Abstract
STI571 (formerly CGP57148) and AG957 are small molecule inhibitors of the protein tyrosine kinase (PTK) p145abland its oncogenic derivative p210bcr-abl. AG490 is an inhibitor of the PTK Janus kinase 2 (JAK2). No direct comparison of these inhibitors has previously been reported, so this study compared their effects on factor-dependent FDC-P1, 32D, and MO7e cells and their p210bcr-abl-expressing factor-independent derivatives. STI571 was a more potent inhibitor of3H-thymidine incorporation in p210bcr-abl-expressing cells than was AG957, and it showed superior discrimination between inhibitory effects on parental cell lines and effects on their p210bcr-abl-expressing derivatives. Assays performed with and without growth factor demonstrated that STI571 but not AG957 reversed the p210bcr-abl-driven factor independence of cell lines. p210bcr-abl-expressing cells were less sensitive to AG490 than to AG957 or STI571. However, for p210bcr-abl-expressing clones from all 3 cell lines, synergistic inhibition was demonstrated between STI571 and concentrations of AG490 with no independent inhibitory effect. Inhibition of nucleic acid synthesis with AG957 treatment was associated with reduced cell numbers, reduced viability, and small pyknotic apoptotic cells. At concentrations of STI571 that reversed the p210bcr-abl factor-independent phenotype, STI571 treatment and growth factor deprivation together were sufficient to induce apoptosis. This study concludes that, for the cell lines studied, (1) STI571 is a more potent and more selective inhibitor of a p210bcr-abl-dependent phenotype than AG957; (2) AG490 synergizes with STI571 to enhance its inhibitory effect on p210bcr-abl-driven proliferation; and (3) the combination of p210bcr-abl-tyrosine kinase inhibition and growth factor signal withdrawal can be sufficient to induce apoptotic death of transformed cells.
Introduction
The tyrosine kinase protein (PTK) product of the BCR-ABL fusion gene that results from the t(9;22) translocation1-5 of chronic myelogenous leukemia (CML) and some acute leukemias is an attractive therapeutic target. This has been particularly so since the demonstration that a myeloproliferative syndrome results from the overexpression of the commonest fusion protein resulting from this translocation (p210bcr-abl) in the bone marrow cells of mice,6,7 although the degree to which the murine model recapitulates human CML is somewhat dependent on the murine genetic background.8 That the overexpression of p210bcr-abl is sufficient to drive the disease phenotype suggests that a selective inhibitor of the p210bcr-abl, or even an inhibitor active against both wild-type p145abl and its oncogenic variants such as p210bcr-abl, may be able to suppress or reverse the CML disease phenotype. Because the kinase activity of the BCR-ABL fusion protein is integral to its transforming ability,9 this has led to the development of several small molecules with inhibitory activity against p145abl and/or p210bcr-abl.
One particularly promising inhibitor is STI571 (formerly known as CGP57148), a 2-phenylaminopyrimidine class molecule that was designed, based on the structure of the adenosine triphosphate (ATP)-binding site of PTKs, and was selected for its specificity for the ABL tyrosine kinase.10 STI571 is equipotent at inhibiting p145abl and p210bcr-abl,10 but it is not completely selective for ABL kinases and shows similar inhibitory potency in biochemical evaluations on the platelet-derived growth factor receptor (PDGF-R)10 and c-kit, the receptor for stem cell factor.11 In vitro cellular studies confirmed the ability of STI571 to reverse the p210bcr-abl-driven conversion of a cell line from hematopoietic growth factor dependence to factor independence and showed selective in vivo antitumor activity against tumor-forming p210bcr-abl-positive cell lines in murine models.10 Similar in vitro effects of STI571 have been shown for a PDGF-R-driven cellular phenotype.12 STI571 has now entered early phase clinical studies, and preliminary data indicate it to be safe and to have clinical activity.13 14
Another ABL inhibitor is AG957, a tyrphostin identified in a large-scale evaluation of molecules designed as competitive antagonists of ATP binding to PTKs.15 Unlike STI571, it is a more potent inhibitor of p210bcr-abl than p145abl (50% inhibitory concentrations [IC50s], 1 and 7.1 μM, respectively),15 although this is less potent than that reported for STI571 in similar noncellular biochemical assays (0.025 μM).10 AG957 inhibits proliferation of the BCR-ABL-positive cell line K562 (derived from a CML patient)16, and related tyrphostin inhibitors promote differentiation of this cell line.17 Recently, AG957 was shown to have greater potency against some subpopulations of BCR-ABL-positive hematopoietic progenitors than genotypically normal progenitors isolated from CML patients.18 Despite its action on the ABL kinases, AG957 is not totally specific; for example, it is a more potent inhibitor of the epidermal growth factor receptor (EGF-R) (IC50, 0.25 μM).15
Although both these semi-selective ABL inhibitors have each been studied individually, there is no direct side-by-side comparison of them, even though the separately published data cited above suggests that STI571 is the more potent ABL kinase inhibitor. Because both STI571 and AG957 have assumed prominence as relatively “specific” BCR-ABL inhibitors, we undertook these studies to directly compare their effects on a p210bcr-abl-dependent cellular phenotype.
AG490 is not an effective ABL inhibitor, but rather it is a tyrphostin that has received attention because of its inhibitory effects on the nonreceptor PTK Janus kinase 2 (JAK2),19 which is critical in signaling from many hematopoietic growth factors.20JAK2 activity is implicated in the pathogenesis of some leukemias,21 its expression is significantly increased in others,19 and a signaling interaction between the JAK2 and BCR-ABL proteins is recognized.22 AG490 is not, however, totally specific for JAK2 (eg, it also inhibits JAK3).23We were interested to include AG490 in our comparison as a non–ABL-inhibiting tyrphostin control, but we also hypothesized that its actions to interfere with JAK signaling pathways might provide additive or even synergistic inhibitory effects on leukemic cells.
