The rate of reconstitution following hematopoietic stem cell (HSC) transplantation differs widely depending on the tissue source of the cells infused. To test the hypothesis that variability in engraftment kinetics is related to differences in the efficiency with which intravenously transplanted HSCs “home” to the bone marrow (BM), the homing properties of murine fetal liver (FL), adult BM, and mobilized peripheral blood (MPB) cells were compared. Lethally irradiated mice transplanted with 2 × 106 FL, BM, or MPB cells exhibited sequentially slower recovery of circulating leukocytes and platelets that correlates with the progressively lower frequency of colony-forming cells (CFCs) in these tissues. However, differences in the rate and degree of early and long-term reconstitution were maintained even after infusing equal numbers of CFCs derived from FL, BM, and MPB. To compare the homing of progenitors from these tissues, cells were labeled with fluorescent PKH26 dye and injected into lethally irradiated hosts. Three hours later, PKH26+ cells were reisolated from the BM and spleen by fluorescence-activated cell sorting and assayed for in vitro CFCs. Despite the higher level of very late antigen (VLA)-2, VLA-4, and VLA-5 on Sca-1+c-kit+ cells from FL compared to BM, 10-fold fewer FL CFCs homed to hematopoietic organs than those from BM. MPB cells homed slightly better, but still less efficiently than BM cells. Therefore, clonogenic cells from different tissues exhibit striking variations in homing efficiency that does not necessarily correlate with engraftment kinetics. Homing is likely counterbalanced by intrinsic differences in proliferative potential that ultimately determine the rate of hematopoietic reconstitution.
Introduction
Hematopoietic recovery of cancer patients following high-dose chemotherapy and stem cell transplantation is characterized by a period of neutropenia and thrombocytopenia, which varies in length depending on the number and tissue source of the hematopoietic stem cells (HSCs) infused.1 For example, stem cells derived from bone marrow (BM) require about 25 days to regenerate clinically safe levels of circulating neutrophils (> 500/μL) and platelets (> 20 000/μL).1 Cytokine-mobilized peripheral blood cells promote accelerated hematopoietic reconstitution (about 9-11 days) and mobilized peripheral blood (MPB) has consequently replaced BM as the preferred source of stem/progenitor cells for clinical transplantation.2,3 Umbilical cord blood (CB) is also gaining favor as a source of HSCs with extensive proliferative and differentiative potential, particularly in pediatric patients where small stem cell numbers do not limit its use. However, CB stem cell engraftment is significantly delayed (about 45 days) compared to BM and MPB.4 Several differences in the biology of HSCs from these tissues may account for this variation in engraftment kinetics. For example, whereas fetal stem cells cycle rapidly in response to stimulation by cytokines that regulate their expansion during mid gestational development,5,6 clonogenic cells that have been mobilized from the BM into the blood by cytokine treatment or hematopoietic stress are largely quiescent.7 Stem cell cycle progression is associated with a concomitant but reversible loss of long-term engraftment potential.8-10 One might thus speculate that differences in the rate of hematopoietic reconstitution following, for example, MPB or CB transplantation are related to variations in the cycling of HSCs from these sources.11However, we recently showed that although quiescent BM cells (in G0/G1) provide better long-term repopulation than cycling BM cells (in S+G2/M), both populations regenerate circulating blood cells with similar kinetics during the first several weeks after transplantation.12 Cycle activation of primitive hematopoietic cells therefore does not appear to have the same deleterious effects on early engraftment as it does on long-term hematopoietic potential.
An alternative explanation for the variable engraftment kinetics of different hematopoietic cell types is that clonogenic cells isolated from distinct anatomic locations or at different times during ontogeny express a unique spectrum of adhesion molecules that convey a developmental stage-specific propensity to interact with stromal cells in particular hematopoietic niches. Human CD34+ CB cells express lower levels of very late antigen (VLA)-2, -3, and -5, and higher levels of lymphocyte function-associated antigen (LFA)-1, intracellular adhesion molecule (ICAM)-1, and L-selectin than CD34+ BM cells.13 Interestingly, despite their common prenatal origin, human CB and fetal liver (FL) CD34+cells also differ in integrin expression and adhesive behavior. Compared to BM, significantly more CB and fewer FL progenitors adhere to marrow stroma or fibronectin-coated substrates in vitro.14 In contrast, high proliferative potential colony-forming cells (HPP-CFCs) from murine day 15 FL adhere much more strongly to BM-derived stromal cells than to FL stroma in vitro, consistent with the preferential migration of primitive progenitors from the FL to the medullary sites of hematopoiesis during ontogeny.15 The specific adhesive interactions that underlie the localization, migration, and regulation of primitive hematopoietic cells within their native microenvironment likely also define their potential to “home” to the BM in a transplant setting. Efficient homing of intravenously transplanted stem cells presumably represents the first step toward timely hematopoietic engraftment. Indeed, it has been noted that the log of the number of CD34+L-selectin+ and CD34+CD44+ cells reinfused after transplantation of granulocyte-monocyte colony-stimulating factor (GM-CSF)–MPB cells correlates better with the time to reach 500 ≥ neutrophils/μL than does the log of the number of CD34+cells or granulocyte-macrophage colony-forming units (CFU-GMs) reinfused.16
As a first step toward elucidating the underlying cause of variation in stem cell engraftment kinetics, and with the ultimate goal of manipulating this process to facilitate rapid hematopoietic reconstitution, we used a murine transplant model to compare the in vivo homing properties of clonogenic cells from adult BM, day 14 FL, and MPB cells. Our results indicate a sequentially greater ability of MPB, BM, and FL cells to rapidly regenerate circulating blood cells in myeloablated recipients even when the numbers of each cell type infused are normalized to contain identical numbers of hematopoietic progenitors. Consistent with the superior engraftment potential of whole FL cells, the Sca-1+c-kit+ subpopulation, which contains all of the repopulating cells, expressed higher levels of several adhesion molecules known to play a key role in stem cell homing than Sca-1+c-kit+ cells from BM or MPB. Surprisingly, in vivo homing assays demonstrated that, compared to BM CFCs, 6- to 12-fold fewer FL CFCs localize to the BM and spleen during the first few hours after transplantation. MPB cells homed slightly better, but still less efficiently than BM cells. These studies document striking variations in the homing efficiency of hematopoietic progenitor cells from different tissues, but suggest that inefficient homing is not necessarily an impediment to rapid hematologic reconstitution by transplanted stem/progenitor cells if their intrinsic potential for proliferation and multilineage differentiation is high.
