Abstract
Acquired chromosomal anomalies (most commonly translocations) in lymphoma and leukemia usually result in either activation of a quiescent gene (by means of immunoglobulin or T-cell–receptor promotors) and expression of an intact protein product, or creation of a fusion gene encoding a chimeric protein. This review summarizes current immunocytochemical studies of these 2 categories of oncogenic protein, with emphasis on the clinical relevance of their detection in diagnostic samples. Among the quiescent genes activated by rearrangement, expression of cyclin D1 (due to rearrangement of theCCND1 [BCL-1] gene) is a near-specific marker of t(11;14) in mantle cell lymphoma; BCL-2 expression distinguishes follicular lymphoma cells from their nonneoplastic counterparts in reactive germinal centers and appears to be an independent prognostic marker in diffuse large cell lymphoma; andTAL-1 (SCL) expression identifies T-cell acute lymphoblastic neoplasms in which this gene is activated. The protein products of other genes activated by chromosomal rearrangement have a role as markers of either lineage (eg, PAX-5 [B-cell–specific activator protein] for B cells, including B-lymphoblastic neoplasms), or maturation stage (eg, BCL-6 for germinal-center and activated B cells and MUM-1/IRF4 for plasma cells). Currently, no hybrid protein encoded by fusion genes is reliably detectable by antibodies recognizing unique junctional epitopes (ie, epitopes absent from the wild-type constituent proteins). Nevertheless, staining for promyelocytic leukemia (PML) protein will detect acute PML with t(15;17) because the microspeckled nuclear labeling pattern for PML-RARα is highly distinctive. Similarly, antibodies to the anaplastic lymphoma kinase (ALK) tyrosine kinase are valuable (because wild-type ALK is not found in normal lymphoid tissue) in detecting neoplasms (CD30-positive large T-cell lymphomas) with t(2;5) or its variants. Thus, immunocytochemical detection of the products of many rearranged genes in lymphoma and leukemia can be clinically informative and provide information on cellular and subcellular protein expression that cannot be inferred from studies based on messenger RNA.
Introduction
Translocations, deletions, and other nonrandom chromosomal abnormalities1,2 play a central role in the pathogenesis of many human hematologic malignant diseases. A growing number of studies are revealing the ways in which these chromosomal alterations affect specific genes, such as those encoding nuclear transcription factors. However, any gene implicated in the neoplastic process can act only through the protein it encodes. Many of the consequences of chromosomal changes with respect to abnormal protein expression have been inferred indirectly by analysis of messenger RNA (mRNA) transcription, but it is clear that protein and mRNA levels do not always correlate. For example, germinal-center B cells appear to contain BCL-2 message but little protein,3,4and the same is true for TAL-1 in erythroid cells.5 Many examples of the opposite combination (high protein and little or no message) also exist, such as BCL-2 in mature lymphocytes6,7 and elastase in mature myeloid cells.8 Antibody-based detection of the protein is therefore required, but this frequently cannot be done because of a lack of suitable reagents. Consequently, we are often ignorant not only about patterns of expression of oncogenic proteins in hematologic neoplasms but also about possible abnormalities in the distribution and subcellular localization of these proteins.
Most of the currently recognized genetic alterations in human leukemias and lymphomas result in either activation of a quiescent gene or creation of a hybrid gene encoding a chimeric protein. Table1 summarizes the genetic abnormalities that have been studied using antibodies specific for the protein products of genes involved in such rearrangements. We here review the immunocytochemical studies that have been done with these antibodies and assess, for each protein, whether abnormalities demonstrable by immunocytochemistry are of clinical value in determining diagnosis or predicting prognosis. Immunocytochemical findings that are of value are discussed below, whereas those for which no clear clinical applications have been observed are described briefly in Table2.
Genes activated by chromosomal changes
The CCND1 (BCL-1) gene and its protein product (cyclin D1)
CCND1 (BCL-1) gene.
The (11;14)(q13;q32) translocation,9 an anomaly often found in mantle cell (centrocytic) lymphoma10,11 and occasionally in other B-cell neoplasms, notably myeloma,12,13 juxtaposes the CCND1(BCL-1) locus encoding cyclin D1 on chromosome 11 to an immunoglobulin-enhancer sequence on chromosome 14.9 TheCCND1 gene is transcriptionally silent in normal lymphohemopoietic tissues14,15; thus, expression of the protein may promote neoplastic cell proliferation by perpetuating the transition from G1 to S.16
Antibodies to cyclin D1.
Several antibodies recognizing cyclin D1 have been described (Table3),14-18 but hematopathologists know that immunocytochemical detection of cyclin D1 in routinely processed tissues is not always easy.19,20 It is possible that cyclin D1 is so closely associated with other molecules in the nucleus that epitopes are masked. Whatever the cause of the difficulty, reliable staining can usually be obtained by using an optimized technique (eg, by incubating the antibody overnight with sections previously subjected to microwave heating in the presence of EDTA), as described in detail elsewhere.19-21
Cyclin D1 expression in normal tissues.
Cyclin D1 in lymphoid neoplasia.
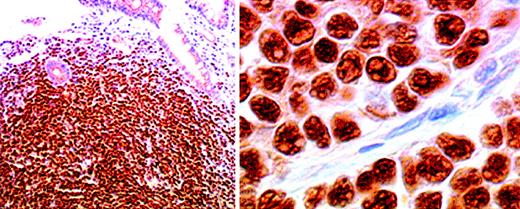
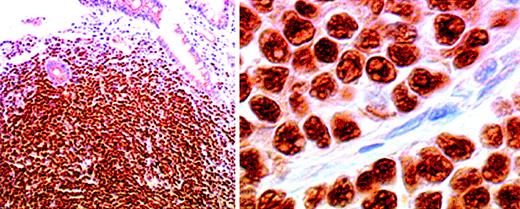
Most mantle cell lymphomas express cyclin D1,22-25although the staining intensity and the percentage of positive cells differ from case to case.15 Immunostaining is usually seen in a diffuse pattern in cell nuclei,15,24 but cytoplasmic positivity is also occasionally observed.17 The blastoid form of mantle cell lymphoma,26 characterized by frequent rearrangement of the CCND1 (BCL-1) gene, a high mitotic index, and a poor prognosis,27 shows no difference in the pattern or frequency of cyclin D1 staining.28Expression of cyclin D1 has also been observed in cases of multiple lymphomatous polyposis (Figure 1), the intestinal form of mantle cell lymphoma.11
Cyclin D1 immunostaining of neoplastic cell nuclei in a case of lymphomatoid polyposis, shown at low power (left) and high power (right).
An immunoperoxidase technique in a paraffin section was used. Original magnification left, × 100; right, × 800.
Cyclin D1 immunostaining of neoplastic cell nuclei in a case of lymphomatoid polyposis, shown at low power (left) and high power (right).
An immunoperoxidase technique in a paraffin section was used. Original magnification left, × 100; right, × 800.
Cyclin D1 expression is not completely specific for mantle cell lymphoma29,30: it has been found in plasmacytoma/myeloma, especially in cases with t(11;14)31,32; in sporadic cases of B-cell chronic lymphocytic leukemia (B-CLL), some of which show t(11;14) and CCND1 rearrangement,33 and in hairy cell leukemia, although levels of cyclin D1 are lower than in mantle cell lymphoma and no CCND1 (BCL-1) gene rearrangements are detected.33,34 Expression of CCND1(BCL-1) mRNA due to t(11;14) has been found in some cases of splenic marginal zone lymphoma,13 but no protein has been detected by immunohistological methods in this disease.35
Clinical applications of cyclin D1 immunostaining.
Detection of cyclin D1 by immunohistochemistry can be added to other criteria for the diagnosis of mantle cell lymphoma,11,30,36 particularly when a poor biopsy or an unusual growth pattern hinders recognition of the disease. Because mantle cell lymphoma has a range of morphologic features (from a small cell tumor to a blastoid proliferation11,26) and growth patterns (diffuse, nodular, or mantle zone23,37), a near-specific marker is clearly valuable, particularly because survival in this disease is significantly worse than that in other small cell B-cell lymphomas.11,37,38 In addition, cyclin D1 immunostaining may distinguish multiple lymphomatous polyposis from other indolent intestinal lymphomas, and the blastoid variant of mantle cell lymphoma (BCL-1 positive) from B-lymphoblastic lymphoma/leukemia (BCL-1 negative).28 Cyclin D1 immunostaining has also been used to identify cases of mantle cell lymphoma in a leukemic phase (a poor prognostic condition) and to differentiate them from other atypical chronic lymphoproliferative disorders.39 40
The BCL-2 gene and its protein product
BCL-2 gene.
The (14;18) chromosomal translocation in follicular lymphoma involves the BCL-2 gene at the chromosome 18 breakpoint, which encodes a 26-kd protein with limited homology to an Epstein-Barr viral protein (BHRF-1).41 Because breakpoints occur in the 3′ untranslated region, full-length protein is expressed after juxtaposition of the gene to the immunoglobulin heavy-chain promoter.41 Extensive studies have documented the role of BCL-2 in blocking apoptosis in many cell types and in response to a variety of stimuli.41 Many cellular and viral homologues have also been identified.
Antibodies to BCL-2.
BCL-2 in normal tissues.
BCL-2 is associated with mitochondria and cell membranes43and is widely distributed in both hemopoietic and nonhemopoietic cells.7,42 However, BCL-2 is absent from B-lymphoid cells in the germinal centers of B-cell follicles and from cortical thymocytes,7,44-46 and it ceases to be detectable when cells are transformed (eg, by mitogens).7
BCL-2 in hematologic neoplasia.