Methods and materials
Cell lines
The following cell lines used were all as previously described: FDC-P124 and its derivative cell lines transfected to overexpress human p210bcr-abl under control of either a retroviral promoter (FDrv210, 3 independently derived clones C, F, and H) or a weaker human BCR promoter (FDbcr210, 3 independently derived clones A, E, and G),25 32D cells26 and their human p210bcr-abl-overexpressing derivative 32Dp210, a gift of Dr B. Druker (Portland, OR),10 MO7e cells27 and their human p210bcr-abl-overexpressing derivative, a gift of Dr B. Druker,10 and K562 cells.28 29
All cell lines were propagated in RPMI 1640 medium (Gibco BRL, Grand Island, NY) supplemented with 10% fetal calf serum (FCS) (CSL Ltd, Parkville, Victoria, Australia) at 37°C in an atmosphere of 5% CO2 in air. Cultures of the factor-dependent FDC-P1 and 32D cell lines were further supplemented with 10% WEHI-3BD-conditioned medium as a source of interleukin-3 (IL-3), and of MO7e with 10 ng/mL human granulocyte-macrophage colony-stimulating factor (GM-CSF), a gift from Amgen (Thousand Oaks, CA). All BCR-ABL-expressing transfectants were maintained in medium without growth factor.
Reagents
The tyrphostin inhibitors AG95715 and AG49019 were provided as white powders by Dr A. Levitski (Hebrew University of Jerusalem, Jerusalem, Israel). Mass spectroscopic analysis by electrospray (Quattro II, Micromass, Manchester, United Kingdom) confirmed these to be of the calculated molecular mass (273 Da and 294 Da, respectively). Stock solutions at 50 mM in dimethyl sulfoxide (DMSO) were prepared and stored as aliquots at −20°C, from which fresh working solutions were prepared in RPMI 1640 for each experiment.
STI57110 was a gift of Dr E. Buchdunger (Novartis, Basel, Switzerland). A 10-mM stock solution in phosphate-buffered saline (PBS) was prepared and stored at −20°C, from which fresh working solutions were prepared in RPMI 1640 for each experiment.
Immunofluorescence and flow cytometry
p210bcr-abl and p145abl expression in cell lines was detected by indirect immunofluorescence FACS profiling.25 Briefly, cells were fixed for 10 minutes at room temperature in 1% paraformaldehyde in PBS and permeabilized in 0.3% saponin and 0.5% Triton X-100 in PBS with 1% FCS at 4°C for 15 minutes prior to staining with the monoclonal anti-ABL antibody 24-21 (Oncogene Science, Manhasset, NY), using a fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G (IgG) (Pharmingen, San Diego, CA) as secondary antibody. Cells were analyzed in a FACScan (Becton Dickinson, San Jose, CA).
Cell proliferation assays
Assays were performed in duplicate across 96-well microtiter plates and set up with robotic assistance (Biomek 2000, Beckman, Fullerton, CA). Pre-diluted inhibitor from frozen stocks was added to the first well to achieve the desired starting concentration in 200 μL and titrated as serial 2-fold dilutions across the plate, leaving 100 μL of medium with inhibitor per well. Cells were harvested and washed twice, and aliquots of 4 × 104 cells in 100 μL were added to each medium-containing well to make a final assay volume of 200 μL. The assay medium was RPMI 1640 and 0.5% FCS with or without the appropriate growth factor and contained 1 μCi of3H-thymidine. Cells were exposed to the inhibitors for either 30 minutes or 18 hours. For 30-minute exposures, cells were washed 2 times after a 30-minute incubation in inhibitor-containing medium, and the medium was replaced with fresh medium containing 1 μCi of 3H-thymidine for 18 hours but lacking inhibitor. In assays testing for synergistic effects between inhibitors, the second (nontitrated) inhibitor was added in a volume of 5 μL, making a total final assay volume of 205 μL. Cells were harvested onto a glass fiber filter, and 3H-thymidine incorporation was counted on a microplate scintillation counter (Top Count.NXT; Canberra Packard, Meriden, CT).
In some experiments, cell number was determined by setting up the assay under identical conditions except that the final assay volume was 2 mL, but the concentration of cells remained at 2 × 105/mL at the start of the assay. After 18-hour exposure, total cell number was determined, and proportional cell viability was measured by trypan blue dye exclusion with the use of a hemocytometer. In parallel, cytospin preparations of cells were prepared and stained with May-Grunwald-Giemsa and examined at ×100 to ×1000 magnification.
DNA integrity analysis
Cytosolic and nuclear DNA was prepared from 5 × 106 cells. Briefly, to prepare cytosolic DNA, cells were lysed in 500 μL of lysis buffer (0.5% Triton X-100, 20 mM Tris-HCl, 1 mM EDTA, pH = 7.4) for 5 minutes on ice. Lysates were spun at 13 000 rpm in a bench-top Eppendorf centrifuge (Heraeus “Biofuge pico,” Osterode, Germany) for 20 minutes, the supernatant was transferred to a fresh tube, and DNA was precipitated with NaCl/isopropanol. To prepare nuclear DNA, the pellets remaining from cytosolic DNA preparations were washed twice in PBS and incubated overnight at 55°C in 750 μL lysate buffer (50 mM Tris-HCl, 0.1 M EDTA, 0.1 M NaCl, 1% SDS, pH = 8.0) to which 40 μL Pronase 20 mg/mL had been added. Then, 310 μL 5 M NaCl was added, samples were spun as above, 800 μL of the supernatant was transferred to a new tube, and DNA was precipitated with 500 μL isopropanol. DNA was pelleted, washed with 70% ethanol, air-dried, electrophoresed through a 0.8% agarose gel, and viewed by ethidium bromide staining and UV illumination.