Materials and methods
Animals
Six- to 8-week-old C57BL/6 (B6) mice (Ptprcb [Ly-5.2]) were used as BM and blood cell donors. Age-matched B6 or B6.SJL (Ptprca[Ly-5.1]) mice were used as recipients as indicated. Timed-pregnant female B6 mice were purchased from Harlan Sprague Dawley (Indianapolis, IN). All other mice were purchased via the National Cancer Institute Animal Program from Charles River Laboratories (Frederick, MD) and maintained under specific pathogen-free conditions in the animal facility of the University of Kentucky Chandler Medical Center. All experiments were approved by the Institutional Animal Care and Use Committee.
Isolation of hematopoietic cells
BM cells were flushed from femurs and tibiae into Hanks balanced salt solution containing 2% fetal bovine serum (FBS; HF medium) using a 21-gauge needle and 3-mL syringe. MPB cells were obtained from mice that had been injected 6 days previously with a single intraperitoneal dose of 200 mg/kg body weight cyclophosphamide (Sigma Chemical, St Louis, MO) and then subcutaneously on 5 subsequent days with 5 μg recombinant human granulocyte colony-stimulating factor (G-CSF; Amgen, Thousand Oaks, CA). Blood was collected from the retro-orbital sinus about 24 hours after the fifth G-CSF injection. FL cells were obtained from day 14 postcoitus embryos (1 or 2 pooled litters) by mashing excised livers through a 40-μm nylon mesh using the plunger from a 3-mL syringe. Erythrocytes were eliminated from each cell population by hypotonic lysis, and the remaining leukocytes suspended in HF medium.
Measurement of short-term hematopoietic reconstitution kinetics and long-term repopulating ability
B6.SJL mice were exposed to 9 Gy total body γ-irradiation administered in 2 doses of 4.5 Gy about 3 hours apart just before transplantation. Ablated animals were injected intravenously with 2 × 106 nucleated BM, FL, or MPB cells, or in separate experiments, with 1.2 × 106 FL or 6.7 × 106 MPB cells containing the same number of CFCs as 2 × 106 BM cells. Mice were bled from the retro-orbital sinus 3, 6, 9, 12, 15, 18, 25, 32, 42, 56, and 120 days after transplantation. Until day 25, only half the mice in each cohort were analyzed alternately at each time so that no individual animal was bled more frequently than every 7 days. Circulating leukocyte, erythrocyte, and platelet counts were measured by analysis of 40 μL blood using a System 9118+ Hematology Series Cell Counter (Biochem Immunosystems, Allentown, PA). At selected times, blood samples were also stained with a donor-specific anti–Ly-5.2 monoclonal antibody (MoAb) (clone ALI4A2) conjugated with fluorescein isothiocyanate (FITC)– and phycoerythrin (PE)–conjugated MoAbs specific for B (anti-CD45R/B220; clone RA3-6B2) or T lymphocytes (anti–Thy-1.2; clone 30H12), or granulocytes (anti–Ly6G/Gr-1; clone RB6-8C5) and macrophages (anti–CD11b/Mac-1; clone M1/70). Multilineage progeny of transplanted stem/progenitor cells were quantitated using a FACScan instrument (Becton Dickinson Immunocytometry Systems, San Jose, CA). After 120 days, BM cells were assayed for CFCs and injected into lethally irradiated B6.SJL mice (0.5 femurs/mouse) for secondary repopulation. Secondary mice were analyzed 10 weeks later for donor-derived peripheral blood leukocytes and BM CFCs as described above.
CFC assays
Cells were plated in duplicate 35-mm culture dishes (Nalge Nunc International, Rochester, NY) containing 1.1 mL MethoCult M3134 medium (1% methylcellulose) supplemented with 30% FBS, 1% bovine serum albumin (Stemcell Technologies, Vancouver, BC), 2 mMl-glutamine, 3% conditioned medium from COS cells transfected with a complementary DNA (cDNA) encoding murine stem cell factor (SCF), 1% pokeweed mitogen-stimulated spleen cell conditioned medium (Stemcell Technologies), and 3 U/mL recombinant human erythropoietin (Amgen). Plating densities for BM, FL, and MPB cells were 3 × 104, 104, and 105cells/dish, respectively. Total colonies (derived from granulocyte, erythrocyte, macrophage, megakaryocyte colony-forming units [CFU-GEMMs]; granulocyte, macrophage, granulocyte-macrophage colony forming units [CFU-G/M/GMs]; and erythroid burst-forming units [BFU-Es]) were counted 12 days later using an inverted microscope.
