and PMN from healthy controls with the nucleoside inhibitors, AZT and 3TC, and the protease inhibitor, Indinavir, singly and in combination, had no effect on LTB4 synthetic capacity (Table 2). This was also true for cells cotreated with GM-CSF and antiretroviral therapy in vitro. Therefore, the data suggest that the defect in 5-LO metabolism is a reflection of the underlying disease and not its therapy.
DISCUSSION
With the progression of HIV disease, CD4 T-lymphocyte depletion results in dysfunction of the normal cytokine profile. This results in the reduction in levels of 1 such mediator, GM-CSF,19-21 which is known to upregulate 5-LO metabolism.23 Such an abnormality might contribute to the reduced LT synthetic capacity, which occurs in AM, PBM, and PMN. We hypothesized that replacement therapy with GM-CSF would restore cellular 5-LO metabolism. The major findings of this study show that (1) PBM from AIDS patients display a selective reduction in 5-LO metabolism, which is associated with reduced 5-LO and FLAP expression. (2) PBM LT synthetic capacity was selectively increased after GM-CSF therapy and was associated with an increase in 5-LO and FLAP, but not cPLA2 expression. (3) Treatment of AIDS subjects with GM-CSF resulted in increased PMN 5-LO metabolism and AA release. This was associated with an increase in 5-LO, FLAP, and cPLA2 expression.
In view of the previously published data from our laboratory that 5-LO metabolism was reduced in AM from AIDS subjects, we anticipated that LT synthetic capacity would be also reduced in PBM. However, the defect in 5-LO metabolism was less dramatic in PBM, which may be explained in part by the environment in which the two cell types reside. AM are resident in the lung where they are exposed to a higher percentage of activated memory CD4 T lymphocytes than are present in the peripheral circulation. Cytokines like GM-CSF are released by activated lymphocytes and may contribute to the upregulation of LT synthesis, which occurs as PBM move into the lung and differentiate into AM.18,22 This hypothesis is supported by the evidence that reduced 5-LO metabolism and 5-LO and FLAP expression is observed in AM from a CD4 T-cell–depleted murine model.33 Therefore, a reduction in CD4 T-lymphocyte count would be expected to have a greater impact on AM than PBM 5-LO metabolism.
PMN dysfunction occurs in AIDS despite the fact that there is no evidence of integration of HIV into the cellular genome. Our laboratory has previously described a dramatic decrease in PMN 5-LO metabolism in AIDS subjects, which was associated with a decrease in FLAP expression.11 These observations strongly suggest that the primary cause for a defect in LT synthesis in AIDS is the result of an alteration of the environmental milieu, most likely from cytokine dysfunction. Furthermore, our in vitro treatments indicate that antiretroviral therapy does not account for the decrease in 5-LO metabolism. An explanation for the dramatic reduction in LT synthetic capacity in PMN may be accounted for by the concomitant reduction in G-CSF levels in AIDS,34 compounding the effect of the reduction the GM-CSF levels on 5-LO metabolism. We did not measure GM-CSF levels in healthy controls or in AIDS patients, but these studies have been performed by other investigators.19GM-CSF therapy partially restored PMN LT synthetic capacity to a degree, which is similar to that of G-CSF therapy. This is consistent with previous reports demonstrating that GM-CSF in vitro upregulated both 5-LO and FLAP expression in PMN via transcriptional and posttranscriptional mechanisms.23 24
PBM from AIDS patients responded to GM-CSF therapy by selective upregulation of the 5-LO pathway, with no change in cPLA2expression. This data is in contrast to the effect of GM-CSF treatment in vitro on PBM from control subjects.35 In these studies, cPLA2 expression and associated AA release was selectively increased, with a resultant increase in LT synthesis. However, there was no effect of GM-CSF on 5-LO or FLAP expression in PBM from healthy controls. This could be explained by differences between PBM from healthy controls and HIV-infected subjects. Reduced in vivo exposure to GM-CSF, with resultant reduction in 5-LO metabolism, may make PBM from AIDS subjects more responsive to the cytokine’s effects. Alternatively, it could be explained by a difference in cellular response to in vivo versus in vitro treatment with GM-CSF. Studies are in progress to examine the effect of GM-CSF therapy on 5-LO metabolism in healthy controls. Importantly, the effect of GM-CSF on PBM and PMN 5-LO metabolism is further augmented in vivo with the increase in both the absolute PBM and PMN count. Notably, the effect of GM-CSF on cellular 5-LO metabolism could be on all leukocytes in the circulation, or alternatively GM-CSF could induce new PBM and PMN from the bone marrow, which have normal levels of these enzymes.