Statistics
Unless otherwise stated, data presented in figures are means of duplicate assays. Figures present data generated simultaneously in a representative experiment. Experiments were replicated 3 times or more except for the data shown in Figure 5 and 6 (2 replicates) and in Figure 3C (1 experiment). The effect of IL-3 on p210bcr-abl-expressing cell lines (Table1) was evaluated with a 2-sided sign test.
Results
Confirmation of BCR-ABL overexpression
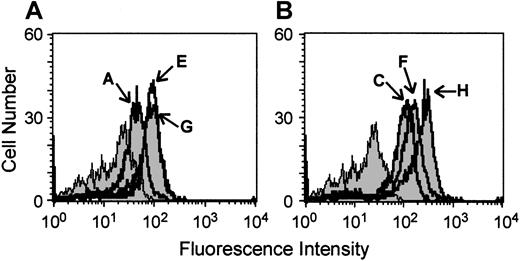
The FDC-P1 cell line and its derivative clones FDbcr210A, FDbcr210E, FDbcr210G, FDrv210C, FDrv210H, and FDrv210F were studied to confirm p210bcr-abl overexpression because they formed the basis of all initial observations in these studies. Flow cytometric detection of ABL protein expression by indirect immunofluorescence was used to parallel the previous characterizations of these cell lines,25 employing the anti-ABL antibody 24-21 directed against the C-terminus of ABL, which hence recognizes both p145abl and p210bcr-abl. This process demonstrated that FDbcr clones and FDrv clones showed higher fluorescence intensity than parental FDC-P1 cells (Figure 1). The clones driving the BCR-ABL p210 complementary DNA (cDNA) from the stronger retroviral promoter showed higher level expression (clone H > F > C) than those driven from the weaker BCR promoter (clone A < E and G), confirming retention of transduced p210bcr-abl expression despite the passaging and storage of these cell lines since previous studies.25 For some studies (eg, those comparing AG957 and STI571), the clones FDrv210H and FDbcr210E were selected as being representative of each group, because they showed the highest level of BCR-ABL expression. K562 cells, which have previously been shown to overexpress BCR-ABL,29 were confirmed to overexpress ABL, based on higher fluorescence intensity observed compared to negative controls omitting one or both detection antibodies (data not shown).
High-level ABL expression in BCR-ABL-transfected cell lines used in these studies.
Fluorescence intensity profiles of parental FDC-P1 cells (shaded) and its derivative clones FDbcr210A, E, and G (solid lines, panel A) and FDrv210C, F, and H (solid lines, panel B). Cells were permeabilized and stained with an anti-ABL antibody detected by a fluorescein-labeled secondary antibody.
High-level ABL expression in BCR-ABL-transfected cell lines used in these studies.
Fluorescence intensity profiles of parental FDC-P1 cells (shaded) and its derivative clones FDbcr210A, E, and G (solid lines, panel A) and FDrv210C, F, and H (solid lines, panel B). Cells were permeabilized and stained with an anti-ABL antibody detected by a fluorescein-labeled secondary antibody.
Effects of inhibitors in proliferation assays
To evaluate the effects of inhibitors on proliferation of p210bcr-abl-expressing cell lines, we measured3H-thymidine incorporation. Because both AG957 and AG490 are hydrophobic and 50-mM stocks were prepared in DMSO, we first ascertained that there was no effect of this vehicle in the residual concentrations remaining in the final assay incubations for the method of serial dilution in the medium employed (Figure2A).
Comparison of anti-proliferative effects of AG957, AG490, and STI571 on BCR-ABL-expressing cell lines.
3H-thymidine incorporation in varying concentrations of vehicle AG957, AG490, and STI571 in the presence or absence of growth factor. (A) Comparison of AG957, AG490, and vehicle in presence of IL-3 for the cell line FDbcr210E; 18-hour exposure to inhibitor. (B) Comparison of AG957 and STI571 ± IL-3 for untransfected FDC-P1 cells; 18-hour incubation in inhibitor. (C) Comparison of AG957 and STI571 ± IL-3 for the BCR-ABL-expressing cell line FDbcr210E; 18-hour incubation in inhibitor. (D) Comparison of AG957 and STI571 ± IL-3 for the BCR-ABL-expressing cell line FDbcr210E; 30-minute incubation in inhibitor. (E) Comparison of AG957 and STI571 for the human BCR-ABL-expressing cell line K562; 18-hour incubation in inhibitor in absence of IL-3.
Comparison of anti-proliferative effects of AG957, AG490, and STI571 on BCR-ABL-expressing cell lines.
3H-thymidine incorporation in varying concentrations of vehicle AG957, AG490, and STI571 in the presence or absence of growth factor. (A) Comparison of AG957, AG490, and vehicle in presence of IL-3 for the cell line FDbcr210E; 18-hour exposure to inhibitor. (B) Comparison of AG957 and STI571 ± IL-3 for untransfected FDC-P1 cells; 18-hour incubation in inhibitor. (C) Comparison of AG957 and STI571 ± IL-3 for the BCR-ABL-expressing cell line FDbcr210E; 18-hour incubation in inhibitor. (D) Comparison of AG957 and STI571 ± IL-3 for the BCR-ABL-expressing cell line FDbcr210E; 30-minute incubation in inhibitor. (E) Comparison of AG957 and STI571 for the human BCR-ABL-expressing cell line K562; 18-hour incubation in inhibitor in absence of IL-3.