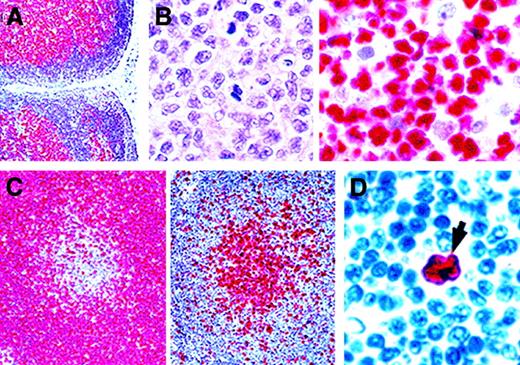
BCL-2 protein is expressed in most cases of follicular lymphoma,47 both in the majority with t(14;18) and in many without it.48,49 However, a few cases lack both protein expression and rearrangement.50 BCL-2 protein is also expressed in many lymphoid and myeloid neoplasms,7,46,51-56 although it is usually absent or expressed at low levels in Burkitt lymphoma53,57 and anaplastic lymphoma kinase (ALK)–positive anaplastic large cell lymphoma (ALCL),58,59 possibly because of the high rate of cell proliferation.60 BCL-2 protein is also often absent or present at low levels in large B-cell tumors at extranodal sites, including the gastrointestinal tract.51,61,62 In contrast, lymph node–based B-cell neoplasms are commonly positive for BCL-2.54 Furthermore, although low-grade mucosa-associated lymphoid tissue (MALT) lymphomas of the gastrointestinal tract are BCL-2 positive,61 they often contain areas of larger cells that lack BCL-2 (Figure2A),63,64 probably representing early transformation to large cell neoplasms. This may be relevant to observations that large B-cell neoplasms arising from MALT are BCL-2 negative51 and that proliferative markers in nodal lymphomas are inversely related to expression of BCL-2.60
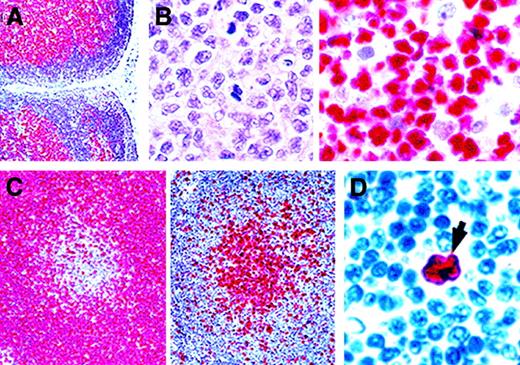
BCL-2 immunostaining of neoplastic lymphoid tissue.
(A) In a low-grade MALT lymphoma, BCL-2 is expressed by the diffuse neoplastic infiltrate lying beneath the intestinal mucosa (Muc). BCL-2 is absent from reactive germinal centers (asterisks) in the tumor and from areas of high-grade transformation. The high-power view (bottom) shows the border between a low-grade area and a high-grade area. The arrows indicate large BCL-2–negative neoplastic cells. Original magnification top, × 200; bottom, × 800. (B) A pseudonegative follicular lymphoma in which the cells in a neoplastic follicle (Foll) lack BCL-2. However, a higher-power examination of the boxed area (center and bottom) shows that many of the BCL-2–positive small interfollicular cells (arrows) are neoplastic centrocytes (small cleaved cells). (Alkaline phosphatase–antialkaline phosphatase [APAAP] technique in paraffin sections; reproduced with permission from American Journal of Pathology47.) Original magnification top, × 250; center, × 400; bottom, × 800.
BCL-2 immunostaining of neoplastic lymphoid tissue.
(A) In a low-grade MALT lymphoma, BCL-2 is expressed by the diffuse neoplastic infiltrate lying beneath the intestinal mucosa (Muc). BCL-2 is absent from reactive germinal centers (asterisks) in the tumor and from areas of high-grade transformation. The high-power view (bottom) shows the border between a low-grade area and a high-grade area. The arrows indicate large BCL-2–negative neoplastic cells. Original magnification top, × 200; bottom, × 800. (B) A pseudonegative follicular lymphoma in which the cells in a neoplastic follicle (Foll) lack BCL-2. However, a higher-power examination of the boxed area (center and bottom) shows that many of the BCL-2–positive small interfollicular cells (arrows) are neoplastic centrocytes (small cleaved cells). (Alkaline phosphatase–antialkaline phosphatase [APAAP] technique in paraffin sections; reproduced with permission from American Journal of Pathology47.) Original magnification top, × 250; center, × 400; bottom, × 800.
In follicular lymphomas,47 BCL-2 expression may also be heterogeneous, and the BCL-2–negative cells tend to be centroblasts (large noncleaved cells) rather than centrocytes (Figure 2B). As a result, cases at the aggressive end of the morphologic spectrum may appear to be BCL-2 negative when, in reality, few cells express the protein. This “pseudonegative” pattern is accentuated by the tendency of centrocytes to migrate away from neoplastic follicles so that large nodules of BCL-2–negative centroblasts are observed, with intervening smaller neoplastic cells that can be mistaken for normal cells (Figure 2B).47 It is possible that the BCL-2–negative large cell component is more sensitive to chemotherapy than is the small cell component, which may therefore persist and account for the indolent progression of the disease.
BCL-2 protein is found in Reed-Sternberg cells in one third to three fourths of all biopsy specimens from patients with Hodgkin disease (HD),45,65-67 including a few who have also had follicular lymphoma,68 but this presumably reflects its ubiquitous presence in lymphoid cells. However, the neoplastic cells of lymphocyte predominance HD (lymphocytic and histiocytic [L&H] or popcorn cells) tend to be BCL-2 negative,69,70 thereby illustrating the distinct nature of this HD subtype.11
Clinical applications of BCL-2 immunostaining.
Although the ubiquitous distribution of BCL-2 protein limits its value for diagnosis, it is widely used for distinguishing reactive lymphoid follicles, which are BCL-2 negative, from neoplastic follicles and proliferation centers (found in chronic lymphocytic leukemia46,71-73), both of which are usually BCL-2 positive. In MALT lymphoma, for example, the reactive lymphoid follicles characteristically associated with this tumor (which may be confused with follicular lymphoma) are clearly negative (Figure2A),64 at least until they have undergone follicular colonization by neoplastic cells.74 A similar phenomenon has been observed in mantle cell lymphomas that localize around germinal centers (Figure3C).52
BCL-6 immunostaining of normal and neoplastic lymphoid cells.
(A) BCL-6 expression in germinal centers in a lymph node showing follicular hyperplasia. (B) Diffuse large B-cell lymphoma. At left is a section stained with hematoxylin and eosin, and at right is a sample with BCL-6 immunostaining showing strong nuclear expression. (C) A trapped germinal center in a mantle lymphoma is BCL-2 negative (left) and BCL-6 positive (right). (D) BCL-6 expression by a popcorn/L&H cell (arrow) in nodular lymphocyte predominance HD (APAAP technique in paraffin sections; reproduced with permission from American Journal of Pathology106). Original magnification A, × 100; B left, × 800; B right, × 800; C left, × 200; C right, × 200; D, × 800.
BCL-6 immunostaining of normal and neoplastic lymphoid cells.
(A) BCL-6 expression in germinal centers in a lymph node showing follicular hyperplasia. (B) Diffuse large B-cell lymphoma. At left is a section stained with hematoxylin and eosin, and at right is a sample with BCL-6 immunostaining showing strong nuclear expression. (C) A trapped germinal center in a mantle lymphoma is BCL-2 negative (left) and BCL-6 positive (right). (D) BCL-6 expression by a popcorn/L&H cell (arrow) in nodular lymphocyte predominance HD (APAAP technique in paraffin sections; reproduced with permission from American Journal of Pathology106). Original magnification A, × 100; B left, × 800; B right, × 800; C left, × 200; C right, × 200; D, × 800.
BCL-2 may also be of value as a marker for identifying infiltration by follicular lymphoma cells in bone marrow trephine biopsy specimens.75,76 Aggregates of normal or reactive lymphoid cells often stain only weakly (although T cells label more strongly than B cells) and have nondescript nuclear morphologic features. In contrast, follicular lymphoma cells show strong cytoplasmic BCL-2 labeling, which tends to emphasize their characteristic cleft nuclear morphologic features (Figure 2B), and this is often a valuable adjunct to the classic criterion of a paratrabecular infiltration pattern. It is also possible that cases of “Burkitt-like” lymphoma can be distinguished from epidemic/sporadic Burkitt lymphoma by the expression of BCL-2 protein,57 as can cases of lymphoblastic lymphoma/leukemia,77 although further studies of this issue are needed.
It has been suggested that the presence of high levels of BCL-2 protein promotes survival of neoplastic cells78 or confers drug resistance79 (or both) and thus is a marker of a poor prognosis. Many clinical studies have sought evidence of such a correlation, and an association between higher cellular BCL-2 levels and poorer disease-free survival at periods up to 10 years was found in several studies of diffuse large cell lymphoma (DLCL).80-86 There is also some evidence for a prognostic influence of BCL-2 levels in acute myeloid leukemia.87-91However, for other hematologic malignant diseases (HD,92follicular lymphoma,84,93,94 chronic lymphocytic leukemia,95,96 and acute lymphoblastic leukemia [ALL]97-99), the data are limited or equivocal.
Diffuse lymphomas therefore represent the most promising area for future studies of BCL-2 expression and offer the longer-term prospect of strategies for specifically inhibiting BCL-2.86However, few if any oncologists currently take BCL-2 levels into account when treating individual patients, and this variable has not been used in any controlled trials to assign patients with lymphoma to more aggressive treatment protocols.
The BCL-6 gene and its protein product
BCL-6 gene.
Studies in knockout mice found that BCL-6 protein is required for germinal-center formation, antibody-affinity maturation, and T-helper-2–mediated responses.100 BCL-6 appears to inhibit differentiation of germinal-center B cells toward plasma cells by binding to signal transducers and activators of transcription 3, thereby preventing the expression of Blimp-1 (a major regulator of plasma cell development).101
Chromosomal translocations involving the 5′ noncoding domain of theBCL-6 gene102 at band 3q27 are observed in about 40% of diffuse large B-cell lymphomas103 and 10% to 15% of follicular lymphomas,103 juxtaposing the gene to promoters from a variety of partner chromosomes (most commonly in immunoglobulin chain loci). Mutations of the regulatory part of theBCL-6 gene are virtually absent in ALL and mantle cell lymphoma, but they occur frequently in germinal-center and postgerminal-center lymphomas, including follicular lymphomas, DLCLs, and Burkitt lymphomas.104 However, mutations of theBCL-6 gene have also been found in normal germinal-center B cells,105 and it is not clear what role they play in lymphomagenesis.