Phenotyping for integrin expression
Aliquots of 106 BM, FL, or MPB cells in 100 μL HF medium were stained with saturating amounts of PE-conjugated anti–Sca-1 (clone E13-161.7), allophycocyanin (APC)-conjugated anti–c-kit (clone 2B8), and biotinylated MoAbs specific to one of the following murine adhesion molecules: CD18 (β2 common chain; clone C71/16), CD29 (β1 common chain; clone Ha 2/5), CD34 (mucosialin; clone RAM34), CD43 (leukosialin; clone S7), CD44 (Pgp-1 or H-CAM; clone IM7), CD49d (VLA α4 chain; clone R1-2), or CD49e (VLA α5 chain; clone 5H10-27). CD49a (VLA α1 chain) was detected with an unconjugated hamster antimouse MoAb (clone Ha 31/8) followed by biotinylated mouse antihamster MoAb (clone G192-1). CD49b (VLA α2 chain) was detected using a PE-conjugated MoAb (clone HMα2), so in this case anti–Sca-1 was conjugated with FITC. Cells were incubated for 30 minutes on ice then washed twice in HF. Binding of biotinylated antibodies was visualized by staining for 30 minutes on ice with FITC-conjugated streptavidin (SA-FITC). Appropriate control samples that were either left unstained or stained with isotype-matched biotinylated rat IgG2a (clone R35-95) or IgG2b(clone A95-1) antibodies, or individual PE-, APC-, or FITC-conjugated antibodies to establish instrument compensation settings were included. All antibodies and SA-FITC were purchased from Pharmingen (San Diego, CA). Stained cells were finally resuspended in HF containing 2 μg/mL propidium iodide (PI) and analyzed on a dual-laser Vantage fluorescence-activated cell sorter (FACS; Becton Dickinson). Viable (PI−) cells were gated on the Sca-1+c-kit+ subpopulation for comparison of integrin expression.
In vivo homing assay
The homing efficiency of hematopoietic progenitor cells was assayed by labeling BM, FL, or MPB cells with the red fluorescent dye PKH26 (Sigma) as previously described.17 An aliquot of PKH26+ cells was assayed for CFCs, and the remaining (107-108) cells were injected into a single lethally irradiated B6 mouse. Three hours later, BM and spleen cells were procured in HF, washed, subjected to red blood cell lysis, and suspended in HF containing 2 μg/mL PI for analysis using a FACScan flow cytometer. The percent of transplanted PKH26+cells that had homed to each organ was calculated as follows: % homing = [(A × B) / C] × 100%, where A equals the percent PKH26+ cells determined by flow cytometry. B equals the total organ cellularity and C equals the number of cells transplanted. List mode files were comprised of 10 000 to 100 000 total events, of which about 70% were PI− (dead cells being derived from the irradiated host). PKH26+ cells in the PI−gate ranged from 100 to 30 000 in number (0.25%-40%) depending on the tissue origin of the transplanted cells and the recipient organ analyzed. These frequencies are consistent with other studies using PKH26 staining to track the homing of different BM cell populations.18 19
In measuring homing to the marrow, cells from both femora and tibiae were assumed to represent 25% of the entire organ.20 To measure CFC homing, PKH26+ cells were reisolated from the BM and spleen by FACS. An aliquot of sorted cells was reanalyzed to assess percent purity and this value was used to correct cell counts before plating in semisolid medium. The percent of transplanted CFCs that had homed to each organ was calculated as follows: % CFC homing = [(A × B × D) / E] × 100%, where D equals CFC frequency among sorted PKH26+ cells, and E equals number of CFCs injected as determined by assay of PKH26-labeled cells prior to transplant.
Statistical analysis
The statistical significance of differences between means (derived by pooling all values from replicated experiments) was assessed using the 2-tailed t test assuming unequal variances.
Results
Differential engraftment kinetics of murine FL, adult BM, and MPB cells
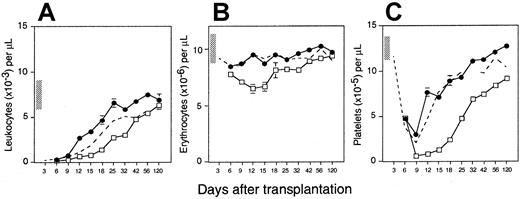
To compare the hematopoietic reconstitution potential of murine BM, day 14 FL cells, and MPB cells, 3 groups of lethally irradiated Ly-5.1 mice (n = 12-21) were transplanted with 2 × 106Ly-5.2+ cells of each type. Circulating blood cells were counted once or twice weekly from 3 days to 4 months after transplantation to measure early engraftment kinetics. Until day 15, the rate of white blood cell recovery was indistinguishable among the 3 groups (Figure 1A). Thereafter, MPB, BM, and FL cells exhibited a sequentially increasing capacity to regenerate circulating leukocytes. Mice transplanted with FL cells recovered to pretransplant levels within 25 days and exhibited supernormal counts thereafter until normalization by day 120. In contrast, recipients of adult BM and MPB cells required 56 days to achieve leukocyte counts on the low end of the normal range. This difference in hematopoietic reconstitution by the 3 cell types was particularly evident between about 3 and 6 weeks after transplantation, when leukocyte counts were significantly higher in recipients of FL versus BM (P < .05) or MPB (P < .05) cells, and in recipients of BM versus MPB cells (P < .01).
Hematopoietic reconstitution kinetics of equal numbers of adult mouse BM, FL, and MPB cells.