FDC-P1 cells are dependent on IL-3, whereas their p210bcr-abl-expressing derivatives have acquired factor independence. This phenotypic change provided a useful opportunity to test for the reversal of a p210bcr-abl-dependent biological effect in vitro. The dependence of parental FDC-P1 cell proliferation on IL-3 is illustrated in Figure 2B: In the presence of IL-3, the median IC50s for inhibition by AG957 and AG490 were 5.0 and 12.5 μM (Figure 2B and Table 1). Interestingly, these IC50s were similar to those of all 6 clones of p210bcr-abl-expressing derivatives, and there was little effect of adding IL-3 to the incubations in the presence of inhibitor, suggesting that little of the inhibitory effect reflected reversal of the p210bcr-abl-driven factor independence. However, the effect of STI571 was quite different (Table1 and Figure 2B,C). Parental FDC-P1 cells displayed an IC50for STI571 of more than 10 μM, whereas 2 p210bcr-abl-expressing clones were significantly more sensitive (median IC50s, 0.65 and 2 μM). Furthermore, unlike AG957, STI571 reverted both these p210bcr-abl-expressing clones to IL-3 responsiveness (Figure 2C). This abrogation of a phenotypic consequence of p210bcr-abl overexpression (reversion to the parental cell phenotype of IL-3 responsiveness) implicates p210bcr-abl inhibition as a functionally significant component of the actions of STI571 in the context of these IL-3-dependent cell lines.
The superiority of STI571 compared to AG957 was replicated for other cell lines and their p210bcr-abl-expressing derivatives. Parental 32D cells were 25-fold more sensitive to AG957 than STI571 (consistent with AG957s nonspecific effect on parental FDC-P1 cells), and significant STI571-mediated reversion of p210bcr-abl-expressing 32Dp210 cells to IL-3 responsiveness was demonstrated (Table 1). Similar observations applied to MO7e and its GM-CSF-independent p210bcr-abl-expressing derivative MO7p210 (Table1).
The initial assays all involved 18-hour incubations in the presence of inhibitor. We also evaluated the effect of a 30-minute exposure to inhibitor (Figure 2D). There was a significant inhibition by AG957 (IC50 > 10 μM) but again little rescue of this effect by IL-3. In contrast, although the cells were now less sensitive to STI571, IL-3 rescue of STI571 inhibition was demonstrated.
We compared the effects of AG957 and STI571 on K562 cells, a human BCR-ABL-positive cell line that has been employed previously in studies of these 2 and related inhibitors.10,16 17 K562 cells were 10 times more sensitive to STI571 than to AG957 (Figure 2E and Table1). Although K562 cells are growth factor independent, the exact role of BCR-ABL in conferring the independence of this cell line to any particular growth factor is not known, and so comparative experiments with and without growth factor were not performed.
Synergistic inhibition by STI571 and AG490
Because AG490 is known to inhibit JAK219 and JAK2 is a component of signaling mediated by many cytokines, including IL-3 and GM-CSF,20 we tested whether there was any synergistic effect between STI571-mediated inhibition and AG490.
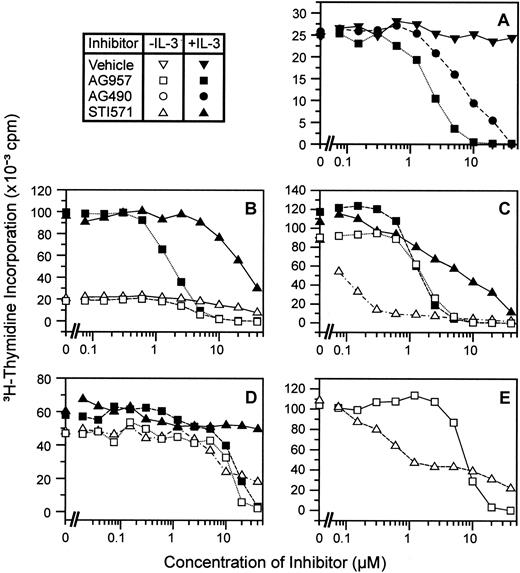
Data are presented for the FDC-P1-derivative cell line expressing the highest level of p210bcr-abl (FDrv210H) and 18-hour assay incubations (Figure 3A). STI571-mediated IL-3-rescuable inhibition is displayed for this cell line (Figure 3A). AG490 alone had no effect on proliferation of this cell line at a concentration of 5 μM. However, when this no-effect concentration of AG490 was added to STI571, significant synergy occurred with a suppression of maximal 3H-incorporation by 42%. AG490 (5 μM) did not suppress all IL-3-mediated signaling, and IL-3 rescue could still be demonstrated in its presence. Similar observations were obtained for studies with the cell line FDbcr210E (data not shown).
Synergistic inhibitory effects of STI571 and AG490 on BCR-ABL-expressing cell lines.
(A) Experiments using the cell line FDrv210H (18-hour incubation in inhibitors), titrating STI571 in a constant concentration of AG490. In panels A and B, solid (+IL-3) and open (−IL-3) symbols identify curves for varying concentrations of AG490 alone (●,○) STI571 alone (▴,▵), and serial dilutions of STI571 in a constant concentration AG490 [5 μM] (♦,⋄). (B) Experiment using the cell line 32Dp210 (18-hour incubation in inhibitors), titrating STI571 in a constant concentration of AG490. (C) For a constant STI571 concentration (0 or 0.01 μM), AG490 was added at the concentrations shown (MO7p210 cell line; 18-hour incubation in inhibitors without GM-CSF).
Synergistic inhibitory effects of STI571 and AG490 on BCR-ABL-expressing cell lines.
(A) Experiments using the cell line FDrv210H (18-hour incubation in inhibitors), titrating STI571 in a constant concentration of AG490. In panels A and B, solid (+IL-3) and open (−IL-3) symbols identify curves for varying concentrations of AG490 alone (●,○) STI571 alone (▴,▵), and serial dilutions of STI571 in a constant concentration AG490 [5 μM] (♦,⋄). (B) Experiment using the cell line 32Dp210 (18-hour incubation in inhibitors), titrating STI571 in a constant concentration of AG490. (C) For a constant STI571 concentration (0 or 0.01 μM), AG490 was added at the concentrations shown (MO7p210 cell line; 18-hour incubation in inhibitors without GM-CSF).