Antibodies to BCL-6.
BCL-6 in normal tissues.
In lymphoid tissues, BCL-6 protein is expressed in germinal-center B cells in secondary follicles (Table 3 and Figure3A)106-111 in both the dark (centroblast-rich) and light (centrocyte-rich) zones.106-110 It is also detectable in a few CD4-positive T cells, both in the germinal centers and in interfollicular areas,107,108 and in perifollicular CD30+ lymphoid cells.107 In addition, BCL-6 protein is expressed in thymocytes, mainly in the cortex.112 Outside the lymphohemopoietic system, expression of BCL-6 has been observed in squamous epithelia.106,113 In all positive cells, BCL-6 protein localizes to the nucleus in a diffuse or microgranular pattern.106-108 110
BCL-6 in lymphoid neoplasia.
Strong nuclear expression of BCL-6 occurs frequently in follicular lymphomas, paralleling its presence in germinal-center cells, their normal counterpart. BCL-6 expression is also found in most Burkitt lymphomas and diffuse large B-cell lymphomas (Figure 3B), regardless of whether the gene is rearranged.106-108,110,114 In lymphomas associated with human immunodeficiency virus, expression of BCL-6 and expression of the Epstein-Barr viral protein LMP1 appear to be mutually exclusive.115
Neoplasms of B cells unrelated to germinal centers (eg, B-cell precursor malignant diseases, B-CLL, mantle cell and marginal zone lymphomas, and hairy cell leukemia) consistently lack BCL-6 protein (Figure 3C),106 in keeping with its absence from the putative normal counterparts of these neoplasms.11,116Among T-cell lymphomas, BCL-6 expression has been detected in about 50% of ALCLs117 and T-cell lymphoblastic lymphomas.112 Reed-Sternberg cells in classic HD are usually BCL-6 negative, but the tumor cells (L&H cells) in lymphocyte predominance HD are strongly positive (Figure 3D),118 in keeping with their probable germinal-center origin.69 119
Clinical applications of BCL-6 immunostaining.
BCL-6 is a valuable marker of B cells of germinal-center origin.106,110,120 BCL-6 immunostaining, used in conjunction with BCL-2, may be useful in characterizing nodular lymphoid regions, since these can represent neoplastic germinal centers (indicative of follicular lymphoma), trapped residual germinal centers (eg, in MALT and mantle cell lymphomas; Figure 3C), or proliferation centers (in B-CLL).121 Labeling for BCL-6 may also help in the recognition of lymphocyte predominance HD (Figure3D).118 122
The MUM1/IRF4 gene and its protein product
MUM1/IRF4 gene.
The 14q+ cytogenetic anomaly in multiple myeloma represents a cryptic t(6;14)(q25;p32) that juxtaposes the immunoglobulin heavy-chain locus to a gene known as MUM1 or IRF4.123The MUM1/IRF4 gene encodes a transcription factor thought to play a key role in lymphoid development, since IRF4-deficient mice have a block in peripheral B-cell maturation (absence of germinal centers and plasma cells) associated with a lack of cytotoxic T-cell responses.124
Antibody to MUM1/IRF4.
A recently generated mAb (MUM1p)125 against human MUM1/IRF4 protein is suitable for immunostaining routinely prepared paraffin sections and for Western blotting, in which it detects a 50-kd band of expected molecular weight.
MUM1/IRF4 in normal tissues.
In normal lymphoid tissue (Table 3), MUM1/IRF4 protein is detected mainly in plasma cells and in a small number of germinal-center B cells, which are usually BCL-6 negative and nonproliferating (Ki67 negative) and found mainly in the light zone of the germinal center.125 In addition, MUM1/IRF4 is expressed in a small percentage of T cells and in most perifollicular CD30-positive cells,125 in keeping with its expression by peripheral blood T cells after stimulation with phytohemagglutinin.125 In all positive cells, MUM1/IRF4 protein expression is primarily nuclear and shows a microgranular and diffuse pattern, with sparing of nucleoli.125
MUM1/IRF4 in lymphoid neoplasia.
In accordance with its expression at late stages of B-cell differentiation and after T-cell activation, MUM1/IRF4 protein is strongly expressed in lymphoplasmacytic lymphoma/immunocytoma, about 75% of diffuse large B-cell lymphomas, primary effusion lymphomas,126 HD, multiple myeloma, and ALCL.125,127 128
Clinical applications of MUM1/IRF4 immunostaining.
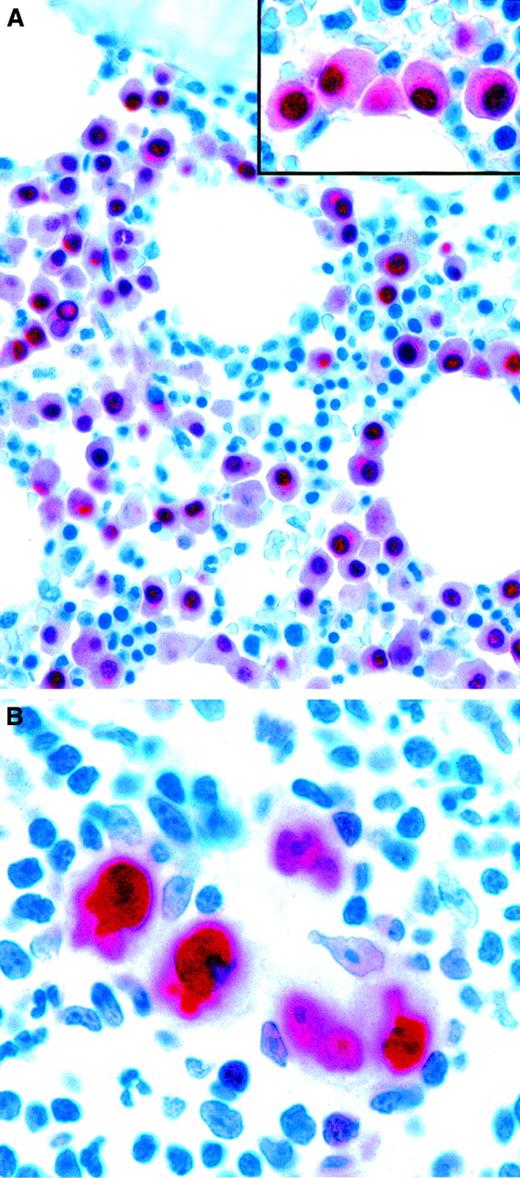
Because of the restriction of MUM1/IRF4 to late-stage B cells, it is potentially valuable (used in combination with other plasma cell markers, such as CD138) for recognizing myelomas (Figure4A). Furthermore, its consistent expression in neoplastic cells in HD (Figure 4B) can be of diagnostic help when the staining highlights Reed-Sternberg and Hodgkin cells in a tissue biopsy specimen (eg, of bone marrow).
Expression of the plasma cell–associated marker MUM1/IRF4 in neoplastic lymphoid cells.
(A) Bone marrow biopsy specimen showing infiltration by multiple myeloma. Monoclonal MUM1/IRF4 is present in the nuclei and the perinuclear area in neoplastic plasma cells. Residual hemopoietic elements are unlabeled. The inset shows higher magnification-view of the same field. (B) Strong labeling of tumor cells in classic HD (APAAP technique in paraffin sections). Original magnification A, × 600; A inset, × 1000; B, × 1000.
Expression of the plasma cell–associated marker MUM1/IRF4 in neoplastic lymphoid cells.
(A) Bone marrow biopsy specimen showing infiltration by multiple myeloma. Monoclonal MUM1/IRF4 is present in the nuclei and the perinuclear area in neoplastic plasma cells. Residual hemopoietic elements are unlabeled. The inset shows higher magnification-view of the same field. (B) Strong labeling of tumor cells in classic HD (APAAP technique in paraffin sections). Original magnification A, × 600; A inset, × 1000; B, × 1000.
The PAX-5 gene and its protein product
PAX-5 gene.
The PAX-5 gene (a member of the paired-box PAXgene family) encodes the transcription factor B-cell–specific activator protein (BSAP), which plays a key role in B-lymphoid development129 and is also involved in the embryonic development of the central nervous system and testis. In the t(8;14)(p13;q32), which is characteristic of small lymphocytic lymphoma with plasmacytoid differentiation (immunocytoma), the PAX-5 gene (on 9p13) is juxtaposed to the immunoglobulin heavy-chain gene (on 14q32).130
Antibodies to PAX-5.
Expression of PAX-5 in normal tissues.
In tissue sections, PAX-5 protein can be stained in B-cell follicles but not plasma cells.131 Unlike B-cell–associated transcription factors such as BCL-6 and MUM1, which are also expressed in activated T cells,106,107,125 PAX-5 appears to be B-cell specific131 (Table 3). Experimental studies have shown that PAX-5 protein is expressed in B-lymphoid precursors, but no data on direct immunostaining in human bone marrow have been reported.
Expression of PAX-5 in lymphohemopoietic neoplasms.
PAX-5 has been found in many B-cell lymphomas (with the strongest expression in follicular, mantle cell, and DLCLs)131 but not in T-cell neoplasms.
Clinical applications of PAX-5 immunostaining.
