Abstract
Hematopoietic stem cells (HSCs) have self-renewal capacity and multilineage developmental potentials. The molecular mechanisms that control the self-renewal of HSCs are still largely unknown. Here, a systematic approach using bioinformatics and array hybridization techniques to analyze gene expression profiles in HSCs is described. To enrich mRNAs predominantly expressed in uncommitted cell lineages, 54 000 cDNA clones generated from a highly enriched population of HSCs and a mixed population of stem and early multipotent progenitor (MPP) cells were arrayed on nylon membranes (macroarray or high-density array), and subtracted with cDNA probes derived from mature lineage cells including spleen, thymus, and bone marrow. Five thousand cDNA clones with very low hybridization signals were selected for sequencing and further analysis using microarrays on glass slides. Two populations of cells, HSCs and MPP cells, were compared for differential gene expression using microarray analysis. HSCs have the ability to self-renew, while MPP cells have lost the capacity for self-renewal. A large number of genes that were differentially expressed by enriched populations of HSCs and MPP cells were identified. These included transcription factors, signaling molecules, and previously unknown genes.
Introduction
Hematopoiesis is a dynamic process with significant complexity in which a subset of hematopoietic stem cells (HSCs) gives rise to cells of both the myeloid and lymphoid lineages.1In addition, HSCs have the ability to self-renew to produce more HSCs. This property allows HSCs to repopulate the bone marrow of lethally irradiated congenic hosts.
Mouse HSCs isolated with Thy1.1loc-kit+Sca-1hiLin−/lomarkers using fluorescence activated cell sorting (FACS) represent only approximately 0.05% of mouse bone marrow cells and these cells can fully reconstitute all blood cell elements.2-4 This population of cells has been further divided into 3 subpopulations4,5: long-term HSCs (LT-HSCs), short-term HSCs (ST-HSCs), and multipotent progenitor (MPP) cells, according to their abilities to support hematopoiesis and self-renewal. These 3 populations of cells can be arranged in a lineage according to a progressive loss of the ability to self-renew.6 The LT-HSC population has extensive self-renewal ability and supports long-term reconstituting ability (> 6 months), representing approximately 0.005% to 0.01% of bone marrow cells.4 ST-HSCs with limited self-renewal ability briefly contribute to hematopoiesis (6-8 weeks). MPP cells cannot self-renew but can reconstitute bone marrow for less than 4 weeks.5 Efflux of dyes such as rhodamine-123 (Rh) can be used to separate early hematopoietic cells into HSC and early progenitor subpopulations by flow cytometry (see Pohlmann et al7 and Weissman8 for detailed description). Rh is a mitochondria-binding fluorescent dye, and can be effluxed from the cell by the ABC transporter including the multidrug-resistance (MDR) gene product, P glycoprotein.12LT-HSCs, which are relatively quiescent,13 have high ABC transporter activity, thus the population of cells that stain most weakly with this dye is highly enriched for LT-HSCs. Alternatively, LT-HSCs, ST-HSCs, and MPP cells can be identified according to the expression levels of lineage-associated antigens such as Mac-1 and CD4.4 5
The rarity of the HSCs, coupled with an inability to maintain these cells in culture for a significant period, has greatly hindered the biochemical and molecular characterization of these cells. Although various growth factors can induce stem cells to proliferate in vitro, no known combination of growth factors has been shown to promote sustained self-renewal of the HSCs. Rather, stem cells induced to proliferate in vitro invariably undergo concomitant differentiation. HSC functions are thought to be regulated by interactions of stem cells with stromal cells.14 The dynamic process of HSC development, including self-renewal, expansion, and maturation, involves interactions between the intrinsic genetic program and extrinsic signals from stromal cells. This dynamic process is accompanied by global changes in gene expression profiles involving molecular events such as signal transduction, transcriptional and translational regulation, and chromatin modification. In the present study, we intend to systematically look into these molecular events by analyzing the gene expression profiles of self-renewing HSCs and non–self-renewing MPP cells. Therefore, any changes in the components of these aforementioned molecular events may provide insight into understanding the molecular mechanisms in controlling self-renewal of HSCs.
Materials and methods
Isolation of HSCs and generation of cDNA libraries
For cDNA library A, 4.7 × 109 bone marrow cells were collected from the femurs and tibias of 60 C57BL/6J mice (6-8 weeks old). These bone marrow cells were incubated with rat monoclonal antibodies against lineage-positive markers (Pharmingen, San Jose, CA) including CD4 (RM4-4), CD2 (RM2-5), CD45R/B220 (RA3-6B2), Gr-1 (RB6-8C5), Mac-1 (M1/70), TER-119 (TER-119), and IL-7Rα chain (B12-1); lineage negative (Lin−/lo) cells were enriched by twice depleting lineage-positive cells through incubation with antibody-coated (sheep antirat IgG) Dynabeads M-450 (Dynal). The remaining Lin−/lo cells were stained with Sca-1 fluorescein isothiocyanate (FITC) for isolation of Lin−/loSca-1+ cells.
The cDNA library A was constructed from 1.3 × 106Lin−/loSca-1+ cells described above using the ZAP expression cDNA synthesis kit following the manufacturer's procedure (Stratagene). Briefly, total RNA was isolated from the Lin−/loSca-1+ cells using Trizol Reagent (BRL). The cDNA was synthesized first by reverse transcription using Superscript II (Invitrogen, Carlsbad, CA) and then by DNA synthesis using Klenow DNA polymerase (Invitrogen). The cDNA inserts were cut with EcoRI and XhoI restriction enzymes and cloned into the EcoRI/XhoI sites of λZAPExp vector. The plasmids (pBK; Stratagene, La Jolla, CA) bearing the cDNA inserts were excised from their parent vector λZAPExp using helper phage according to the manufacturer's protocol. The primary cDNA library contained 36 000 clones.