Studies with 32Dp210 cells replicated this phenomenon (Figure 3B). For this cell line also, 5 μM AG490 was a no-effect concentration for this agent alone. Figure 3B displays the typical effect of STI571, with an IC50 of 0.03 μM in the absence of IL-3 and more than 2 μM with IL-3. Addition of 5 μM AG490 suppressed maximal proliferation by approximately 31%. Figure 3C demonstrates this synergistic effect for a third cell line, MO7p210. Furthermore, this titration demonstrates that for a given no-effect concentration of STI571 on this cell line of 0.01 μM, the cells are sensitized to the synergistic inhibitory effect of increasing no-effect doses of AG490. Consistent with the notion that this synergy was dependent on inhibition of p210bcr-abl by STI571, synergy was not detected between STI571 and AG490 for nontransfected FDC-P1, 32D, and MO7e cell lines.
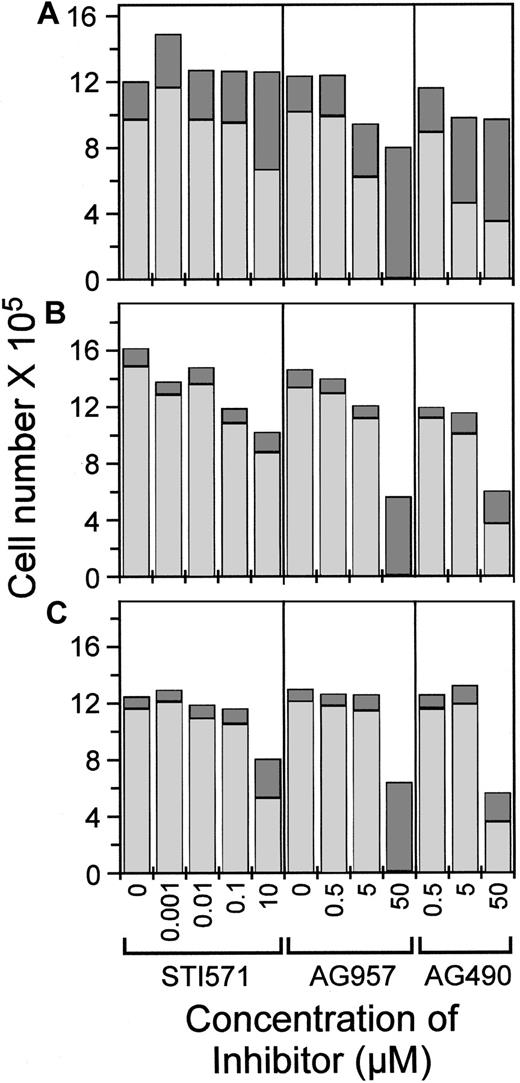
Effects of inhibitors on cell numbers and viability
Because 3H-thymidine incorporation only indirectly measures cellular proliferation by the surrogate of nucleic acid synthesis, we determined whether cell numbers and viability were changing over the duration of these relatively short proliferation assays.
Even in 10 μM of STI571, FDC-P1 cells in IL-3 proliferated (because the total cell number exceeded the starting cell number by 3.1-fold) (Figure 4A), although there was an increased proportion of dead cells, correlating with the 20% below-maximal 3H-thymidine incorporation observed for these conditions. Approximately 80% to 85% of FDC-P1 cells were viable over noninhibitory concentrations of STI571, but there was increased death (54% viable) at 10 μM STI571. For both AG957 and AG490, FDC-P1 cell numbers were 26% and 18% lower than control at 5 μM inhibitor, and there was increased death. No FDC-P1 cells were viable after 18 hours in 50 μM AG957.
Total cell number and viability following assays with 18-hour incubation in AG957, AG490, and STI571.
Total cell number shown as the sum of dead (darkly shaded column part) and viable (lightly shaded column part), for the concentrations of STI571, AG957, and AG490 shown. Assays started with 4 × 105 cells in 2 mL. (A) FDC-P1 cell in media + IL-3. (B) FDbcr210E cells in media + IL-3. (C) FDbcr210E cells in media without IL-3.
Total cell number and viability following assays with 18-hour incubation in AG957, AG490, and STI571.
Total cell number shown as the sum of dead (darkly shaded column part) and viable (lightly shaded column part), for the concentrations of STI571, AG957, and AG490 shown. Assays started with 4 × 105 cells in 2 mL. (A) FDC-P1 cell in media + IL-3. (B) FDbcr210E cells in media + IL-3. (C) FDbcr210E cells in media without IL-3.
FDbcr210E cells in IL-3 also proliferated under all assay conditions (Figure 4B), with total cell number increasing in the assay period by 2.5- to 4-fold, except in high tyrphostin concentrations that also suppressed 3H-thymidine incorporation (Figure 2A,C). At 50 μM, AG957 killed all cells in 18 hours, whereas 65% of cells remained viable in AG490. In the absence of IL-3, the profile of final cell numbers was essentially similar, except that the fold increase overall approximated 3-fold rather than 3- to 4-fold, consistent with the previously documented residual IL-3 responsiveness of this particular FDC-P1-derived p210bcr-abl-expressing clone25 (Figure 4C). The different total cell yields in STI571 with and without IL-3 (comparing Figure 4B,C) reflect the effect of the restoration of IL-3 dependence for this particular clone.