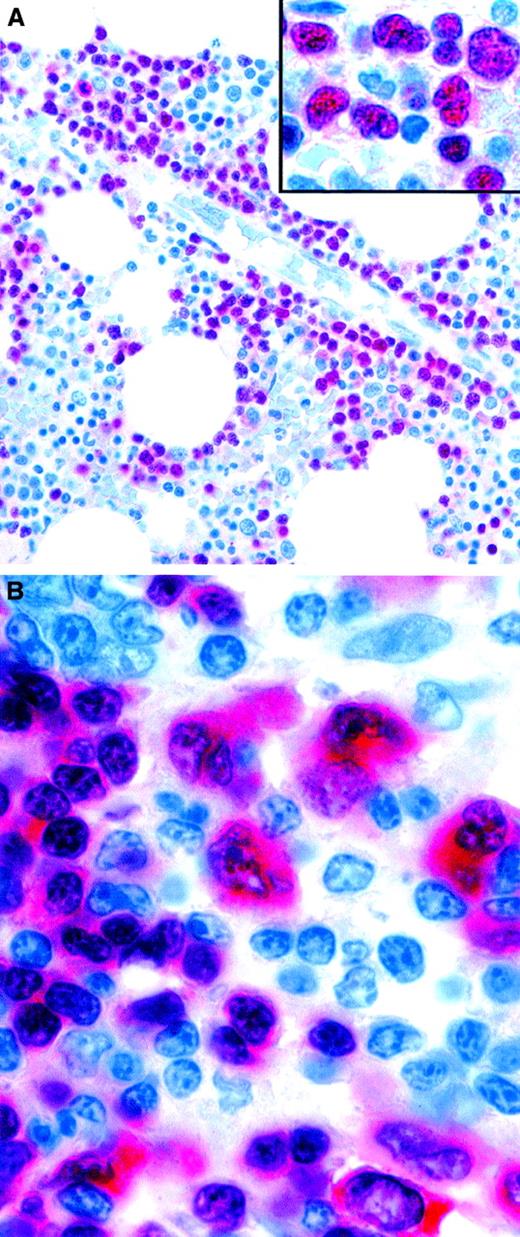
PAX-5 may prove to be a valuable diagnostic marker in paraffin-embedded biopsy specimens of B-lymphoblastic neoplasms because it is expressed strongly in such samples and is negative in T-cell lymphoblastic proliferations (Figure 5A; B.F., unpublished data, 2001). HD sometimes mimics ALCL, and PAX-5 may be useful in such cases because Reed-Sternberg cells are usually positive132 (Figure 5B), whereas ALCLs (both ALK positive and ALK negative) are consistently negative for PAX-5.
Expression of the PAX-5 (BSAP) transcription factor in neoplastic lymphoid cells.
(A) Pro-B acute lymphoblastic leukemia cells in a bone marrow biopsy specimen are selectively stained. The inset is a higher magnification, showing the irregular nuclei of PAX-5–positive leukemic cells. (B) Tumor cells of classic HD showing nuclear labeling, as well as cytoplasmic staining. Normal residual mantle zone B cells also express PAX-5 (APAAP technique in paraffin sections). Original magnification A, × 400; A inset, × 800; B, × 1000.
Expression of the PAX-5 (BSAP) transcription factor in neoplastic lymphoid cells.
(A) Pro-B acute lymphoblastic leukemia cells in a bone marrow biopsy specimen are selectively stained. The inset is a higher magnification, showing the irregular nuclei of PAX-5–positive leukemic cells. (B) Tumor cells of classic HD showing nuclear labeling, as well as cytoplasmic staining. Normal residual mantle zone B cells also express PAX-5 (APAAP technique in paraffin sections). Original magnification A, × 400; A inset, × 800; B, × 1000.
The TAL-1 gene and its protein product
TAL-1 gene.
The TAL-1 gene (also known as SCL orTCL-5)133 at 1p32 encodes a 42-kd nuclear protein134 that is essential for mammalian hematopoiesis133 and normal yolk sac angiogenesis.135 Structural alterations in theTAL-1 gene represent the most frequent molecular lesions in T-cell ALL (T-ALL).1,133 In up to 25% of childhood cases, a submicroscopical 90–kilobase-pair deletion on the 5′ side of the gene133 brings it under the influence of promoters for theSIL gene. Much less commonly, the gene is rearranged by a chromosome translocation; this is usually t(1;14)(p32;q11), which associates the gene with the TCRδlocus.133 136
Antibodies to TAL-1.
TAL-1 in normal tissues.
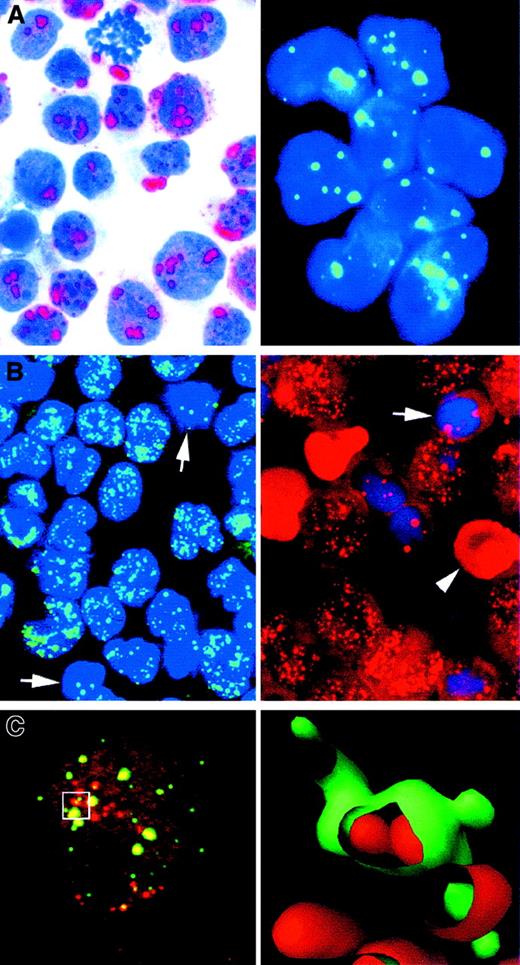
Studies of TAL-1 protein or TAL-1 mRNA found that the gene is expressed in some, but not all, hemopoietic lineages (notably, erythroid precursors, megakaryocytes, and mast cells; Figure6A),137,139,140 but it is not detectable in even the most immature T cells (thymocytes and CD2-positive precursor lymphoid cells in fetal liver).141TAL-1 immunostaining is localized to the nucleus (Figure 6A-C),137 characteristically in the form of small dots (Figure 6C),137 whose number is greater than that of PML-positive structures. In mitotic cells, TAL-1 is consistently cytoplasmic.137
TAL-1 expression in normal hemopoietic cells and neoplastic cell lines.
(A) At left is a sample from fetal liver that shows numerous scattered TAL-1–positive nuclei. At right is a sample with double staining for TAL-1 (in brown) in combination with cytokeratin (CK), transferrin receptor (TfR), or platelet glycoprotein (Plt) (all in red) that identifies the TAL-1–positive cells, many of which are intrasinusoidal (arrow), as erythroid precursors and megakaryocytes. (B) The erythroleukemic HEL cell line shows strong nuclear positivity. Nucleoli are unstained. (C) TAL-1 is also expressed in the K562 erythroid cell line, localized to discrete intranuclear bodies and often in an annular pattern (immunoperoxidase [A-C] and APAAP [A] techniques in cryostat sections [A] and cytospin preparations [B,C]; reproduced with permission from Blood137). Original magnification A left, × 100; A right, × 800; B, × 800; C, × 1000.
TAL-1 expression in normal hemopoietic cells and neoplastic cell lines.
(A) At left is a sample from fetal liver that shows numerous scattered TAL-1–positive nuclei. At right is a sample with double staining for TAL-1 (in brown) in combination with cytokeratin (CK), transferrin receptor (TfR), or platelet glycoprotein (Plt) (all in red) that identifies the TAL-1–positive cells, many of which are intrasinusoidal (arrow), as erythroid precursors and megakaryocytes. (B) The erythroleukemic HEL cell line shows strong nuclear positivity. Nucleoli are unstained. (C) TAL-1 is also expressed in the K562 erythroid cell line, localized to discrete intranuclear bodies and often in an annular pattern (immunoperoxidase [A-C] and APAAP [A] techniques in cryostat sections [A] and cytospin preparations [B,C]; reproduced with permission from Blood137). Original magnification A left, × 100; A right, × 800; B, × 800; C, × 1000.
TAL-1 in hematologic neoplasia.
The TAL-1 gene is activated in about one fourth of all cases of T-ALL, but the link with expression of TAL-1 protein remains unclear. In several T-ALL lines, mRNA and/or protein was found in the absence of detectable gene rearrangement,137,142 andTAL-1 mRNA has been detected by reverse transcriptase–polymerase chain reaction (RT-PCR) in most cases of T-ALL.143,144 However, this may derive from normal monocytes and T cells in the samples145: the results of an immunocytochemical study suggested that TAL-1 protein is only rarely expressed by leukemic cells in T-ALL when the gene is not rearranged.146
Clinical applications of TAL-1 immunostaining.
In a study by Chetty et al,147 samples from approximately 50% of T-ALL cases showed nuclear labeling, but the investigations were done in paraffin-embedded tissue, on which the available mAbs do not provide clean labeling. However, immunocytochemical labeling of fresh T-ALL samples that were also studied with molecular biologic techniques suggested that cases in which the TAL-1 gene is rearranged can be detected by immunocytochemistry.146There appears to be no correlation between TAL-1 gene rearrangement and clinical behavior in T-ALL.148
Fusion genes encoding chimeric proteins
Several chromosomal translocations cause fusion of unrelated genes rather than overexpression of intact quiescent genes, and these hybrid genes encode tumor-associated chimeric proteins.1 2 This situation has reawakened interest in producing tumor-specific antibodies, but unfortunately, antibodies specific for the novel junctional regions in these chimeric proteins have proved to be difficult to raise. In addition, genes may break at different points and thus give rise to more than one fusion protein, with different junctional regions. In spite of these obstacles, progress has been made by using antibodies directed against nonjunctional parts of chimeric proteins, since such antibodies can detect relocalization (eg, PML) or de novo expression induced by gene fusion (eg, ALK).
The (15;17) translocation and its variants
The PML and RARα genes.
The (15;17) chromosomal translocation, a specific marker for acute promyelocytic leukemia (APL),149 fuses the retinoic acid receptor-α (RARα) gene to a growth suppressor gene (PML) that encodes a protein thought to be involved in regulation of p53 acetylation and premature senescence induced by oncogenic Ras.150 The PML-RARα fusion protein may play a key role in the pathogenesis of APL by blocking terminal differentiation and inhibiting apoptotic cell death,149and studies indicate that it acts by recruiting histone deacetylase through the RARα portion of the chimeric protein.151,152In the rare cases of APL carrying variants of the t(15;17) (Table 1), the RARα gene is fused to the nucleophosmin(NPM), PLZF, or nuclear mitotic apparatus(NuMA) gene.153
Antibodies to PML.