Lethally irradiated Ly-5.1 mice were injected with 2 × 106 Ly-5 congenic day 14 FL cells (●; n = 12 mice from 3 experiments), adult BM cells (○; n = 12 mice from 3 experiments), or cyclophosphamide/G-CSF MPB cells (■; n = 21 mice from 2 experiments). Shown are the mean ± SEM number of peripheral blood leukocytes (A), erythrocytes (B), and platelets (C) counted on the indicated days after transplantation. The vertical bars along the y-axis define the ranges of blood counts in normal Ly-5.1 mice. SEs not shown are too small for the scale used. Note that the time after transplantation is not depicted on a linear scale.
Hematopoietic reconstitution kinetics of equal numbers of adult mouse BM, FL, and MPB cells.
Lethally irradiated Ly-5.1 mice were injected with 2 × 106 Ly-5 congenic day 14 FL cells (●; n = 12 mice from 3 experiments), adult BM cells (○; n = 12 mice from 3 experiments), or cyclophosphamide/G-CSF MPB cells (■; n = 21 mice from 2 experiments). Shown are the mean ± SEM number of peripheral blood leukocytes (A), erythrocytes (B), and platelets (C) counted on the indicated days after transplantation. The vertical bars along the y-axis define the ranges of blood counts in normal Ly-5.1 mice. SEs not shown are too small for the scale used. Note that the time after transplantation is not depicted on a linear scale.
BM and FL cells were similarly effective in alleviating radiation-induced anemia (Figure 1B). In contrast, recipients of MPB cells exhibited significantly lower erythrocyte counts than mice in both of the other 2 groups at all times between days 12 and 56 after transplantation (P < .01). Recipients of BM and FL cells also recovered platelets at similar rates, although counts in the latter group were significantly higher after day 42 of analysis (P < .05). Once again, MPB cells regenerated circulating platelets much more slowly than either BM or FL cells (Figure 1C).
Four months after transplantation, the majority of circulating leukocytes in all mice were derived from donor stem cells (Table1). The superior engraftment potential of FL compared to both BM (P < .01) and MPB cells (P < .01) was again illustrated by the sequential decrease in overall donor cell contribution in recipients of these cells (96% versus 76% versus 70%, respectively). This trend extended to B lymphocytes and myeloid cells. FL cells also rendered the highest level of T-cell engraftment, but in this compartment only, recipients of MPB cells contained a higher proportion of donor-derived progeny than mice transplanted with BM cells (57% versus 34%).
Hematopoietic progenitor cells recovered to 2-fold higher levels in recipients of BM and FL cells (about 66 000/femur) than in mice transplanted with MPB (about 33 000/femur) (Table 1). Marrow cells from all reconstituted animals were transplanted into secondary lethally irradiated Ly-5.1 mice. Ten weeks later, identical trends in hematopoietic reconstitution were observed (Table2), albeit at slightly lower absolute levels of engraftment as is typical of serial transplantation studies. The only exception to this trend was that all 3 groups of secondary recipients recovered similar numbers of CFCs (about 37 000/femur). Thus, FL cells exhibited a significantly higher capacity for long-term, multilineage hematopoietic reconstitution than equal numbers of BM or MPB cells.
Differences in hematopoietic progenitor cell content do not account for the sequentially decreasing engraftment potential of FL, BM, and MPB cells
To test the possibility that differences in the rate and degree of engraftment by equal numbers of BM, FL, and MPB cells may be due to differences in the number of progenitor cells infused, the 3 populations were assayed for in vitro CFCs. Murine FL, BM, and MPB cells contained an average of 360 ± 20 (n = 6), 217 ± 10 (n = 20), and 65 ± 5 (n = 9) CFCs/105 cells, respectively. By comparison, normal peripheral blood contained 11 ± 1 CFCs/106 cells, thus confirming the efficiency of the mobilization regimen. The 2 × 106 FL, BM, and MPB cells transplanted in the experiments above thus contained an average of 7200 ± 400, 4340 ± 200, and 1300 ± 100 CFCs, respectively. FL and MPB grafts were thus normalized to the CFC content of 2 × 106 BM cells and transplanted into a new cohort of irradiated hosts (ie, 1.2 × 106 FL cells/mouse and 6.7 × 106 MPB cells/mouse). Retrospective analysis of the actual CFC content of these grafts indicated that they did contain the expected number of CFCs: 4080 or 4920 for 11 and 9 mice, respectively, injected with FL cells (2 experiments), and 4020, 4422, or 3950 for 11, 13, and 4 mice, respectively, injected with MPB cells (3 experiments). Figure 2A shows that even after reducing the number of FL cells injected to correct for their higher progenitor content, recipient mice exhibited higher numbers of circulating leukocytes than BM cell recipients at all times after day 12 of transplantation (P < .05) except day 32. The only effect of transplanting fewer FL CFCs was to eliminate the rebound of circulating leukocytes to supernormal numbers between days 32 and 56 (compare Figures 1 and 2). As in Figure 1, recovery of erythrocytes and platelets in mice injected with equal numbers of BM- or FL-derived CFCs was indistinguishable. In contrast, mice transplanted with 3.4-fold more MPB cells (to correct for their lower frequency of CFCs) still engrafted all 3 lineages, particularly platelets, more slowly than BM cells (Figure 2).
Engraftment kinetics of adult mouse BM, FL, and MPB cells containing equal numbers of hematopoietic progenitors.