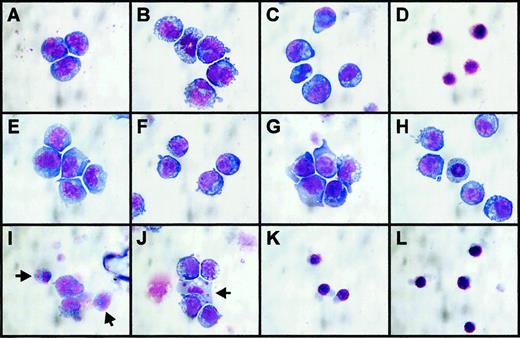
Induction of apoptosis by inhibitors
There were significant morphologic changes in cells under the various incubation conditions. Reducing the concentration of FCS from 10% to 0.5% for the assay itself resulted in FDC-P1 cells assuming a vacuolated appearance and increased diameter with increased nuclear pleiotrophy (Figure 5A,B), but mitotic figures indicated proliferation continued (Figure 5B). Adding STI571 at a high but noninhibitory concentration (10 μM) did not further affect cell appearance (Figure 5C). A toxic concentration of AG957 (50 μM) resulted in shrunken cells with pyknotic nuclei (Figure 5D), consistent with the nonviability of these cells (Figure 4A), but AG957 concentrations as low as 5 μM resulted in a similar appearance (data not shown). Transfected p210bcr-abl-expressing FDC-P1 cells had an identical appearance to nontransfected FDC-P1 cells in 10% FCS (Figure 5A,E) and retained a more normal appearance under low serum conditions, whether or not IL-3 was present (Figure 5F,G), consistent with a resistance to this type of cellular stress. At the no-effect dose of AG490 used in the synergy studies above, as well as being no anti-proliferative effect, there was no effect on cell morphology (Figure 5H). At doses of STI571 inhibiting proliferation in the absence of IL-3 (1-10 μM), apoptotic cellular death was widely evident (Figure 5I), and IL-3 rescue of proliferation was associated with a reduced prevalence of these morphologic changes of apoptosis, although some apoptotic cells remained evident (Figure 5J). Regardless of whether IL-3 was present or not, anti-proliferative doses of AG957 (5-50 μM) resulted in FDrv210H cells assuming an identical morphology to that of dead parental FDC-P1 cells (Figure 5K,L).
Morphology of cell lines incubated with AG957, AG490, and STI571 for 18 hours.
May-Grunwald-Giemsa stained cytospin preparations of cells incubated for 18 hours in media with FCS, growth factor, and inhibitor concentrations as listed. (A) FDC-P1 cells, 10% FCS + IL-3, no inhibitors. (B) FDC-P1 cells, 0.5% FCS + IL-3, no inhibitors. (C) FDC-P1 cells, 0.5% FCS + IL-3, STI571 = 10 μM. (D) FDC-P1 cells, 0.5% FCS + IL-3, AG957 = 50 μM. (E) FDrv210H cells, 10% FCS no IL-3, no inhibitors, (F) FDrv210H cells, 0.5% FCS no IL-3, no inhibitors. (G) FDrv210H cells, 0.5% FCS + IL-3, no inhibitors. (H) FDrv210H cells, 0.5% FCS + IL-3, AG490 = 5 μM. (I) FDrv210H cells, 0.5% FCS no IL-3, STI571 = 10 μM; arrows indicate 2 cells with fragmenting apoptotic nuclei. (J) FDrv210H cells, 0.5% FCS + IL-3, STI571 = 10 μM; arrow indicates one cell with fragmented apoptotic nucleus. (K) FDrv210H cells, 0.5% FCS no IL-3, AG957 = 50 μM. (L) FDrv210H cells, 0.5% FCS + IL-3, AG957 = 50 μM.
Morphology of cell lines incubated with AG957, AG490, and STI571 for 18 hours.
May-Grunwald-Giemsa stained cytospin preparations of cells incubated for 18 hours in media with FCS, growth factor, and inhibitor concentrations as listed. (A) FDC-P1 cells, 10% FCS + IL-3, no inhibitors. (B) FDC-P1 cells, 0.5% FCS + IL-3, no inhibitors. (C) FDC-P1 cells, 0.5% FCS + IL-3, STI571 = 10 μM. (D) FDC-P1 cells, 0.5% FCS + IL-3, AG957 = 50 μM. (E) FDrv210H cells, 10% FCS no IL-3, no inhibitors, (F) FDrv210H cells, 0.5% FCS no IL-3, no inhibitors. (G) FDrv210H cells, 0.5% FCS + IL-3, no inhibitors. (H) FDrv210H cells, 0.5% FCS + IL-3, AG490 = 5 μM. (I) FDrv210H cells, 0.5% FCS no IL-3, STI571 = 10 μM; arrows indicate 2 cells with fragmenting apoptotic nuclei. (J) FDrv210H cells, 0.5% FCS + IL-3, STI571 = 10 μM; arrow indicates one cell with fragmented apoptotic nucleus. (K) FDrv210H cells, 0.5% FCS no IL-3, AG957 = 50 μM. (L) FDrv210H cells, 0.5% FCS + IL-3, AG957 = 50 μM.