Most PML isoforms and PML-RARα fusion proteins can be detected by immunocytochemical labeling with mAb PG-M3154 (Table 3), which recognizes an epitope near the cysteine-rich (putative DNA-binding) region. Another anti-PML mAb (5E10)155 has been described, but it is directed against an epitope probably located in a region (between amino acids 448 and 466) that is lost in some PML-RARα forms (eg, type S and bcr3 PML-RARα; Table3).156 Anti-PML antibodies fail to identify endogenous PML in Western blotting analyses,154 possibly because of the small amounts of each PML isoform (13 have been identified157) resolved by gel electrophoresis.
PML in normal tissues.
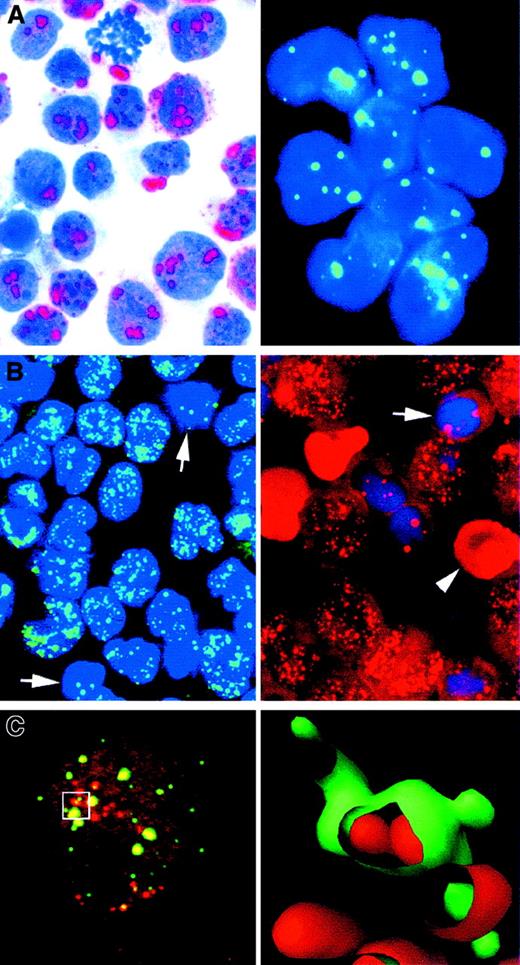
Wild-type PML protein is expressed ubiquitously, but levels vary greatly according to cell type.154,158-160 PML protein is localized to discrete nuclear dots (average, 10 dots/nucleus) not associated with nucleoli (Figure7A).154,156,161-163 The diameter of these dots ranges from 0.5 to 1 μm,162 and they correspond to nuclear domains (PML oncogenic domains [PODs])164,165 reorganized previously by electron microscopy as “nuclear bodies”166 and by immunocytochemistry as “nuclear dots” or “nuclear domain 10.”167 These macromolecular protein-rich complexes are tightly bound to the nuclear matrix155,163,164 and are known to contain several other proteins, including Sp100, NDP52, PIC1/SUMO-1, and Int-6.165,168-174 Progressive accretion of proteins gives rise to a characteristic ringlike appearance,154,156 162 especially in cells overexpressing PML.
Staining for wild-type and hybrid PML proteins.
(A) Speckled nuclear staining, representing wild-type PML, in transfected U937 cells (left) and in a case of FAB-M5 acute leukemia (right). (B) Nuclear microspeckled staining pattern indicating the presence of hybrid PML-RARα protein in 2 cases of APL. Single arrows indicate normal residual marrow cells; and arrowhead, a red blood cell (APAAP and immunofluorescent techniques in cytospin preparations; reproduced with permission from Blood177). (C) Laser-scan confocal microscopy of the KG1 myeloid cell line double stained for PML (green) and PLZF (red). At left is the confocal image. At right is a 3-dimensional reconstruction of the area outlined on the left panel, showing the spatial relation between PML and PLZF in the nuclear bodies. Original magnification A-C, × 1000.
Staining for wild-type and hybrid PML proteins.
(A) Speckled nuclear staining, representing wild-type PML, in transfected U937 cells (left) and in a case of FAB-M5 acute leukemia (right). (B) Nuclear microspeckled staining pattern indicating the presence of hybrid PML-RARα protein in 2 cases of APL. Single arrows indicate normal residual marrow cells; and arrowhead, a red blood cell (APAAP and immunofluorescent techniques in cytospin preparations; reproduced with permission from Blood177). (C) Laser-scan confocal microscopy of the KG1 myeloid cell line double stained for PML (green) and PLZF (red). At left is the confocal image. At right is a 3-dimensional reconstruction of the area outlined on the left panel, showing the spatial relation between PML and PLZF in the nuclear bodies. Original magnification A-C, × 1000.
PML in hematologic neoplasia.
In leukemias other than APL (and in lymphomas), PML labeling consistently shows a wild-type speckled pattern (Figure 7A, right). In cells containing the PML-RARα fusion protein (eg, in APL blasts and transfected cells), the PML nuclear bodies are disrupted and the wild-type speckled pattern is replaced by a microspeckled pattern (Figure 7B) consisting of many tiny dots (often too numerous to count) in which PML-RARα and PML colocalize.156,161,162,164Treatment of APL cells in vitro or in vivo with all-trans-retinoic-acid (ATRA) or arsenic trioxide (As2O3) restores the wild-type speckled PML pattern.156,161,162,164,175 Wild-type PML is often expressed at higher levels in Reed-Sternberg cells in HD than in neoplastic cells of other lymphoid malignant diseases.160
Clinical applications of PML immunostaining.
APL can be diagnosed rapidly by showing the microgranular PML immunocytochemical labeling pattern (Figure 7B).176-180This appearance is specific for APL with t(15;17), since the variants t(11;17) and t(5;17) do not disrupt the wild-type speckled pattern.181-184 Immunostaining must be done on fresh samples (smears, cytospin preparations, or frozen sections), since fixation and paraffin embedding change the wild-type speckled pattern to a diffuse pattern.160,177 Immunocytochemistry is especially useful in recognizing the microgranular variant of APL (M3V)—which is characterized by sparse cytoplasmic granules and marked nuclear irregularity, with folding, lobulation, or convolution—because it can be difficult to distinguish M3V from acute myelomonocytic or monocytic leukemia (M4 and M5B).185 The immunocytochemical diagnosis of APL is clinically valuable because the disease responds to treatment with ATRA186,187 and As2O3.188 Variant translocations are not detected by immunostaining for PML, but they are rare and at least some of them, such as t(11;17), do not respond to ATRA treatment.149 189
The (11;17) and (5;17) translocations.
In the rare t(11;17) and t(5;17) anomalies, the portion of RARα present in the PML-RARα chimeric protein is fused to the Kruppel-like transcription factor PLZF or NuMA (the nuclear mitotic apparatus protein) (both of which are encoded by genes on chromosome 11), or to the amino-terminal of NPM.190-192 There are conflicting reports on the subcellular distribution of the PLZF protein: studies have observed localization to small punctate nuclear domains different from PODs,193 colocalization of PLZF and PML in nuclear bodies,194 and localization to 0.3- to 0.5-μm nuclear bodies (PLZF bodies) adjacent to but functionally distinct from PML nuclear bodies (Figure 7C).195 However, it is agreed that PLZF-RARα and PML-RARα colocalize in the same microspeckles.194,195 PLZF is expressed mainly in CD34+ progenitors196 but not in more mature myeloid cells, and it may also be involved in central nervous system differentiation.197 Because of the rarity of these APL molecular variants, no clinical studies of the value of antibodies to their products have been reported.
The (2;5)(p23;q35) translocation and its variants
The NPM and ALK genes.
The (2;5)(p23;q35) translocation, which is associated with ALCL,198-200 fuses the NPM gene, which encodes the ubiquitously expressed nucleolar phosphoprotein NPM, on chromosome 5 to the ALK gene, which encodes a receptor tyrosine kinase.201,202 The resulting NPM-ALK hybrid protein (also known as p80) contains 40% of the amino-terminal portion of NPM linked to the entire intracytoplasmic domain of ALK.203-206 Six variant translocations in which ALK fuses to a partner other than NPM have been identified in human tumors207-213 (Table4), but the classic NPM-ALK anomaly accounts for more than 80% of ALK fusion genes. TheALK gene appears not to be transcribed in normal lymphoid cells, but the promoter or promoters for NPM or other partners are assumed to induce transcription of the ALKfusion genes in lymphoma cells.201 NPM-ALK kinase activity is induced as a result of cross-linking by the NPM portion or one of the other fusion partners. Initial estimates of the frequency of t(2;5) and the resulting NPM-ALK gene in ALCL varied widely,214-219 but most of this uncertainty has since been resolved through immunocytochemical studies of ALK expression.
Antibodies to ALK.
The laboratory that identified the p80 protein (later shown to be identical to NPM-ALK) prepared an affinity-purified polyclonal antibody to the kinase domain,220-222 and several immunocytochemical studies were conducted with samples made available to other researchers.218,219,223,224 A second polyclonal antibody (anti-ALK 11) has been used on a more limited scale for biochemical and immunocytochemical studies.225,226However, most studies of ALK protein are now done with mAbs. Two such reagents have been described: ALK1 and ALKc. These recognize the cytoplasmic portion of ALK227,228 and are both suitable for immunolabeling paraffin-embedded biopsy specimens and for Western blot analyses.227-230
Antibodies to NPM.
MAbs against the carboxy-terminal portion of NPM were described more than 15 years ago.231 More recently, 3 paraffin-reactive antibodies recognizing epitopes on the carboxy-terminal portion (which is lost in the NPM-ALK hybrid kinase) or the amino-terminal portion (present in NPM-ALK) have been raised.232
Expression of ALK and NPM in normal tissues and cell lines.