Lethally irradiated Ly-5.1 mice were injected with 1.2 × 106 Ly-5 congenic day 14 FL cells (●; n = 20 mice from 2 experiments) or 6.7 × 106cyclophosphamide/G-CSF MPB cells (■; n = 28 mice from 3 experiments), each containing the same number of CFCs as 2 × 106 BM cells (dashed line; reproduced from Figure1). Shown are the mean ± SEM number of peripheral blood leukocytes (A), erythrocytes (B), and platelets (C) counted on the indicated days after transplantation. The vertical bars along the y-axis define the ranges of blood counts in normal Ly-5.1 mice. SEs not shown are too small for the scale used. Note that the time after transplantation is not depicted on a linear scale.
Engraftment kinetics of adult mouse BM, FL, and MPB cells containing equal numbers of hematopoietic progenitors.
Lethally irradiated Ly-5.1 mice were injected with 1.2 × 106 Ly-5 congenic day 14 FL cells (●; n = 20 mice from 2 experiments) or 6.7 × 106cyclophosphamide/G-CSF MPB cells (■; n = 28 mice from 3 experiments), each containing the same number of CFCs as 2 × 106 BM cells (dashed line; reproduced from Figure1). Shown are the mean ± SEM number of peripheral blood leukocytes (A), erythrocytes (B), and platelets (C) counted on the indicated days after transplantation. The vertical bars along the y-axis define the ranges of blood counts in normal Ly-5.1 mice. SEs not shown are too small for the scale used. Note that the time after transplantation is not depicted on a linear scale.
After 4 months, recipients of CFC-normalized transplants were analyzed for the degree of donor cell contribution to lymphoid and myeloid lineages. Engraftment patterns were similar to the previous experiments in which mice were transplanted with 2 × 106 cells (Table 1). The only effect of transplanting fewer FL cells was a reduction in T-cell chimerism (from 84% to 57%;P < .01). This difference was even more pronounced after secondary transplantation of FL cells, when T-lymphoid progeny decreased from 75% to 40%; P < .01; Table 2). Increasing the number of MPB cells transplanted increased the femoral CFC content of primary recipients slightly, but this was not statistically significant. No significant improvement in engraftment was noted in secondary mice transplanted with 6.7 × 106versus 2 × 106 MPB cells (Table 2). These results suggest that hematopoietic progenitors from BM, FL, or MPB exhibit intrinsic differences in initial engrafting ability and subsequent proliferative potential that determine the rate of hematologic recovery after transplantation.
FL, BM, and MPB cells differ in integrin expression
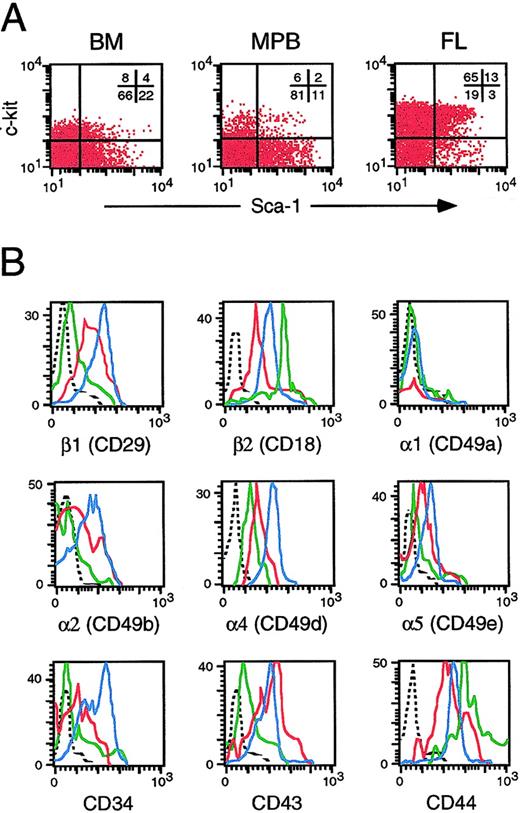
We hypothesized that the differences in engraftment potential of hematopoietic cells from FL, BM, and MPB may be due to differences in their homing efficiency in irradiated adult recipients. As a first step toward testing this possibility, we examined the expression of 9 adhesion molecules on primitive Sca-1+c-kit+ cells that have previously been shown to contain all of the early and long-term engrafting activity in these tissues. This subpopulation represents 2% to 4% of BM and MPB cells (Figure 3). Consistent with their higher frequency of CFCs and superior engraftment potential, FL cells contained considerably more c-kit+ (78%) and Sca-1 (16%) cells. The peak channel fluorescence of Sca-1+c-kit+ FL cells expressing VLA β1, α2, α4, and α5 chains was 5- to 10-fold higher than their counterparts from BM and MPB. Indeed, Sca-1+c-kit+ cells from FL, BM, and MPB exhibited a hierarchical decrease in expression of these 4 integrin subunits, as well as CD34, that mirrored their sequentially poorer engraftment potential (Figure 3). The peak channel fluorescence of Sca-1+c-kit+ FL and BM cells expressing CD43 was also about 15-fold higher than that of MPB Sca-1+c-kit+ cells. CD44 (Pgp-1/Ly-24) and β2 common chain were the only molecules that were expressed at 5- to 15-fold higher levels on primitive MPB cells than cells from FL or BM. None of the 3 tissues contained a significant proportion of Sca-1+c-kit+ cells expressing α1. Therefore, Sca-1+c-kit+cells, which contain all transplantable stem and progenitor cells, differed widely between BM, FL, and MPB in terms of their expression of several adhesion molecules implicated in hematopoietic cell trafficking.