The cDNA library B was made from 2.8 × 104 twice-sorted Lin−/loSca-1+Thy-1.1loc-kit+HSCs. Isolation of a population of Lin−/loThy-1.1loc-kit+Sca-1+ HSCs, RNA extraction, cDNA synthesis, and polymerase chain reaction (PCR) amplification have been described previously.15 There were 7.5 × 106 clones in the original library.
Probe preparation for macroarray and microarray analyses
For preparation of cDNA probes used for macroarray hybridization, total RNA isolated from lineage-positive cells derived from thymus, spleen, and bone marrow was used as template to generate the first strand of cDNAs using SuperScript II (Invitrogen), incorporating 33P-dCTP into the cDNA probes during the reverse transcription.
For preparation of cDNA probes used for microarray hybridization, c-kit-phycoerythrin (PE) (2B8) and Sca-1-biotin (D7)/Streptavidin-Cy-Chrome (Pharmingen) were used to stain Lin−/lo cells prior to separation using a Vantage fluorescence-activated cell sorter (Becton Dickinson, San Jose, CA). In order to isolate HSCs from MPP cells, Rh (0.1 μg/mL; Sigma, St Louis, MO) was used to separate Lin−/loc-kit+Sca-1+ cells into Rhlo (lowest 15%) and Rhhi (highest 15%) populations. We were able to obtain 1.4 × 104RhloLin−/lo c-kit+Sca-1+and 1.5 × 104RhhiLin−/loc-kit+Sca-1+cells from 15 mice. The postsorting analysis showed that the purity of the sorted Lin−/loc-kit+Sca-1+cells was 98%. Total RNA was isolated from equal numbers of RhloLin−/lo c-kit+Sca-1+and RhhiLin−/loc-kit+Sca-1+cells (1 × 104). In order to amplify the cDNA for microarray hybridization, PCR amplification was performed using pSMART cDNA synthesis strategy following the manufacturer's procedure (ClonTech, San Jose, CA). Briefly, VNdT20(5′-GACTCTAGAGCGGCCGCCTTTTTTTTTTTTTTTTTTTTVN-3′, V = A, G, C; n = T, A, G, C) was used for the first-strand cDNA synthesis. 5′-cap oligo (5′-TACGGCTGCGAGAAGACGACAGAAGGG-3′) was used for the second-strand cDNA synthesis. Both the VNdT and 5′-cap primer (5′-TACGGCTGCGA GAAGACGACAGAA-3′) were used for the PCR amplification with limited amplification cycles (20 cycles were used in this study). Cy3-dCTP (for Rhlo cells) and Cy5-dCTP (for Rhhi cells) fluorescence were incorporated during the PCR reaction. The unincorporated nucleotides were removed through a Millipore column (Millipore, Bedford, MA). The optical density (OD)200-700nm of the probes was measured using a spectrophotometer. The incorporation rate was calculated subsequently according to the ratio of the absorbance of Cy3 (523 nm) and Cy5 (633 nm) over the DNA absorbance at 260 nm.
Macroarray preparation, hybridization, and analysis
To prepare for macroarray, the 2 HSC cDNA libraries, A and B, described above were plated onto LB agarose/ampicillin plates in 24 × 24-cm Q-trays (Genetix, Christchurch, England) with densities of 500 to 2000 colonies per tray, and incubated overnight at 37°C. The bacterial colonies were then picked up by a Q-bot robotic system (Genetix) and inoculated into 384-well plates. Thus, each cDNA clone was addressed by its position in the plate. The macroarrays were generated by spotting bacterial colonies from 384-well plates onto 24 × 24-cm nylon membranes using Q-bot (Genetix). Thirty-six thousand cDNA clones from library A and 18 000 clones from library B were spotted in duplicate, and the position of each clone on the membrane was recorded according to its address in the 384-well plate. These macroarray membranes were incubated on the top of LB-agarose plus antibiotics at 30°C overnight in Q-trays. DNAs were denatured by soaking the membrane in 0.5 N NaOH/1.5 M NaCl for 10 minutes and neutralized with 0.5 M Tris-Cl, pH 8.0/1.5 M NaCl for 5 minutes. DNAs were fixed by ultraviolet (UV) crosslinking (UVP, Upland, CA). For hybridization, membranes were prehybridized for 2 hours at 60°C in 100 mL solution (12.5 mM PEG8000; 250 mM NaCl; 85 mM Na2HPO4; 7.5 mM H3PO4[86%]; 243 mM sodium dodecyl sulfate [SDS]; 10 mM ethylenediaminetetraacetic acid [EDTA]; 32 mM NaOH, pH 7.2) containing 100 μg/mL of denatured salmon sperm DNA and 100 μg/mL denatured mouse Cot-1 DNA. Denatured 33P-labeled probes from mature cell lineages were added to the prehybridized solution and incubated overnight in roller bottle or shaking bath at 60°C. Membranes were washed twice with 2x sodium chloride sodium citrate (SSC)/1% SDS at room temperature for 15 minutes, and then once in 0.2x SSC/0.1% SDS at 60°C for 30 minutes. Membranes were exposed to a phosphor imager screen and scanned using a phosphoimage scanner (Molecular Dynamics, Sunnyvale, CA). The hybridization result was analyzed using Spotfinder for image processing (H. Hammersmark and R.B., University of Washington, Seattle). Since each cDNA clone was spotted in duplicate, an average signal intensity of the duplicate was used. Local background hybridization signals surrounding each spot were subtracted prior to comparing spot intensity. Quantitative comparison of signal intensities was analyzed using Microsoft Excel. In order to avoid false-negative signals generated from slow or nongrowing bacterial colonies, a probe derived from the vector sequence was hybridized to the macroarray in parallel. The negative selection process was performed by selecting cDNA clones with positive signals for vector hybridization and negative or very weak signals for the cDNA probes derived from lineage-positive cells. Using this process, 3000 of 36 000 cDNA clones and 2000 of 18 000 cDNA clones were selected from library A and B, respectively, for microarray analysis.