ALK protein cannot be detected in tissues of human adults, except in a few cells in the nervous system that show weak labeling.227,228 RT-PCR has detected NPM-ALKand ATIC-ALK mRNA at low levels in normal and reactive lymphoid cells,233,234 but ALK-positive circulating cells cannot be detected by immunocytochemistry.227 Cell lines with t(2;5) are ALK positive, as are some neuroblastoma cell lines235 and the rhabdomyosarcoma line Rh30,227 all of which express full-length ALK protein.201,227 In contrast to ALK, NPM can be detected in most human cells by immunocytochemical techniques, usually confined to cell nuclei.232
Expression of ALK in lymphoid neoplasia.
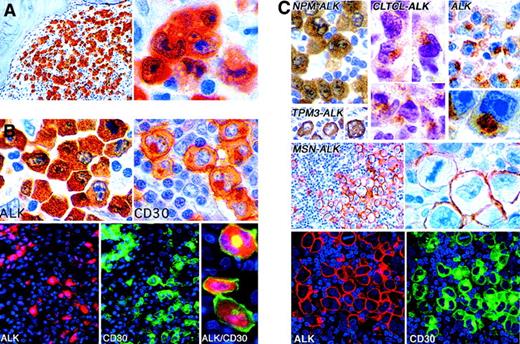
The absence of ALK protein from normal lymphoid tissue means that positive ALK staining is a near-specific marker for lymphomas containing a hybrid ALK gene (Figure8). When a lymphoma expresses ALK, the subcellular labeling pattern is informative (Table 4). Thus, in cases with the classic t(2;5), ALK is observed not only in the cytoplasm but also in the nucleus (Figure 8A), whereas tumors expressing ALK fusion proteins other than NPM-ALK show no nuclear labeling because they do not dimerize with wild-type NPM (Figure8C).227,228,230,236,237 Furthermore, 2 ALK fusion proteins (CTCL-ALK and moesin-ALK) were found to have unique immunohistochemical staining patterns (granular cytoplasmic and membrane-associated, respectively; Figure 8C).210,212 Staining for the amino-terminal of NPM can also be informative because it is detected in both the cytoplasm and the nucleus in tumor cells with t(2;5),232 whereas staining in lymphomas with variant translocations is restricted to the nucleus.238 Western blotting of cryostat-section extracts can also be used to characterize variant ALK proteins, whose molecular weights differ from that of NPM-ALK.239
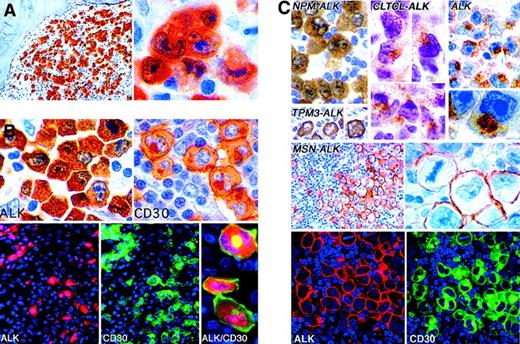
Immunostaining of lymphomas for ALK proteins.
(A) Labeling for NPM-ALK in the nucleus and cytoplasm in a lymphoma with anaplastic large cell morphologic characteristics. (B) A t(2;5)-positive large cell lymphoma showing the contrast between NPM-ALK expression (cytoplasmic with some nuclear staining) and CD30 staining (surface membrane and Golgi-associated labeling). The double fluorescent images at bottom show that the same cells express CD30 and ALK but in different patterns. (C) Different patterns of ALK immunostaining in lymphomas. All tumors contain a hybrid ALK protein, except for that marked ALK, an example of the rare subtype of large B-cell lymphoma (CD30-negative and positive for epithelial membrane antigen) in which full-length wild-type ALK protein is expressed. (Immunoperoxidase and immunofluorescence techniques in paraffin sections; reproduced with permission from Blood227,258 and Laboratory Investigation212.) Original magnification A left, × 250; A right, × 1000; B top, × 1000; B bottom (ALK and CD30, × 400; ALK/CD30, × 1000); C, × 400 to × 1000.
Immunostaining of lymphomas for ALK proteins.
(A) Labeling for NPM-ALK in the nucleus and cytoplasm in a lymphoma with anaplastic large cell morphologic characteristics. (B) A t(2;5)-positive large cell lymphoma showing the contrast between NPM-ALK expression (cytoplasmic with some nuclear staining) and CD30 staining (surface membrane and Golgi-associated labeling). The double fluorescent images at bottom show that the same cells express CD30 and ALK but in different patterns. (C) Different patterns of ALK immunostaining in lymphomas. All tumors contain a hybrid ALK protein, except for that marked ALK, an example of the rare subtype of large B-cell lymphoma (CD30-negative and positive for epithelial membrane antigen) in which full-length wild-type ALK protein is expressed. (Immunoperoxidase and immunofluorescence techniques in paraffin sections; reproduced with permission from Blood227,258 and Laboratory Investigation212.) Original magnification A left, × 250; A right, × 1000; B top, × 1000; B bottom (ALK and CD30, × 400; ALK/CD30, × 1000); C, × 400 to × 1000.
Extensive immunohistologic studies have shown that ALK-positive tumors correspond only partly to what pathologists diagnosed in the past as ALCL.217,219,223,227,228,237 ALK-positive lymphomas typically contain a population of medium to large hallmark neoplastic cells, with a reniform eccentric nucleus and a juxtanuclear hof in the Golgi region.222,230 However, the morphologic variation is wide228-230,240,241 and includes cases with a relatively uniform proliferation of hallmark cells,242tumors in which many bizarre large cells (sometimes with a sarcomatoid appearance) are observed, and neoplasms containing large numbers of macrophages (the lymphohistiocytic pattern) or other reactive cells.11 243-245
ALK-positive lymphomas may contain areas of small cells that also express ALK protein.228,230 This indicates that the large cells do not represent cytologic transformation of a small cell tumor after acquisition of an ALK translocation. In cutaneous T-cell lymphoma, cellular transformation can create this mixed large and small cell pattern, but these cases are ALK negative and lack theNPM-ALK gene.246 When the small cell component is prominent,247 the tumor is sometimes referred to as the “small cell variant” of ALK-positive lymphoma.228,230,236,237 There is no reason, however, to think of this as a different disease, although spread of the disease to the peripheral blood and bone marrow may be more likely in cases of this sort.248 249
ALK-positive lymphomas usually express T-cell markers and/or cytotoxic granule proteins, CD30, and epithelial membrane antigen and c-Myc.219,222,228,230,250 Unlike ALK-negative cases, ALK-positive ALCLs are consistently BCL2 negative.58,59 We have found that tumors that are diagnosed morphologically as ALCL but have a B-cell phenotype are always ALK negative,228,251and they probably represent one end of the morphologic spectrum of diffuse large B-cell neoplasia.11,251 Primary CD30-positive cutaneous lymphomas are also ALK negative, in keeping with the absence of the NPM-ALK gene.252Finally, the controversial proposed “Hodgkin-like” subtype of ALCL11 is almost always ALK negative219,227,228,230 (as are Reed-Sternberg cells219,223,224,227 228) and probably represents true HD.
Most important, lymphomas defined by ALK labeling appear to be clinically homogeneous, regardless of whether they have the classic t(2;5) or one of its variants. They are usually found in young men presenting with advanced disease (stage III-IV), are often associated with systemic symptoms (especially fever) and involvement of extranodal sites (especially skin, bone, and soft tissues), respond well to chemotherapy, and have a favorable outcome.221,237,253,254Whether the high proliferative rate of the tumor,255 the host immune response to the ALK protein,256 or both these factors contribute to this pattern must be clarified in additional studies. In contrast, ALK-negative lymphomas with anaplastic large cell morphologic features tend to occur in older patients and usually have an unfavorable prognosis.221,253,254 257
Rare cases of ALK-positive large B-cell lymphoma have been described. These are characterized by immunoblastic (rather than anaplastic) morphologic features, expression of full-length ALK, and cytoplasmic IgA (Figure 8C).258 The ALK-positive lymphomas with a B-cell phenotype described by Gascoyne et al254 may belong to this rare category, but there is insufficient information to confirm this.
ALK protein is not detectable in most nonhematopoietic neoplasms. The only exceptions are a few cases of rhabdomyosarcoma228and neuroblastoma,235 both of which produce full-length ALK, possibly reflecting their primitive origin. In contrast, in the rare entity known as “inflammatory myofibroblastic tumor,” ALK expression is commonly found and appears to reflect the presence of an ALK fusion protein (eg, TPM3-ALK, TPM4-ALK, or clathrin-ALK).208 259-261
Clinical applications of anti-ALK antibodies.
Immunohistologic staining for ALK in lymphoid tissue biopsy specimens is now a widely used hematopathologic marker for diagnosing the clinicopathologic entity associated with t(2;5) and its variants, for which the name “ALK-positive lymphoma”228,230,236seems most appropriate (rather than “ALCL”). ALK labeling can also be of value in discerning low levels of tumor tissue infiltration in such cases (eg, in bone marrow trephine specimens249,253) and identifying cases in which the neoplastic cells are obscured by macrophages.229 The small cell variant of ALK-positive lymphoma247 can also be distinguished, because of its positive labeling for ALK, from peripheral T-cell lymphomas.228 Moreover, inflammatory myofibroblastic tumors208 that express the ALK protein as a result of translocations involving the ALK gene can also be diagnosed by using ALK labeling, and the absence of CD30 distinguishes them from ALK-positive lymphomas.
Other translocations.
Two translocations involving fusion of NPM with genes other than ALK have been described, and both are rare. The (5;17)(q32;q21) translocation in APL fuses the NPM gene to the RARα gene,191 and the t(3;5)(q25.1;q34), which is found occasionally in myelodysplastic syndrome/acute myeloid leukemia,262 fuses NPM to the myelodysplasia/myeloid leukemia factor 1 (MLF1) gene. It was reported that the resulting NPM-MLF1 hybrid protein localizes to the nucleus, whereas wild-type MLF1 protein is found in the cytoplasm,262 thus indicating that the NPM moiety targets MLF1 to a novel site.