Integrin expression on phenotypically primitive adult mouse BM, FL, and MPB cells.
Cells were stained with MoAbs specific for Sca-1, c-kit, and each of the indicated adhesion molecules. Panel A shows the distribution of viable cells in each tissue according to Sca-1 and c-kit expression. Panel B shows differences in integrin expression on Sca-1+c-kit+ cells from FL (blue histograms), BM (red histograms), and MPB (green histograms) cells. Dashed lines represent background staining with appropriate isotype control antibodies. Data are representative of results obtained in 3 independent experiments.
Integrin expression on phenotypically primitive adult mouse BM, FL, and MPB cells.
Cells were stained with MoAbs specific for Sca-1, c-kit, and each of the indicated adhesion molecules. Panel A shows the distribution of viable cells in each tissue according to Sca-1 and c-kit expression. Panel B shows differences in integrin expression on Sca-1+c-kit+ cells from FL (blue histograms), BM (red histograms), and MPB (green histograms) cells. Dashed lines represent background staining with appropriate isotype control antibodies. Data are representative of results obtained in 3 independent experiments.
Differential homing of hematopoietic progenitor cells from murine FL, BM, or MPB
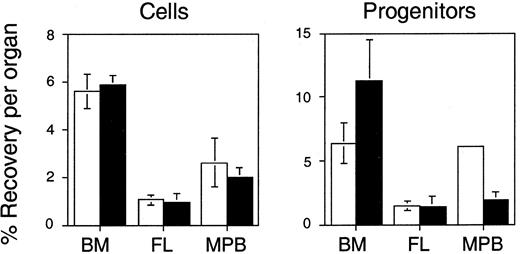
The differences in integrin expression on Sca-1+c-kit+ cells from FL, BM, and MPB cells suggest that primitive hematopoietic cells from these tissues may vary in their propensity to home to and engraft the BM after intravenous transplantation. However, because even Sca-1+c-kit+ cells are still heterogeneous and contain many cells without clonogenic potential, we used an in vivo homing assay to track functional progenitors to the hematopoietic organs after transplantation. Hematopoietic cells were labeled with PKH26 dye and injected into lethally irradiated syngeneic recipients (one per experiment). Three hours later, PKH26+ cells in the BM and spleen were quantitated by flow cytometry, reisolated by FACS, and assayed for CFCs. We have previously shown that 5.6% ± 0.7% and 5.9% ± 0.4% of transplanted BM cells are recovered in the BM and spleen, respectively, at this time.17 A similar proportion of BM-derived CFCs home to the marrow (6.4% ± 1.6%), whereas seeding of the spleen is 2-fold higher (11.3% ± 3.2%).17 We now report the results of homing assays of FL and MPB cells, which were performed contemporaneous to our earlier studies of BM cell homing, reproduced here for comparison. Surprisingly, despite their superior repopulating ability, FL cells homed 6- to 12-fold less efficiently than BM cells in vivo. Only 1% to 1.5% of transplanted FL cells or CFCs were recovered in the BM or spleen 3 hours after intravenous injection (Figure4). Homing of MPB was only slightly better with 2.6% to 2% of nucleated cells detectable in the BM and spleen, respectively. Homing of MPB-derived CFCs was more difficult to measure because of their unexpectedly high sensitivity to the toxic effects of the PKH26 staining procedure than BM or FL CFCs. Because of this, viable MPB CFCs were detected in BM in only one experiment (at 6.1% of input numbers) and in spleen in only 2 experiments (at 1.9% ± 0.6% of input numbers) (Figure 4). Although it is difficult to draw firm conclusions from these limited data, it appears that, similar to FL, MPB CFCs home less efficiently to the BM and spleen than progenitors isolated from BM.
Reduced homing capacity of murine FL and MPB cells compared to BM.
Lethally irradiated mice were transplanted with about 107to 108 PKH26-labeled BM, FL, or MPB cells. Three hours later, PKH26+ cells that had homed to the BM (■) or spleen (▪) were quantitated by flow cytometry and isolated by FACS for CFC assays. Shown are the mean ± SEM percent recovery of donor-derived cells (left) and CFCs (right) per organ relative to numbers injected. See “Materials and methods” for details of recovery calculations. Pooled data are from 3 BM, 5 FL, and 4 MPB cell experiments (homing was assayed in one mouse per experiment). Differences between BM and either FL or MPB cell and CFC homing are significant (P < .05) in all cases except for BM versus MPB-derived CFCs homing to BM where the latter value is derived from a single measurement. Data for BM cells are reproduced from Szilvassy and colleagues.17
Reduced homing capacity of murine FL and MPB cells compared to BM.