Microarray preparation, hybridization, and analysis
cDNA inserts from 5000 clones selected using macroarray analysis described above were PCR-amplified using primers derived from the vector. For library A, ZAP-F (5′-AGTGGATCCAAAGAATTC-3′) and ZAP-R (5′-CTCTAGAAGTACTCTCGAG-3′) were used, and for library B, SubA1 (5′-CTTCGAACCGCGGATATCAGATC-3′) and SubS2 (5′-AAGGTTCCTTCACAAAGATCCCTCGAG-3′) were used. Amplified PCR products were purified using Sephacryl S500 (Pharmacia, Peapack, NJ), mixed 1 to 1 with dimethyl sulfoxide (DMSO) (Amersham, Piscataway, NJ), and spotted in duplicate onto each 75 × 25-mm coated type 7 microarray slide (Amersham) using Genespoter II (Molecular Dynamics). The microarray slides were air-dried and DNA was fixed by UV crosslinking at 500 mJ. The microarray slides were hybridized with Cy3- and Cy5-labeled probes described above. Briefly, the purified probes were concentrated with a speed-vacuum in the dark and resuspended in 20 μL hybridization buffer (5x SSC, 5x Denhardt, 0.1% SDS, 50% formamide, 100 μg/mL denatured salmon sperm DNA, 20 μg/mL polyA60 RNA, 100 μg/mL mouse Cot I DNA). An equal amount of Cy3- and Cy5-labeled probes were combined, denatured, and added onto the microarray slides, and hybridized at 42°C in a hybridization oven or in a humidified chamber for 16 hours to 18 hours. Microarray glass slides were washed twice with 2x SSC/0.1% SDS, and 0.2x SSC/0.1% SDS at 55°C for 5 minutes, and then with 0.1x SSC at room temperature for 1 minute. Glass slides were immersed in water for 10 seconds and dried immediately with N2. The hybridized microarray was scanned with a confocal dual-laser scanner at 523 nm and 633 nm (Molecular Dynamics) and analyzed using the custom array analysis software developed at the University of Washington (H. Hammersmark and R.B.). This array analysis software includes image processing, data normalization, and error analysis as described previously.16
Reverse transcriptase–polymerase chain reaction
Reverse-transcription reactions were carried out using SuperScriptII following the manufacturer's manual (Invitrogen). The following primers were used for PCR: Activin βC, 5′-GACACCTTACTCTGGAGCTG-3′ and 5′-GGGAGGCAGA GTAGATTACA-3′; BA_RA6B66, 5′-TCATGGTATGCCCTCGTGTA-3′ and 5′-AAATGTGTGGGCTTTTCAGG-3′; BA_RA5A82, 5′-AACATGGCTTGGAGAC AACC-3′ and 5′-AAGCCTGGGATTCACTGCTA-3′; BA_RA4B58, 5′-ATGAGGGC CATTGTTACACC-3′ and 5′-TTATGGCCAGCTTGGTTCAC-3′; MSC_RA15C76, 5′-TGATACCCTTGGCTCGAAAC-3′ and 5′-CCAAGTGCTGGGATTAAAGG-3′; MSC_RA16D13, 5′-GGCTCGAAATTAACCCTCAC-3′ and 5′-CCAGATCTCGT TACGGATGG-3′. The PCR condition was 35 cycles consisting of 94°C for 30 seconds, 55°C for 40 seconds, and 72°C for 1 minute.
Competitive repopulation assay
Two hundred fifty cells (either RhloLin−/loSca-1+c-kit+or RhhiLin−/loSca-1+c-kit+) from Ly5.2 mice were mixed with 4 × 104Lin−/lo cells from congenic host Ly5.1 mice17and were then transplanted into lethally irradiated (11 Gy [1100 rads]) Ly5.1 mice by intravenous (IV) injection. Each group included 8 mice. Peripheral blood cells were harvested at different time points after transplantation, as indicated in Figure 2C, and flow cytometric analyses were performed using different specific cell surface markers (CD3, B220, Mac-1, and Gr-1) together with anti-Ly5.2 antibody.