Discussion
Studies of the proteins encoded by genes involved in chromosomal alterations in hematologic neoplasms have lagged behind molecular biologic investigations of these anomalies because of the investment of resources required to make satisfactory antibodies, a process that depends on the availability of antigen. It is sometimes possible to raise highly specific antibodies against synthetic peptides (eg, anti-BCL-27 and PML154), but the best immunogens are probably recombinant proteins, the production of which is not simple. Furthermore, although polyclonal reagents have been used for immunocytochemical studies and Western blotting analyses (in which nonspecific reactions can be to some extent ignored), they may introduce problems of nonspecific reactivity, variation between different samples, and limited availability. Therefore, mAbs are preferable, but reagents that satisfy stringent criteria for specificity are not easy to produce. Antibodies should detect fixation-resistant epitopes in paraffin-embedded tissue, but those that meet this requirement are more difficult to produce.
Despite these problems, specific antibodies to the products of genes involved in hematologic neoplasia have provided major new insights, particularly when the protein target is relevant not only to hematologic neoplasia specifically but also to cell physiology in general. The best of example of this is BCL-2, a protein that was first studied in lymphomas with the t(14;18) but that subsequently was found to be extremely important in apoptosis.
Most antibodies discussed here can detect their targets through biochemical methods (Western blotting or immunoprecipitation). These techniques are particularly applicable to the study of hybrid genes, since they allow chimeric proteins (eg, the products of the hybridBCR-ABL and NPM-ALKgenes239,263,264) to be distinguished from wild-type proteins on the basis of their unique molecular sizes. Biochemical analysis can also be useful when a gene (eg,AML1265) encodes several transcripts. However, compared with immunocytochemistry, biochemical methods are more demanding technically and provide only limited information on tissue distribution and subcellular localization of the proteins.
Immunocytochemistry thus has obvious advantages for studying tissue distribution and localization. We have here discussed genetic anomalies that switch on quiescent genes separately from those that create fusion genes. For the anomalies that switch on genes, a major consideration is the expression pattern of the gene product in normal cells. Immunocytochemical studies of widely expressed genes (eg,c-MYC) generally have little clinical value. In contrast, few normal cells express the products of the BCL-1 andTAL-1 genes, and immunocytochemical staining for these proteins has a role in the diagnosis of mantle cell lymphoma and T-ALL, respectively.
Molecules such as BCL-2 and BCL-6 are in an intermediate position: both are expressed by many cells in the absence of gene rearrangement, but immunocytochemical labeling can be useful in certain situations. For example, BCL-2 is a good marker for the t(14;18) if normal germinal-center B cells, which are consistently BCL-2 negative, are compared with their neoplastic counterparts, follicular lymphoma cells, which are usually BCL-2 positive. Thus, immunocytochemical detection of BCL-2 protein has a diagnostic role in distinguishing between reactive and neoplastic follicle centers, even though it is widely expressed outside the germinal center. BCL-6 is not a comparably specific immunocytochemical marker for gene translocation (even in a restricted context), but it has diagnostic value as a molecule associated with germinal-center cells.
Antibodies to chimeric proteins generated by fusion genes are theoretically less suitable for immunocytochemical analyses, since they are usually raised against one of the 2 constituent proteins and therefore react with both wild-type and hybrid proteins. Antibodies specific for junctional epitopes unique to the chimeric protein should avoid this problem, but despite reports of such epitopes on the BCR-ABL,266,267 AML1-ETO,265 and E2A-PBX1268 chimeric proteins—created respectively by t(9;22), t(8;21), and t(1;19)—the only antibodies that appear potentially suitable for immunocytochemical use are monoclonal reagents specific for the E2A-PBX1 junction.268 These, however, apparently have not been used since their initial description.
Therefore, although in theory it should be possible to produce antibodies specific for hybrid proteins, in practice such antibodies remain elusive. If short recombinant protein sequences (or synthetic peptides) are used as immunogens, the junctional region may not adopt the same 3-dimensional conformation that it has in the chimeric protein in vivo. Furthermore, the junctional sequences may not be immunogenic, an issue that has been addressed, at least with respect to T-cell recognition, in several studies. For example, there is evidence that junctional BCR-ABL peptides can be bound by HLA class I molecules,269,270 and several investigators271 have reported that in vitro immunization with a peptide from p210 BCR-ABL can elicit a specific cytotoxic response. However, it is not yet clear whether the BCR-ABL junctional sequence is immunogenic in vivo.272 Peptides eluted from HLA molecules on fresh chronic myeloid leukemia cells include those from the BCR protein but not junctional sequences.273
Antibodies specific for chimeric oncogene products will therefore always be difficult, if not impossible, to produce. However, the 2 examples discussed here (PML-RARα and NPM-ALK) show that antibodies to normal constituents of a hybrid protein can be used to detect underlying genetic abnormalities. In the case of t(15;17), the intranuclear distribution of the chimeric PML-RARα protein is so distinctively different from that of wild-type PML that the existence of the PML-RARα hybrid gene can reliably be inferred. The presence of the NPM-ALK fusion gene generated by t(2;5) can be assumed when a tumor shows ALK labeling in the nucleus and cytoplasm. When labeling is restricted to the cytoplasm, a variant hybrid ALK gene is likely to be present (since ALK protein is not expressed in normal lymphoid tissues), and labeling and Western blotting with anti-NPM can confirm this. ALK therefore behaves like proteins such as BCL-1 or BCL-2, which are switched on by gene rearrangement. Because of the absence of ALK protein from normal lymphohemopoietic cells, ALK fulfills most of the criteria for that elusive entity—a tumor-specific antigenic marker.
Immunocytochemical labeling for the NPM-ALK chimeric protein illustrates a point of wider relevance: the importance of subcellular localization patterns. Several proteins move from the cytoplasm to the nucleus under physiologic conditions. For example, the dimeric p50/p65 nuclear factor-κβ transcription factor is normally held in the cytoplasm by the inhibitor protein Iκβ, but it translocates to the nucleus when Iκβ is inactivated by phosphorylation.274Epidermal growth factor was also found to relocate to the nucleus, after binding of its ligand.275,276 Such observations are often made when proteins affected by genetic changes in hematologic neoplasia alter their subcellular localization (Table5), and it is frequently concluded that the sites to which proteins relocate must be the sites of their oncogenic action. However, the NPM-ALK chimeric protein, for example, despite its prominent nuclear (and, in particular, nucleolar) localization, almost certainly exerts its lymphomagenic effect within the cytoplasm.277 The chimeric PML-RARα protein shows no tendency to associate with nucleoli; thus, the hybrid NPM-RARα protein that is produced in rare cases of APL (and which presumably also accumulates in nucleoli) probably does not exert its transforming effect at this site.
Another leukemia-associated protein that relocates to a site that may not be relevant to its action is PML, which is displaced in APL cells from its normal target sites (ie, nuclear bodies) through heterodimerization with the PML-RARα fusion protein.156,161,162,164 However, a PML-RARα mutant that cannot dimerize with PML in coimmunoprecipitation experiments and does not delocalize PML from the nuclear bodies is nevertheless also capable of blocking differentiation.278 Moreover, in some cases of APL, there are variant translocations that presumably contribute directly to leukemogenesis but that encode fusion proteins (PLZF-RARα,190 NPM-RARα,191 and NuMA-RARα192) that do not alter the localization of PML, thereby suggesting that interference with retinoid signaling rather than disruption of PML nuclear bodies is the important event.
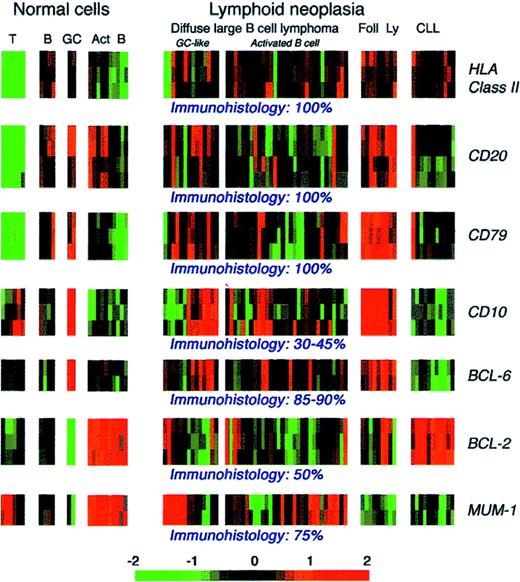
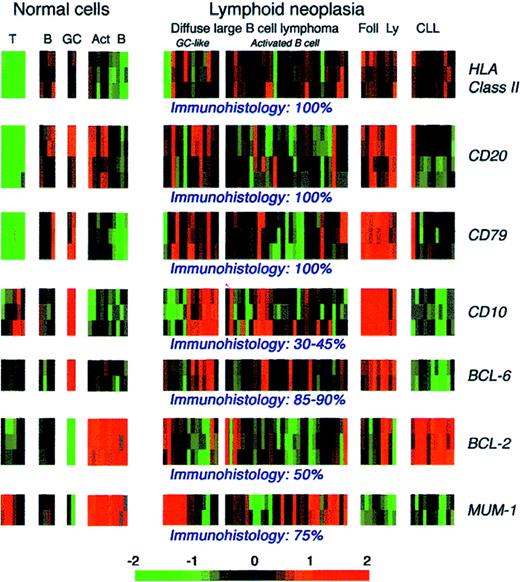
In conclusion, antibodies specific for the products of genes involved in chromosomal changes in leukemia and lymphoma have proved to be valuable both for studying normal cell physiology (eg, BCL-2) and for diagnosis (eg, BCL-1, PML, and NPM-ALK). The scope of antibody-based studies should continue to expand as new genetic changes in lymphoma and leukemia are identified.279 Furthermore, microarray studies of the expression of large numbers of gene sequences are likely to reveal new potential immunocytochemical markers of clinical relevance associated with subtypes of disease280-282 or prognosis.280 However, researchers choosing candidate proteins against which to raise antibodies must recognize that the level of mRNA extracted from a cell suspension (and particularly from a biopsy specimen) does not necessarily correlate with the level of the corresponding protein in the tumor cells. This consideration is relevant to a microarray study of human B-cell lymphoma.282 Figure 9 shows the mRNA expression data from this study for protein markers previously investigated immunohistologically in DLCL. The mRNA patterns for purified cells appear to correlate with protein expression (eg, the absence of B-cell gene expression in T cells and the expression of CD10 and BCL-2 in purified germinal-center cells). However, expression of the proteins studied is known to range in DLCL from less than 40% of cases (CD10) to essentially all cases (class II, CD20, and CD79), and these documented differences in protein expression are not, at least on first inspection, reflected in the mRNA profiles for the corresponding genes, which show little obvious differences.