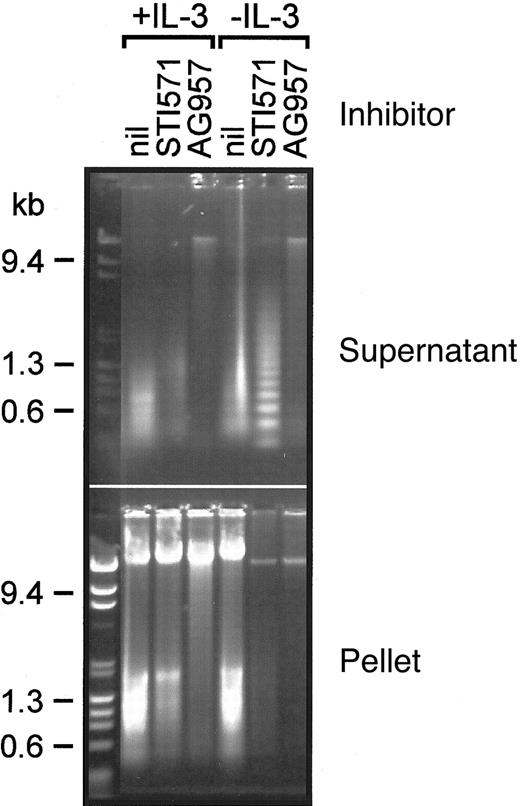
We evaluated the integrity of cellular DNA under these conditions (Figure 6). In the presence of IL-3, most high molecular weight cellular DNA was recovered in the pellet (nuclear) cellular fraction. Despite the appearance of these cells in the presence of 50 μM AG957, only a small proportion of the DNA was in the cytosolic fraction, and this was of relatively high molecular weight. In the absence of IL-3, STI571 10 μM not only inhibited proliferation (Figure 2C) and induced an apoptotic cellular morphology (Figure 5I), but there was also cytosolic recovery of laddered DNA fragments (Figure 6) consistent with classical apoptotic cell death. In the absence of IL-3, AG957 again resulted in recovery of some cytosolic and nuclear high molecular weight DNA, but apoptotic degradation of the DNA was not evident in the cytosolic fraction.
DNA analysis from AG957- and STI571-treated BCR-ABL-expressing cells.
Ethidium bromide-stained agarose gel displaying DNA in cytosolic (supernatant) and nuclear (pellet) fractions of FDbcr210E cells treated for 18 hours in the conditions labeled; inhibitor concentrations were STI571 = 10 μM and AG957 = 50 μM.
DNA analysis from AG957- and STI571-treated BCR-ABL-expressing cells.
Ethidium bromide-stained agarose gel displaying DNA in cytosolic (supernatant) and nuclear (pellet) fractions of FDbcr210E cells treated for 18 hours in the conditions labeled; inhibitor concentrations were STI571 = 10 μM and AG957 = 50 μM.
Discussion
Both AG957 and STI571 have been extensively studied because of their inhibition of p145abl and its oncogenic derivative p210bcr-abl. Within the context of the cell lines we have employed, our studies demonstrate the superiority of STI571 over AG957 as a selective p210bcr-abl inhibitor. Despite the 7-fold superior potency of AG957 for p210bcr-abl over p145abl,15 AG957 was still a much less effective inhibitor of a p210bcr-abl-dependent cellular phenotype (factor independence) than STI571. The similar IC50 of AG957 on nontransfected cell lines and their p210bcr-abl-expressing derivatives suggests the inhibitory effects of AG957 are either unrelated to p210bcr-abl inhibition or are not restricted to p210bcr-abl inhibition. AG957 also appeared more toxic than STI571 because even the lowest inhibitory concentrations induced rapid cell death by apoptosis, whereas, in the presence of growth factor, inhibitory concentrations of STI571 suppressed nuclear DNA replication measured by 3H-thymidine incorporation without significant cell death.
Although the previously reported effects of AG957 on K562 cell proliferation were associated with inhibition of p210bcr-abl kinase activity,16 this association does not prove that the AG957 effects were primarily by its action on p210bcr-abl. Our data suggest that AG957 has other inhibitory effects in its spectrum of activity that also need to be considered in analyses such as these. In a comparison of AG957 effects on normal and CML patient-derived hematopoietic stem cells, although statistically significant differences were seen for some cell types, the discrimination was not biologically great, IC50s being in the range of 12 to 181 μM, and never more than 5.3-fold for any progenitor cell type.18 Indeed, AG957 displays an IC50 for the epidermal growth factor receptor (EGF-R) of 0.25 μM,15 making it a better inhibitor of the EGF-R than of p210bcr-abl, and leaving considerable scope for it to exert inhibitory effects on other kinase molecules. It is even possible that the spectrum of activity of AG957 includes inhibition of kinases critical for signaling from the IL-3 and GM-CSF receptors, which would have masked the recognition of its effect on p210bcr-abl in the assays we performed.
In contrast, the inhibitory effect observed in these and other10 assays strongly implicates an effect of STI571 on the p210bcr-abl kinase. In our own experiments, in the presence of growth factor, STI571 showed up to 17-fold greater potency for p210bcr-abl-expressing derivatives of cell lines than for parental cell lines (Table 1). As a biological test of biochemical specificity, we based our assays on cell lines with a p210bcr-abl-driven specific phenotype—the acquisition of factor independence—and, as has been previously observed,10 STI571 reversed this back to the factor-dependent phenotype of nontransfected cells. To determine its spectrum of activity, STI571 has been specifically tested against a broad range of protein kinases.10,12 Although our experiments and those of others10 indicate that STI571 acts on cellular p210bcr-abl, these surveys of its activity indicate that it is not totally specific. It is equipotent against the kinase activity of PDGF-R10 andc-kit.11 This is unlikely to be of relevance in the assays on which these present studies are based, because we did not supplement media with ligands for these receptors, and FDC-P1 and 32D cells are not known to express activated forms of them, although MO7e cells express c-kit.30 However, this may be important in clinical situations, because, for example in CML, BCR-ABL-expressing early hematopoietic stem cells are likely to expressc-kit and to be responsive to its ligand. Even in the case of the PDGF-R, aberrant activation in hematopoietic cells drives a leukemic phenotype,12 31 and this may contribute to the clinical effectiveness of STI571 in some circumstances.