Results
Construction and normalization of HSC cDNA libraries
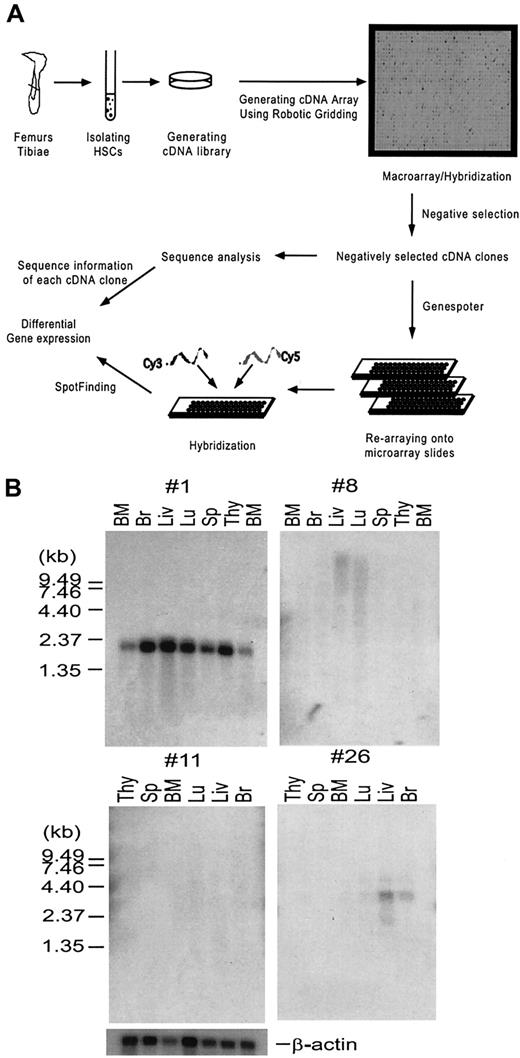
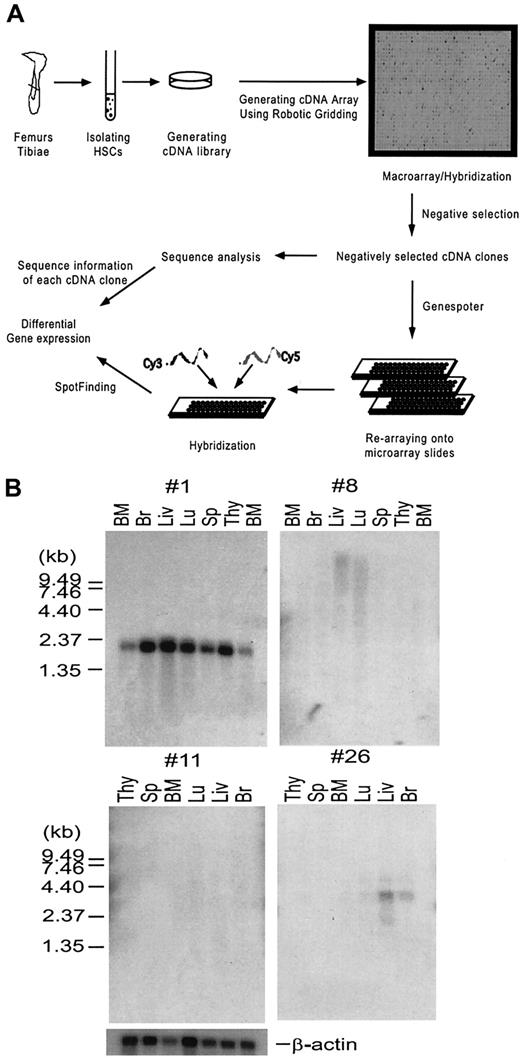
In order to enrich for genes that are predominantly expressed in the uncommitted, or lineage-negative cell populations, and remove highly redundant and housekeeping genes for further analysis, high-density array18 (macroarray) analysis was used and cDNA clones that hybridized to the lineage-positive probes were removed. cDNA clones that had no or very weak hybridization signals to the lineage-positive probes were selected. These negatively selected cDNA clones were subsequently subject to analysis of differential gene expression using a microarray system.19 The strategy for using macroarrays and microarrays to analyze the gene expression patterns of the HSC is illustrated and described in Figure1A. First, 2 populations of HSCs were isolated from mouse bone marrow cells by FACS based on their cell surface antigen expression: the Lin−/loSca-1+population (A) containing MPP cells, LT-HSCs, and ST-HSCs20; and the Lin−/loc-kit+Thy-1loSca-1+population (B) enriched with LT-HSCs.5 Correspondingly, cDNA libraries A and B were generated from each of these 2 complementary populations of HSCs. Sequence analysis of 200 randomly chosen clones from each library indicated that they are very diverse. Bacterial colonies derived from these HSC cDNA libraries were arrayed onto nylon membranes using the Q-bot robotic system to generate the macroarrays, or high-density cDNA arrays. These macroarrays were hybridized with the cDNA probes generated from committed or mature hematopoietic cell lineages of the thymus, bone marrow, and spleen (lineage-positive probes). Clones that hybridized to the lineage-positive probes were eliminated through the process of negative selection (see “Materials and methods”) and the remaining clones were selected and spotted onto microarray type glass slides. These microarrays were used to analyze the gene expression profiles of HSCs and MPP cells by hybridizing with fluorescent-labeled (Cy3 and Cy5) cDNA probes derived from the sorted cell populations of HSCs and MPP cells. This allowed the identification of genes preferentially expressed in the HSCs and MPP cells, respectively.
Enrichment of genes that are predominantly expressed in hematopoietic stem cells.
(A) A schematic drawing illustrating the strategy of systematic analyses of gene expression in hematopoietic stem cells (HSCs). Bacterial colonies from the 2 HSC cDNA libraries A and B described in the text were spotted in duplicates onto 24 × 24 cm nylon membranes using a Q-bot robotic system (Genetix), generating macroarrays. The macroarray membranes were hybridized with 33P-labeled cDNA probes derived from mature cell lineages described in the text in parallel with the vector-derived probe. Hybridization signals were analyzed using Spotfinder software (see “Materials and methods” for detailed description) and converted to numeric numbers using Microsoft Excel. cDNA clones, showing negative or very weak hybridization signals to the cDNA probes derived from lineage-positive cells but positive to the vector-derived probe, were selected. This process was called negative selection. The negatively selected cDNA clones were analyzed by DNA sequencing, and spotted in duplicate onto microarray slides. Total RNA isolated from RhloLin−/loc-kit+Sca-1+or RhhiLin−/loc-kit+Sca-1+HSCs was used to generate Cy3- and Cy5-labeled cDNA probes, respectively, by RT-PCR. The probes were hybridized to the microarray slides and the data were analyzed using array analysis software. Details of the experiment are described in “Materials and methods.” (B) Northern analysis of unknown genes from cDNA library B. Two micrograms of poly A+ RNA purified from indicated tissues was separated on a 1% agarose-formaldehyde gel, transferred to nylon membrane, and then hybridized with a 32P-labeled probe prepared from each unknown clone. Four representative blots are shown.