What is the clinical significance of our findings? LT play an important role in host defense mechanisms, eg, by upregulating phagocytosis,12 expression of cell surface CR3 molecules,13 secretion of O2− and lysosomal hydrolases, and killing of microorganisms.36Furthermore, 5-LO knockout mice, with defective production of LT, display increased susceptibility to bacterial pneumonia.14Therefore, a reduction in PBM, as well as AM and PMN 5-LO metabolism could predispose the host to infection. This would further compound the immunosuppression seen in patients with progression of HIV disease to AIDS. However, we have demonstrated that by treating these subjects with GM-CSF we can reverse the reduction in 5-LO metabolism. Furthermore, other investigators have demonstrated that GM-CSF therapy can boost the host immune defense mechanisms and increase the clearance of microorganisms.37-39 We speculate that the increase in cellular 5-LO metabolism after GM-CSF therapy may account for the mechanism by which the clearance of microorganisms is augmented. GM-CSF may be more useful clinically because it increases both PMN and PBM phagocytosis, whereas G-CSF only affects PMN function. Therapeutic intervention with cytokine therapy may be a useful adjunct for treatment of AIDS patients with opportunistic infections.
Supported by a grant from the Immunex Pharmaceutical Company, the American Lung Association of Michigan, and the General Clinical Research Center at University of Michigan (Grant No. M01-RR00042). M.J.C. was the recipient of National Institutes of Health Grant No. R01-HL-02810. M.P-G. was supported by the National Institutes of Health Grant No. R01-HL471.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Michael J. Coffey, MD, Associate Professor of Internal Medicine, University of Michigan Medical Center, 6301 MSRB III, 1150 W Medical Center Dr, Ann Arbor, MI 48109-0642; e-mail:coffeym@umich.edu.

![Fig. 5. [3H]Eicosanoid profile of stimulated PBM from AIDS subjects before and during therapy with GM-CSF. Cells prelabeled overnight were incubated with 1 μmol/L A23187 for 30 minutes. HPLC elution profiles are displayed for [3H]AA metabolites synthesized by A23187-stimulated PBM from a representative HIV-infected subject on day 1 (A) and on day 4 (B). Peaks were identified by coelution with authentic standards, and the products were expressed as a percentage of incorporated radioactivity. This HPLC profile is representative of 6 subjects whose cells were radiolabeled.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/11/10.1182_blood.v94.11.3897/4/m_blod42312005x.jpeg?Expires=1768856363&Signature=SfsuI6KGxzaC2NOtvcAiV8ZhdGqk1qacgXBMjtAYrLvTCb69nQwkwQ~prKALgB1NWtTxUKq1b9Mfp4VCFlYtRGJU~mDQl1eIe91HpCY-wM4mDB-WhnNFHCnAFRplOLaIIUTBpLEAd6HUxc2l37JuBm7NYQ1Pfp1vUcrEwYhtrH1ZxBHjvOCfePeB5J6egAmRGcs6bjTO2OFnRswG4yJmI85axoB-tg3hBr84kOXOw4LRlO~a4C0cjW1EZS6aLxHwek57jQ7mwfB0EnbUdA9~E0JqvjrMSwzwUZTGYuf~8PvfcWi12N~M4U32Rf4T4jwi9rwghRjqGgqJmEvNyAZAPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. [3H]Eicosanoid profile of stimulated PMN from AIDS subjects before and during therapy with GM-CSF. Prelabeled cells were incubated with 1 μmol/L A23187 for 15 minutes. HPLC elution profiles are displayed for [3H]AA metabolites synthesized by A23187-stimulated PMN from a representative HIV-infected subject on day 1 (A) and on day 4 (B). Peaks were identified by coelution with authentic standards, and the products were expressed as a percentage of incorporated radioactivity. This HPLC profile is representative of 6 subjects whose cells were radiolabeled.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/11/10.1182_blood.v94.11.3897/4/m_blod42312008x.jpeg?Expires=1768856363&Signature=V9FOwTIv1RUweCoMXdliwBMQQnfEnpYvzVpvSs6VuVKv~UErn6oy7X4hA7CO6HUnmyBZthlF32Mtyw-sffzAetZv2t3ApLY8KAmroHPIgznyJkkE8J4f3XSP9MkYgEtL9A-ipnH1oc-JnYaBafQBuhRGGnjn6S7nMH6cSGyPbXHpw3nefTB42nU9Kv~mHydStbSyyQYX8WdbiljzOPDaCk5Q9YbyC-yPr91qdBWGEwsXVW3MC~LNh~PxwtlYKpnFo9nXDKqf75kbmm34QbiwO3zztjjqvIIm8q5gRkXZiq~DG-cCSJYPEpmRozMp81clayJvTd1~vjZDUdc8-pgODQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)