Microarray patterns of mRNA expression for 7 lymphoid-associated genes in normal and neoplastic lymphoid cells.
These data are from a series reported by Alizadeh et al (http://llmpp.nih.gov/lymphoma) that included samples of diffuse large B-cell lymphomas. On the basis of microarray analysis, these tumors were assigned to 2 categories (with different clinical behavior), with one resembling germinal-center cells and the other having an activated B-cell phenotype. The frequency of positive immunostaining for the 7 markers in diffuse large B-cell lymphoma indicated by published studies and the authors' experience is indicated beneath the microarray images. Act B indicates activated B cells; B, B cells; CLL, B-cell chronic lymphocytic lymphoma; Foll Ly, follicular lymphoma; GC, germinal-center cells; and T, T cells.
Microarray patterns of mRNA expression for 7 lymphoid-associated genes in normal and neoplastic lymphoid cells.
These data are from a series reported by Alizadeh et al (http://llmpp.nih.gov/lymphoma) that included samples of diffuse large B-cell lymphomas. On the basis of microarray analysis, these tumors were assigned to 2 categories (with different clinical behavior), with one resembling germinal-center cells and the other having an activated B-cell phenotype. The frequency of positive immunostaining for the 7 markers in diffuse large B-cell lymphoma indicated by published studies and the authors' experience is indicated beneath the microarray images. Act B indicates activated B cells; B, B cells; CLL, B-cell chronic lymphocytic lymphoma; Foll Ly, follicular lymphoma; GC, germinal-center cells; and T, T cells.
Thus, the process of translating new molecular biologic findings into practical antibody-based techniques for assessing human tumor samples will continue to require a combination of judicious discrimination in choosing clinically important molecules and skill in making and characterizing specific antibodies. Given the large number of genes from which to choose the right candidates and the lotterylike nature of mAb production, an element of luck would also help!
This article is dedicated to the memory of Carlo Falini. The authors also wish to express their gratitude to their collaborators who have contributed with great skill over the years to the production and characterization of many of the reagents referred to in this review.
Supported by Associazione Italiana per la Ricerca sul Cancro (B.F.) and the Leukaemia Research Fund of Great Britain (D.Y.M.).
The authors contributed equally to this work.
References
Author notes
Brunangelo Falini, Istituto di Ematologia, Policlinico, Monteluce, 06100 Perugia, Italy.

![Fig. 2. BCL-2 immunostaining of neoplastic lymphoid tissue. / (A) In a low-grade MALT lymphoma, BCL-2 is expressed by the diffuse neoplastic infiltrate lying beneath the intestinal mucosa (Muc). BCL-2 is absent from reactive germinal centers (asterisks) in the tumor and from areas of high-grade transformation. The high-power view (bottom) shows the border between a low-grade area and a high-grade area. The arrows indicate large BCL-2–negative neoplastic cells. Original magnification top, × 200; bottom, × 800. (B) A pseudonegative follicular lymphoma in which the cells in a neoplastic follicle (Foll) lack BCL-2. However, a higher-power examination of the boxed area (center and bottom) shows that many of the BCL-2–positive small interfollicular cells (arrows) are neoplastic centrocytes (small cleaved cells). (Alkaline phosphatase–antialkaline phosphatase [APAAP] technique in paraffin sections; reproduced with permission from American Journal of Pathology47.) Original magnification top, × 250; center, × 400; bottom, × 800.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.409/6/m_h80222021002.jpeg?Expires=1765140566&Signature=IQj~gIVBkCzEuhFvcpHMWB8IVs0lb23lS0wnncBqgljJkyh7YAqtzymowIf~eqEHugJN~LmE14B76IObKETYRBdcsctZ~WoB~pC7SREa2s2YdXkln7Bk4Zm44KhckkrsIcCeH2u-Ev48xZtq5AYLdOsvei0XqxX-HdmfkMCkdad~nnSSJIrXbSH40FxBJtm9TygXDmGU6LG~MmuLSBHULaOLU6aKG3QKOvv-aF1inMykN3eszrUH~HssrgrEr47j0kPRw85zdsoqtJ1QEjR5WjQsU4te~RsKCtgEtMYEP46uFRdifQHzV3qeLK0iPhDsAmbBEIfCkTgX~CweYs4HDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. TAL-1 expression in normal hemopoietic cells and neoplastic cell lines. / (A) At left is a sample from fetal liver that shows numerous scattered TAL-1–positive nuclei. At right is a sample with double staining for TAL-1 (in brown) in combination with cytokeratin (CK), transferrin receptor (TfR), or platelet glycoprotein (Plt) (all in red) that identifies the TAL-1–positive cells, many of which are intrasinusoidal (arrow), as erythroid precursors and megakaryocytes. (B) The erythroleukemic HEL cell line shows strong nuclear positivity. Nucleoli are unstained. (C) TAL-1 is also expressed in the K562 erythroid cell line, localized to discrete intranuclear bodies and often in an annular pattern (immunoperoxidase [A-C] and APAAP [A] techniques in cryostat sections [A] and cytospin preparations [B,C]; reproduced with permission from Blood137). Original magnification A left, × 100; A right, × 800; B, × 800; C, × 1000.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.409/6/m_h80222021006.jpeg?Expires=1765140566&Signature=MYq4PH5EX6hzrqqn4XFn7gVDiiwnN97YwbZb1malQOSQjrf2~vUieS5q6jQSvKXwwvspHH60yyzS6fzT~XisNoew4yLYL4zVNI1ZurZ5HNLm6NcariUN289blupnz8qV0gUoei5bZwNc8AISKksRSS90lIdBJKuAjvDflnP5owiIqt2Tg0D2s-1uUjgBVwbByxLIxY8dNeVGTnb5bZARDUULk~oQmLerYi-5cslcQIVq~gI69GZ~OT4hayKXUT4dLEJdt9EwvTRJgTW~6myua2RoLQ9SkngFr6MKl0-8FFTlOmNh7ZAlU74TUeVId15PJxlAhQruhCOD4giBlbqy0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. BCL-2 immunostaining of neoplastic lymphoid tissue. / (A) In a low-grade MALT lymphoma, BCL-2 is expressed by the diffuse neoplastic infiltrate lying beneath the intestinal mucosa (Muc). BCL-2 is absent from reactive germinal centers (asterisks) in the tumor and from areas of high-grade transformation. The high-power view (bottom) shows the border between a low-grade area and a high-grade area. The arrows indicate large BCL-2–negative neoplastic cells. Original magnification top, × 200; bottom, × 800. (B) A pseudonegative follicular lymphoma in which the cells in a neoplastic follicle (Foll) lack BCL-2. However, a higher-power examination of the boxed area (center and bottom) shows that many of the BCL-2–positive small interfollicular cells (arrows) are neoplastic centrocytes (small cleaved cells). (Alkaline phosphatase–antialkaline phosphatase [APAAP] technique in paraffin sections; reproduced with permission from American Journal of Pathology47.) Original magnification top, × 250; center, × 400; bottom, × 800.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.409/6/m_h80222021002.jpeg?Expires=1765465565&Signature=kNCTVBxEbkb1IVxoawHcHV5QdWFdvO4SyPhbwxC3gOrqjeYUHrR7fKcHFeBFB34oe756R7O1dzk8TSL6UmiwLqqqX2IRScQGSWiWNrJmogJjlwNFzUdQGmK3y6IycBrdB1I68FlWEzZCYZ~v7FPHxeCiYtx5B5gHYQxbTUaIP-EbW4M75BWqwKP~kTac0XyzgOFm3Z8bsEsnQ9noS~FiWcwPjM3ceTeaxmeUBbC6w-FFHcs~s2ClVBRBpv-9qqs8~SM~OSjIojavcD1JGfAd~6n4Nx2EzvNDOreahC4~tyZAozVd8gKXWOuUmdWKNVjGr-7EmKsGemc6p8VQRx6IaQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 6. TAL-1 expression in normal hemopoietic cells and neoplastic cell lines. / (A) At left is a sample from fetal liver that shows numerous scattered TAL-1–positive nuclei. At right is a sample with double staining for TAL-1 (in brown) in combination with cytokeratin (CK), transferrin receptor (TfR), or platelet glycoprotein (Plt) (all in red) that identifies the TAL-1–positive cells, many of which are intrasinusoidal (arrow), as erythroid precursors and megakaryocytes. (B) The erythroleukemic HEL cell line shows strong nuclear positivity. Nucleoli are unstained. (C) TAL-1 is also expressed in the K562 erythroid cell line, localized to discrete intranuclear bodies and often in an annular pattern (immunoperoxidase [A-C] and APAAP [A] techniques in cryostat sections [A] and cytospin preparations [B,C]; reproduced with permission from Blood137). Original magnification A left, × 100; A right, × 800; B, × 800; C, × 1000.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.409/6/m_h80222021006.jpeg?Expires=1765465565&Signature=xUMHveV5zLqVxcnsv9dZcMNzDL-bRU8QzwDjsW44MhsACWNhaVh0X0B0jwKVefPtFeaYgs1CcUI~Mf8-AlgegqefEB~dZVPTgjZ4q8Y5RDRfIOzqLPU3xprM1GVR-cv7jquqAS6bvw3gVV3QJ2feO6uPbP-I~5LuYhMLKwTP1ROBI3YYRZ7skwlDGYF5Ydb7eJxo~O9vEA0iQz0dFpGF38ZDrt~3vsZK8p4PfbatDjkN4Ef8k1JnspUXad1oqq6FR9IuaQtIyCS5Guk0BHjgMtqFiQovDAaXGRPFtGleytJZR54HZXUoydiLsoS7cEs2pVxSNbsF5AScZlkfrbP30A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)