Enrichment of genes that are predominantly expressed in hematopoietic stem cells.
(A) A schematic drawing illustrating the strategy of systematic analyses of gene expression in hematopoietic stem cells (HSCs). Bacterial colonies from the 2 HSC cDNA libraries A and B described in the text were spotted in duplicates onto 24 × 24 cm nylon membranes using a Q-bot robotic system (Genetix), generating macroarrays. The macroarray membranes were hybridized with 33P-labeled cDNA probes derived from mature cell lineages described in the text in parallel with the vector-derived probe. Hybridization signals were analyzed using Spotfinder software (see “Materials and methods” for detailed description) and converted to numeric numbers using Microsoft Excel. cDNA clones, showing negative or very weak hybridization signals to the cDNA probes derived from lineage-positive cells but positive to the vector-derived probe, were selected. This process was called negative selection. The negatively selected cDNA clones were analyzed by DNA sequencing, and spotted in duplicate onto microarray slides. Total RNA isolated from RhloLin−/loc-kit+Sca-1+or RhhiLin−/loc-kit+Sca-1+HSCs was used to generate Cy3- and Cy5-labeled cDNA probes, respectively, by RT-PCR. The probes were hybridized to the microarray slides and the data were analyzed using array analysis software. Details of the experiment are described in “Materials and methods.” (B) Northern analysis of unknown genes from cDNA library B. Two micrograms of poly A+ RNA purified from indicated tissues was separated on a 1% agarose-formaldehyde gel, transferred to nylon membrane, and then hybridized with a 32P-labeled probe prepared from each unknown clone. Four representative blots are shown.
To generate the macroarrays of cDNA libraries A and B, 36 000 clones from library A (made from Lin−/loSca-1+ cells) and 18 000 of 7.5 × 106 cDNA clones from library B (made from Lin−/lo c-kit+Thy-1loSca-1+cells) were arrayed onto nylon membranes (Figure 1), and hybridized with 33P-labeled cDNA probes derived from the hematopoietic lineage-positive populations of cells. The image data were analyzed using Spot-Finding software,16 which converted the image intensity of each spot into numerical data. After analyses, 3000 cDNA clones from macroarray A and 2000 clones from macroarray B that were negative or weakly positive were chosen to further construct the microarray. We have analyzed these negatively selected cDNA clones. A sequence comparison of 384 randomly chosen clones after the negative selection indicated that: (1) the percentage of housekeeping gene sequences represented by sequences encoding for mitochondrial and ribosomal proteins was greatly reduced from 25% to 5%; (2) the percentage of unique genes was increased to 60%; and (3) the percentage of novel sequences, which have no significant homologies with known sequences, was increased to 10% at the time the present manuscript was submitted, albeit the percentage decreased with the rapid progress of human and mouse genomic projects (data not shown).
The sequence analysis of the 2000 negatively selected clones from library B provides a glimpse of the gene expression profile of adult bone marrow enriched with HSCs. A partial annotated list of genes identified in cDNA library B is shown in Table1. Numerous gene encoding transcription factors, chromatin modifiers, transmembrane proteins, and signaling molecules were identified. Based on their molecular and cellular roles in other tissues, contexts, or species, these genes might be likely candidates for playing an essential role in stem cell functions. For example, the bmi-1 gene is a Polycomb-group gene that represses expression of Hox genes during development, and regulates cell proliferation and senescence by down-regulating theink4a locus.21 The ink4a locus expresses 2 genes by alternative splicing: p16 is a G1 cyclin inhibitor and p19ARF stabilizes expression of p53 by binding to Mdm2. Constitutive expression of both bmi-1 and telomerase results in immortalization of epithelial cells.22 Telomerase is activated in most immortal cell lines and cancers, and possibly is involved in the regulation of HSC self-renewal.23 The Notch family has been implicated in the determination of HSC fate.24-26 Recently, constitutive expression of the activated form of Notch1 was shown to be able to immortalize pluripotent, cytokine-dependent HSCs.27
HSCs express genes that are shared by other tissues such as brain, muscle, and germ line cells (Table 1). For example, male-enhanced antigen (Mea) specifically expressed in the testis is involved in spermatogenesis.28 Some of the genes we identified are specific to hematopoietic tissues; for example, RGS18 is expressed in LT-HSCs and ST-HSCs as well as monocytes.15 Among unknown genes, there are genes that code for a variety of important functional domains and several genes with no obvious homology to any known functional domains. Some of the other unknown genes were analyzed by Northern hybridization. Of 10 unknown clones tested, 2 were expressed in all tissues (no. 1), 2 were expressed in some tissues (no. 26), and 6 had no detectable signals in any of the tissues examined (no. 8, no. 11). These 6 clones are candidates for HSC-specific genes (Figure 1B).