We had expected that AG490, as a JAK2 inhibitor,19 would have no significant effect on factor-independent p210bcr-abl-expressing cells. Indeed, in the presence of IL-3, the IC50s observed for transfected p210bcr-abl-expressing cells and their respective parental cell lines were similar for all 3 cell lines evaluated (Table 1). Such a comparison cannot be made in the absence of IL-3, because nontransfected cells do not grow without growth factor. However, once the growth factor dependence of p210bcr-abl-expressing cells was restored by STI571 treatment, addition of AG490 at a dose having no or minimal effect in its own right resulted in a significant further suppression of proliferation. Noting that a combination of growth factor deprivation and p210bcr-abl inhibition by STI571 provided an initiating signal for cell death to proceed by apoptosis, it is tempting to attribute the synergy between STI571 and AG490 to the known ability of AG490 to inhibit JAK2, which is utilized in IL-3 signaling.20 However, AG490's profile of activity has only been tested against a very small number of kinases,19and recently it was shown to also inhibit JAK3.23 There is ample scope for this effect to be mediated by inhibition of another kinase or enzyme yet to be identified as susceptible to inhibition by AG490. Nonetheless, the combination of an effective p210bcr-abl inhibitor such as STI571 and inhibition or antagonism of growth factor signaling might form an efficacious and highly novel signaling-based combination therapy for diseases such as CML. In this regard, it would be of interest to evaluate this synergistic inhibitor combination using cell lines with acquired resistance to inhibition by STI571.32-34
As expected, AG957 killed cells by apoptosis;17p210bcr-abl-expressing FDC-P1 cells in AG957 showed characteristic apoptotic morphologic changes, although we did not detect endonuclease-mediated apoptotic fragmentation of DNA. It is possible, because apoptosis is energy dependent and tyrphostins such as AG957 were designed as competitors of ATP binding to ATP-dependent enzymes, that the apparently broad spectrum of inhibitory activities of AG957 includes paralysis of ATP-dependent steps of the apoptotic enzymic cascade. STI571 has previously been reported to induce apoptosis in BCR-ABL-positive CML cells.35 36 We have shown that for p210bcr-abl-expressing FDC-P1cells, the combination of p210bcr-abl-inhibition by STI571 and growth factor deprivation led not just to arrest of cell growth but also to apoptotic cell death. At STI571 concentrations specifically reversing the p210bcr-abl-dependent phenotype, the restoration of growth factor signaling largely reversed apoptotic death. These observations provide evidence that, although specific inhibition of p210bcr-abl alone may not necessarily kill a p210bcr-abl-driven leukemia, a rationally designed combination of interruptions to signaling pathways may indeed induce death of such cells. In this regard, the nonspecificity of STI571 may be advantageous—its potency against thec-kit receptor (mediating growth factor signals from its ligand stem cell factor) may be contributing to its efficacy in hematologic disease.
Our studies have demonstrated in a head-to-head comparison the superior specificity and potency of STI571 over AG957 as a p210bcr-abl inhibitor. Additionally, our studies of synergy between STI571 and AG490 or growth factor deprivation support the view that there is scope for synergistic combinations of rationally selected signaling-based therapies to provide novel approaches of increased anti-leukemic potency for the treatment of kinase-driven malignancies such as p210bcr-abl-driven CML.
We thank Dr A. Levitski for synthesizing and providing AG957 and AG490 for these studies; Dr B. Druker for providing cell lines and some helpful comments; Dr E. Buchdunger and staff at Novartis for providing STI571; Dr F. Walker for assistance with the proliferation assays; Ms M. Nerrie and Ms D. McPhee for technical assistance; Mr G. Rennie for help with the statistical analysis; Ms E. Passmore for secretarial assistance; and Professor A. W. Burgess, Professor W. Robinson, Dr A. Scott, Dr P. Ekert, and Dr B. Brady for helpful discussions. G. Lieschke is the recipient of a Wellcome Senior Research Fellowship in Medical Sciences in Australia.
Supported by a World Health Organization fellowship (X. Sun).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Graham J. Lieschke, Ludwig Institute for Cancer Research, PO Box 2008, The Royal Melbourne Hospital, Victoria, 3050, Australia; e-mail: graham.lieschke@ludwig.edu.au.

![Fig. 3. Synergistic inhibitory effects of STI571 and AG490 on BCR-ABL-expressing cell lines. / (A) Experiments using the cell line FDrv210H (18-hour incubation in inhibitors), titrating STI571 in a constant concentration of AG490. In panels A and B, solid (+IL-3) and open (−IL-3) symbols identify curves for varying concentrations of AG490 alone (●,○) STI571 alone (▴,▵), and serial dilutions of STI571 in a constant concentration AG490 [5 μM] (♦,⋄). (B) Experiment using the cell line 32Dp210 (18-hour incubation in inhibitors), titrating STI571 in a constant concentration of AG490. (C) For a constant STI571 concentration (0 or 0.01 μM), AG490 was added at the concentrations shown (MO7p210 cell line; 18-hour incubation in inhibitors without GM-CSF).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/97/7/10.1182_blood.v97.7.2008/6/m_h80710843003.jpeg?Expires=1768233063&Signature=yNhkxfn67IPByGBB3roT3IZFlA7coW-TI6LjY87g0Czcj1sC31RZ9lHb9CX6QRNdV-B8g2F-D34jLo1c3V4Oj0vtRvsjzpW7WZDuvy4j2r5miRgPHbjzGb4siLj3jAxuJns~~lWAx6-Mbt-ASlpbZIW7cFEeQ6XNz~~2txyejgGDG3Hf3fovYzL~LXcFTJt70IvQg-rQdE62C4rb61sWnnPHRWmjrkq1Q2yS7MdvR0r8l0oUh9FKolzZjbSCc-ahF90hliGM4J1tJJH0wJt-uSAZGiWm-jGOWMfjuyeyYuZ5294Ce8fRyraTKYBUWep~dPFAlsKpOtTG7CZNEg9HGg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)