Lethally irradiated mice were transplanted with about 107to 108 PKH26-labeled BM, FL, or MPB cells. Three hours later, PKH26+ cells that had homed to the BM (■) or spleen (▪) were quantitated by flow cytometry and isolated by FACS for CFC assays. Shown are the mean ± SEM percent recovery of donor-derived cells (left) and CFCs (right) per organ relative to numbers injected. See “Materials and methods” for details of recovery calculations. Pooled data are from 3 BM, 5 FL, and 4 MPB cell experiments (homing was assayed in one mouse per experiment). Differences between BM and either FL or MPB cell and CFC homing are significant (P < .05) in all cases except for BM versus MPB-derived CFCs homing to BM where the latter value is derived from a single measurement. Data for BM cells are reproduced from Szilvassy and colleagues.17
Discussion
Outcomes of clinical hematopoietic cell transplants over the past several decades have established a clear relationship between the source of HSCs infused and the speed of hematologic recovery.1-4 Although engraftment can be achieved relatively quickly in patients transplanted with MPB or BM cells, particularly when supported by cytokine treatment, clinically significant periods of neutropenia and thrombocytopenia remain a concern. We and others have exploited murine models to demonstrate the efficacy of ex vivo expanded hematopoietic cells as a cellular therapy to augment hematologic recovery after stem cell transplantation.12,21,22 Translation of these findings to clinical practice is now yielding encouraging results.23,24 However, the potential benefits of quantitative progenitor expansion that can be achieved in culture must be considered in the context of simultaneous deleterious changes in the homing capacity of progenitor cells generated in vitro.17Based on these findings, the present studies were conducted to identify potential differences in the homing of unmanipulated hematopoietic cells from different tissues and to test the possibility that variation in engraftment kinetics may be related to differences in homing potential.
Initial experiments compared the early engraftment properties of FL, BM, and MPB cells by measuring the rate at which they were able to regenerate circulating blood cells during the initial months after transplantation. This method had not been used previously to evaluate hematologic recovery by murine FL cells, or for more than 3 weeks by MPB cells. Furthermore, graft sizes were adjusted to contain either equal numbers of nucleated cells or functional hematopoietic progenitors identified by their capacity to generate colonies of mature blood cells in vitro. Our results indicate that murine day 14 FL, adult BM, and MPB cells exhibit dramatic functional differences in engraftment kinetics that are independent of the number of cells or CFCs infused. Mice transplanted with 2 × 106 MPB cells engrafted more slowly than mice injected with an equivalent number of BM cells. Transplanting more MPB cells to account for the lower frequency of CFCs in this tissue had no effect on engraftment kinetics. Recipients of 2 × 106 or 1.2 × 106 FL cells also engrafted with identical kinetics up to day 32, although recipients of 2 × 106 cells then exhibited a transient overshoot of normal leukocyte counts until day 120. In both transplant situations, FL cells regenerated significantly higher numbers of circulating leukocytes and platelets over the initial weeks after injection than did equivalent numbers of BM- or MPB-derived cells or CFCs. Differences in early reconstitution potential were particularly well illustrated on day 25, when recipients of 2 × 106FL, BM, or MPB cells contained 7.5 ± 0.95, 4.5 ± 0.51, and 1.4 ± 0.14 × 103 leukocytes/μL, respectively, and counts in FL recipients only had attained normal values. After a cumulative 6.5 months in primary and secondary hosts, FL cells also contributed significantly more progeny to lymphoid and myeloid compartments than did equal numbers of BM- or MPB-derived cells or CFCs. This superior reconstituting ability of FL cells is consistent with published data demonstrating that FL cells contribute to long-term repopulation (105-245 days) about 5-fold more effectively than BM cells in a competitive repopulation assay.25 However, contrary to our findings, that study did not reveal any differences in short-term (25-33 days) hematopoiesis between the 2 tissues.25 This discrepancy may be due to differences in assay design (competitive versus noncompetitive) or the methods used to measure engraftment (circulating blood counts versus donor/competitor chimerism). Competitive repopulation assays of human CD34+BM and CB cells transplanted together into severe combined immunodeficient mice implanted with human fetal bone fragments revealed similar ontogeny-related differences in engraftment potential.26
The rate of hematopoietic reconstitution by cyclophosphamide/G-CSF MPB cells was similar to that reported in previous, albeit limited, studies of G-CSF MPB.27,28 Molineux and coworkers transplanted lethally irradiated mice with 2 × 106 G-CSF MPB cells from splenectomized BDF1 donors and documented engraftment rates that were similar until day 12 to our results (Figure 1) using 2 × 106 B6 strain MPB cells.27 Thereafter, leukocyte and platelet counts were 2- to 6-fold higher until day 21, the last day evaluated in that study. In a subsequent study, these investigators transplanted 6 × 106 G-CSF MPB cells from BDF1 donors and observed engraftment from 4 to 14 days that was indistinguishable from our data (Figure 2) using 6.7 × 106 cells/mouse.28 Thus, these and our present murine studies indicate that despite its rapid reconstitution potential as established from human clinical transplants, MPB appears to be an inferior source of early engrafting cells when compared to BM, and especially FL, on either a per cell or per progenitor basis. This discrepancy between mice and humans was unexpected and suggests that murine models of MPB cell transplantation may not accurately predict its efficacy in a clinical setting. However, it is important to emphasize that even in mice transplanted with 6.7 × 106 MPB cells, the total number of CFCs infused (about 4340/mouse weighing an average of 25 g, or 1.74 × 105 CFCs/kg recipient body weight) was well below the minimal dose of 2 to 5 × 105 CFU-GM/kg required for optimal engraftment in humans.29 Furthermore, the conclusion that autologous MPB cells promote faster hematologic recovery than autologous BM cells is based on clinical studies in which the MPB group typically receives a much higher CFU-GM or CD34+ cell dose than the BM group.1 Overall, our data suggest a cell-intrinsic, ontogeny-related determinant of engraftment efficiency that cannot be completely overcome simply by increasing graft size or progenitor content.