Previously, Philips and his coworkers described the gene expression profile of Sca-1+AA4.1+c-kit+Lin−/locells isolated from fetal liver HSCs.29 There are a number of genes commonly expressed in both the adult bone marrow HSCs (Lin−/loc-kit+ Thy-1loSca-1hi) generated by this study and the fetal liver HSCs (Sca-1+AA4.1+c-kit+Lin−/lo) generated by Philips et al,29 such as Evi-1, TSA-1, Ramp1, RGS2, ZF216, macroH2A1.2, CD34, Notch1, vascular endothelial ZF1, and calmodulin. (Tables1-3). In comparison to gene expression profiles of neural progenitor cells,30 a number of genes were also found to be expressed in both neural progenitor and hemaptopoietic stem cells. These included histone H2A, interferon-induced factors, guanine triphosphate (GTP)–nucleotide binding protein, nuclear poly(a)-binding protein, GCN5 histone acetyltransferase, and so on, indicating a shared, at least partially, genetic program among HSCs and neural progenitor cells.
Differential gene expression between HSCs and MPP cells
Since Rh staining allows for separation of HSCs into populations enriched for ST-HSC/early progenitor cells and LT-HSCs,9-11 the Lin−/loSca-1+c-kit+ cells were further separated into Rhlo and Rhhipopulations using flow cytometry (Figure2A-B). In order to avoid a potential interference from the intermediate staining of Rh (Rhitm) cells, we chose a symmetrical portion of cells containing either the 15% highest staining or 15% lowest staining for Rh. A competitive repopulation assay was performed to confirm the functional differences between these 2 populations of cells. As Figure 2C shows, the engraftment rate using Rhlo cells is much higher than that using Rhhi cells after transplantation. In addition, the Rhlo cells could support hematopoiesis for up to 6 months after transplantation (Figure 2C) and were able to reconstitute the bone marrow in the secondary transplantation (F. L. and L. L., data not shown). The Rhhi cells, which gave rise to both myeloid and lymphoid lineages, only engrafted the bone marrow for less than 4 weeks (Figure 2C). This result demonstrated that we had obtained 2 distinct cell populations: the Rhlo population of cells were enriched for HSCs and the Rhhi population of cells were enriched for MPP cells.5
Isolation and functional characterization of Rhlo and Rhhi cell populations.
(A) A cartoon showing 2 important populations of cells: LT-HSCs and multipotent progenitors (MPPs). The LT-HSCs can support long-term (up to 6 months) hematopoiesis in competitive repopulation assay. MPP cells can only briefly support (< 4 weeks) hematopoiesis in the same assay. Rhodamine-123 (Rh) was used to separate these 2 populations of cells. (B) Separation of RhloLin−/loSca-1+c-kit+and RhhiLin−/loSca-1+c-kit+cells using flow cytometry. R3: Lin−/loSca-1+c-kit+ cells; R2: Rhlo (15% of cells with the lowest staining); R4: Rhhi (15% of cells with the highest staining). (C) Competitive repopulation assay. Either RhloLin-/loSca-1+c-kit+or RhhiLin-/loSca-1+c-kit+cells (250 cells) derived from the donor Ly5.2 mice were mixed with 4 × 104 Lin−/lo cells from congenic host strain Ly5.1,17 and injected to lethally irradiated (11 Gy [1100 rads]) Ly5.1 mice. Eight mice were used per group. Peripheral blood cells were harvested at different time points after transplantation as indicated. Flow cytometric analyses were performed using different specific cell surface markers (CD3, B220, Mac-1, and Gr-1) together with anti-Ly5.2 antibody. ○ indicates Rhlo lymphoid lineage; ▵, Rhlo myeloid lineage; ●, Rhhi lymphoid lineage; ▴, Rhhi myeloid lineage.
Isolation and functional characterization of Rhlo and Rhhi cell populations.
(A) A cartoon showing 2 important populations of cells: LT-HSCs and multipotent progenitors (MPPs). The LT-HSCs can support long-term (up to 6 months) hematopoiesis in competitive repopulation assay. MPP cells can only briefly support (< 4 weeks) hematopoiesis in the same assay. Rhodamine-123 (Rh) was used to separate these 2 populations of cells. (B) Separation of RhloLin−/loSca-1+c-kit+and RhhiLin−/loSca-1+c-kit+cells using flow cytometry. R3: Lin−/loSca-1+c-kit+ cells; R2: Rhlo (15% of cells with the lowest staining); R4: Rhhi (15% of cells with the highest staining). (C) Competitive repopulation assay. Either RhloLin-/loSca-1+c-kit+or RhhiLin-/loSca-1+c-kit+cells (250 cells) derived from the donor Ly5.2 mice were mixed with 4 × 104 Lin−/lo cells from congenic host strain Ly5.1,17 and injected to lethally irradiated (11 Gy [1100 rads]) Ly5.1 mice. Eight mice were used per group. Peripheral blood cells were harvested at different time points after transplantation as indicated. Flow cytometric analyses were performed using different specific cell surface markers (CD3, B220, Mac-1, and Gr-1) together with anti-Ly5.2 antibody. ○ indicates Rhlo lymphoid lineage; ▵, Rhlo myeloid lineage; ●, Rhhi lymphoid lineage; ▴, Rhhi myeloid lineage.
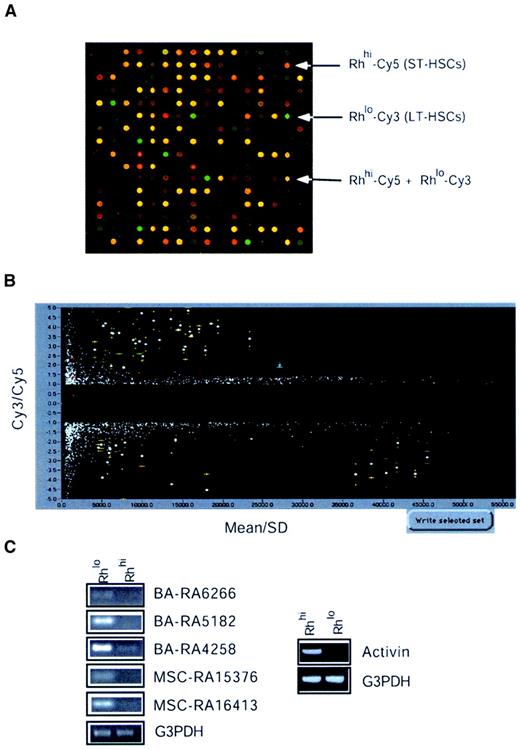
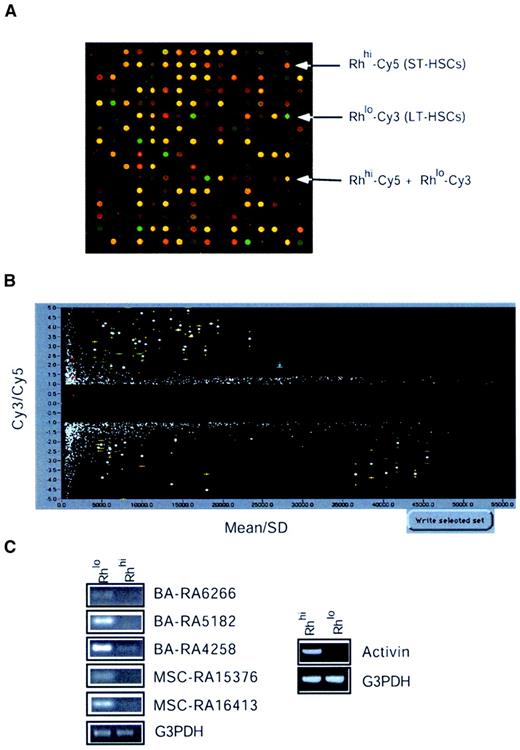
To study differential gene expression among Rhlo and Rhhi cells, we used the microarray glass slides on which the 5000 negatively selected cDNA clones from both libraries A and B had been arrayed in duplicate. These microarrays were hybridized to Cy3- and Cy5-labeled probes generated from Rhlo and Rhhi cells, respectively. Figure3A shows a typical hybridization result showing differentially expressed genes between the Rhlo and the Rhhi cells. Rhlo-Cy3 (green) represents genes that are expressed higher in the enriched HSCs. Rhhi-Cy5 (red) represents genes that are expressed higher in the enriched MPP cells. Yellow represents genes that are expressed in a relatively similar level in both cell populations. Figure 3B shows the results of statistical analysis based on 2 experiments with 4 points (2 points on each slide) representing each cDNA clone, and selection using SpotUnite and SpotSelection software (H. Hammersmark and R.B., see “Materials and methods”). The vertical axis represents the Cy3/Cy5 signal intensity ratio and the horizontal axis was sorted by mean ± standard deviation (sd) (mean indicates average signal intensity of 4 spots for each cDNA insert). We chose cutoff lines that were either 2-fold higher or 2-fold lower for the Cy3/Cy5 intensity ratio. Thus, genes highly expressed in the Rhlo population fell into the upper (positive) region and genes highly expressed in the Rhhi population fell into the lower (negative) region. The spots that fell very close to the left axis represent cDNAs with very weak hybridization signals and, along with spots of significant variation, were ignored. Using this strategy we have identified approximately 30 genes that were highly expressed in the LT-HSCs and around 30 genes that were highly expressed in the MPP cells. These clones were marked with error bars (yellow, Figure 3B). Of these, some were not found in the nonredundant database and therefore were defined as novel or functionally unknown genes (Table 2). To confirm the differential expression of selected genes using microarray analysis, RT-PCR was performed and 15% of the clones were confirmed to be expressed preferentially in HSCs. Activin βC was the only clone from the Rhhi cell group tested that showed predominant expression in MPP cells (Figure 3C).
Microarray analysis of HSC gene expression.
(A) Microarray hybridization and spotfinding. Microarray slides described in Figure 1 were hybridized with cDNA probes prepared from cells. Each cDNA probe was labeled with Cy3 and Cy5 fluorophores separately. A combination of these cDNA probes was hybridized with microarrays A (3000 × 2) and B (2000 × 2). A typical hybridization result was shown using the Cy3-labeled cDNA probe from RhloLin−/loSca-1+c-kit+and the Cy5-labeled probe from RhhiLin−/loSca-1+c-kit+cells. (B) Statistical analysis and selection. A typical example showing the microarray hybridization results obtained from 2 experiments scanned by Genescanner (Molecular Dynamics) and analyzed by SpotUnite and SpotSelection programs (see text for details). (C) RT-PCR assays. To confirm the differential expression of genes between the Rhlo and Rhhi cells, 15% candidate genes were analyzed by RT-PCR as described in “Materials and methods.”
Microarray analysis of HSC gene expression.
(A) Microarray hybridization and spotfinding. Microarray slides described in Figure 1 were hybridized with cDNA probes prepared from cells. Each cDNA probe was labeled with Cy3 and Cy5 fluorophores separately. A combination of these cDNA probes was hybridized with microarrays A (3000 × 2) and B (2000 × 2). A typical hybridization result was shown using the Cy3-labeled cDNA probe from RhloLin−/loSca-1+c-kit+and the Cy5-labeled probe from RhhiLin−/loSca-1+c-kit+cells. (B) Statistical analysis and selection. A typical example showing the microarray hybridization results obtained from 2 experiments scanned by Genescanner (Molecular Dynamics) and analyzed by SpotUnite and SpotSelection programs (see text for details). (C) RT-PCR assays. To confirm the differential expression of genes between the Rhlo and Rhhi cells, 15% candidate genes were analyzed by RT-PCR as described in “Materials and methods.”