To test the hypothesis that tissue-specific differences in engraftment may be related to variability in the homing of stem/progenitor cells, we used an in vivo homing assay to track fluorescently labeled CFCs to the hematopoietic organs of lethally irradiated mice after intravenous transplantation. Three hours was selected as the end-point for this assay based on previous studies demonstrating that CFC homing to the BM reaches plateau levels after this time.17 Surprisingly, we found that FL and MPB cells homed 6-fold and 3-fold less efficiently to BM and spleen than did BM cells (about 1%, 2%, and 6% recovery of input numbers in both organs, respectively, for the 3 tissues). Homing of FL progenitors was also deficient; approximately 1.5% of input CFCs were recovered in the BM and spleen, compared to 6% in BM and 11% in spleen for BM-derived CFCs. Our limited homing data for MPB progenitors also suggest significant impairment compared to BM. These results were surprising in light of the clear superiority of FL cells in rapid and sustained hematopoietic reconstitution compared to BM. Furthermore, Sca-1+c-kit+ cells from FL, which contain all in vitro assayable progenitors and cells with early and long-term hematopoietic reconstitution potential,6,30 expressed 10-fold higher levels of VLA-2, 4, and 5 (ie, the 3 α chains and the β1 common chain) than Sca-1+c-kit+ cells from BM. The pivotal role of VLA-4 in hematopoietic stem/progenitor cell mobilization and homing after transplantation is well documented in both mice and humans.31,32 Expression of CD49d (α4 chain) and CD49e (α5 chain) have also been associated with an early engrafting subpopulation of murine Sca-1+Lin− BM cells.33 Such early engrafting cells also express CD43,33 consistent with our noted expression of this integrin at 15-fold higher levels on FL and BM than on MPB Sca-1+c-kit+ cells. Our finding that MPB cells home less efficiently than BM cells is consistent with their slower engraftment and the lower expression on Sca-1+c-kit+ MPB cells of all integrins analyzed except CD44 (hyaluron-binding receptor) and β2(CD18). CD44 mediates adhesion of myeloid progenitors to BM stromal cells,34 extravasation of lymphocytes across endothelial vessels and their homing to peripheral organs,35 and lodgment of murine spleen colony-forming units in both BM and spleen.36 In contrast, the β2-integrin LFA-1 is not expressed on steady-state BM or MPB progenitors, or on cells that mediate radioprotection in mice.37 Therefore, expression of CD44, but not β2, may partially compensate for other integrins implicated in homing but which are down-regulated during mobilization. It is important to note, however, that although the total level of specific adhesion molecule expression on Sca-1+c-kit+ cells from different tissues correlated well with their observed engraftment, the MoAbs used for phenotyping do not discriminate between functionally active and inactive forms of the integrins examined. It remains possible that FL cells express high levels of conformationally inactive forms of some integrins that could compromise their overall homing ability. Engraftment may also depend on a threshold level of some integrins, above which any level of expression will facilitate efficient homing, and not gradations in total adhesion molecule expression. Low integrin expression levels on primitive MPB cells may thus not necessarily predict inefficient homing, as long as functionally active molecules were expressed at or above the putative threshold. It will be important to address these possibilities in the future.
The surprising discordance between the homing and engraftment kinetics of FL CFCs merit discussion in the context of several aspects of our experimental design. First, in vivo homing studies offer a glimpse at organ distribution at only a single window in time after hematopoietic cell transplantation. The patterns observed after 3 hours may not extend to later times and rapid engraftment by FL cells may be explained by normal, albeit delayed, homing. Homing of FL cells may also vary at different embryonic ages. However, since Wolber and colleagues observed similar defects in the homing of day 12 FL cells when assayed 24 hours after transplantation into irradiated mice (3-fold less than adult BM cells38), we believe that reversal of this trend by collecting FL cells earlier in gestation, or by assaying homing at later times, is unlikely. Second, we measured the homing of in vitro assayable CFCs because quantitative analysis of more primitive HSCs, such as competitive repopulating units,39requires more cells than can be reisolated from the hematopoietic organs after transplantation. The potential role of CFCs in early engraftment is controversial40,41 and some might argue that homing defects identified in FL progenitors may not extend to more primitive stem cells, which, though able to rapidly regenerate circulating blood cells on transplantation, cannot generate colonies in vitro. We and others have previously shown that more differentiated classes of hematopoietic cells do contribute to early engraftment in mice and humans.22-24 It is therefore reasonable to expect that reduced homing of CFCs will negatively affect hematologic recovery. The fact that FL cells engraft rapidly despite reduced homing of progenitor cells, and perhaps their precursors, suggests that this impediment is overcome by the higher proliferative potential of hematopoietic cells isolated early in ontogeny.5,25 30
In summary, our results indicate that progenitor cells from different hematopoietic tissues exhibit wide variations in homing ability in vivo. However, timely hematopoietic engraftment is not necessarily limited by inefficient homing if the transplanted cells possess a high potential for proliferation and differentiation, as exemplified here by FL cells. The ultimate rate of reconstitution after transplanting HSCs from different sources thus appears to be the result of a balance between multiple intrinsic and extrinsic variables. The present experimental system appears well suited to elucidating the various components of the engraftment process, and offers a tool to facilitate its manipulation.
The authors thank Drs Gary Van Zant and Craig Jordan (Blood and Marrow Transplant Program, University of Kentucky) for critically reviewing the manuscript.
Supported by the University of Kentucky Hospital and Department of Internal Medicine, and grant R01-HL61392 (to S.J.S.) from the National Institutes of Health. S.J.S. was supported by a Junior Faculty Scholar Award from the American Society of Hematology.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stephen J. Szilvassy, Hematology/Oncology-BMT, Lucille P. Markey Cancer Center, Rm CC414, 800 Rose St, Lexington, KY 40536-0093; e-mail: szilvas@pop.uky.edu.