The differentially expressed genes fall into a variety of gene families (Table 2). Among the genes that are highly expressed in HSCs are several clones identified as transcription factors. Nfix belongs to the conserved nuclear factor I (NFI) family of transcription/replication proteins. Loss of function of Nfia, one of 4 Nfix family members, causes severe developmental defects, suggesting this family may have distinct roles in vertebrate development.31 Two novel genes that encode a PI3 kinase and a PKA kinase were also identified. The homologue of PKA-like kinase in Schizosaccharomyces pombe, Kin, was found to be important for growth polarity.32 PI3 kinase can be activated by c-kit/stem cell factor receptor signaling and is essential for male fertility.33 Furthermore, PI3 kinase has been known to be essential for cell survival.34 RBM3, an RNA-binding protein, is up-regulated by granulocyte macrophage–colony-stimulating factor (GM-CSF)35 and is closely related to the Y chromosome ribonucleic acid recognition motif (YRRM).36YRRM has been implicated in azoospermia, a male infertility disease, and spermatogenesis.36 This suggests that RBM3 might be involved in the regulation of HSC development. Histone acetyltransferase 1 (HAT1) is also highly expressed in the Rhlo cells. HAT1 belongs to the GCN5-related N-acetyltansferase superfamily and functions to acetylate histone H4, thereby participating in chromatin-related transcriptional regulation.37 Recently HAT1 was found to be involved with telomeric silencing by the transcriptional repression of telomere-proximal genes.38 In contrast, Set oncoprotein, a component of a recently identified protein complex, INHAT, that inhibits the activity of HAT,39 was highly expressed in the Rhhi cell population.
Among the genes that are highly expressed in Rhhi cells is the growth factor Activin βC. Activin βC belongs to the transforming growth factor β (TGF-β) family, which functions in the determination of cell fate and pattern formation during embryogenesis.40 However, the function of Activin βC remains unknown. Other members of the Activin family (A and B) are known to function as dimeric proteins with diverse biologic activities in vertebrate reproduction.41 Expression of Activin βC in the MPP cells suggests that it may be involved in the regulation of HSC differentiation and proliferation. Another gene differentially expressed in the MPP population is Enx-1, a mouse homologue of Drosophila enhancer of zeste and member of the Polycomb family of transcriptional regulators.42 It controls the expression of several homeobox genes. The HoxA9gene was also identified in the Rhhi cells. HoxA9 was found to be involved in translocation t(7;11)(p15;p15), present in acute myeloid leukemia (AML) French-American-British (FAB) classification M2-M4.43 Inactivation ofHoxA9 was shown to have defects in myeloid, erythroid, and lymphoid development in knockout mice.44 Overexpression ofHoxA9 and Meis1 has been shown to induce murine leukemia45 and overexpression of HoxA9 alone can immortalize myeloid progenitors.46
We also identified approximately 70 genes that are expressed in both LT-HSCs and MPP cells (Table 3). Among these is the transcription factor GCN5, a histone acetyltransferase, which interacts with Notch 1 and plays an important role in the recombination recognition sequence binding protein J (RBP-J)–mediated transactivation by Notch 1.47 Loss of GCN5 leads to increased apoptosis and mesodermal defects during mouse development.48 Fiz1, a zinc finger protein with 11 C2H2-type zinc fingers, interacts with receptor tyrosine kinase Flt3.49 Flt3 has been shown to play a role in proliferation and survival of hematopoietic progenitor cells as well as differentiation of early B-lymphoid progenitors, dendritic cells, and natural killer cells.50 TOK-1, a POU-domain–containing transcription factor, is a p21 C-terminal–binding protein and preferentially binds to an active form of cyclin-dependent kinase 2 (CDK2) via p21.51 p21 is a negative cell-cycle regulator and is involved in maintaining the quiescent state of HSCs.52 One of the identified apoptosis-related molecules is survivin. Survivin is an inhibitor of apoptosis and is overexpressed in various human cancers but undetectable in normal tissues.53 Overexpression of survivin resulted in an accelerated S phase shift, resistance to G1 arrest, and activated Cdk2/Cyclin E complex leading to Rb phosphorylation.54
Discussion
The mechanisms that regulate the self-renewal of HSCs are largely unknown. The deterministic model of hematopoiesis suggests that HSC self-renewal is autonomous; thus, expression of specific sets of genes could determine the stem cell fate. Therefore, a systematic approach was used to study differential gene expression profiles in adult HSCs. The ability to isolate a cell population enriched with HSCs allowed us to study the gene expression profile of HSCs. Using macroarray and microarray techniques, we were able to obtain a snapshot of genes expressed by enriched populations of HSCs as well as MPP cells. The identification of multiple genes that regulate proliferation, cell survival, immortalization, and differentiation gives new insights into HSC functions. This is important not only to study their biologic behaviors but also for potential clinical applications. For example, the efficacy of treatment modalities for cancer, such as radiation therapy and many chemotherapy agents, is constrained by dose-limiting bone marrow toxicity. Therefore, the ability to isolate HSCs with self-renewal and repopulating potentials, in vitro amplification of these cells, and transplantation without tumor cells would greatly increase the success rate of current cancer therapies.
Ex vivo–expanded stem cells have been extensively tested for possible use in transplantation. Soluble jagged-1, a Notch ligand, induced the survival and expansion of human stem cells with multipotent repopulating capacity.55 The presence of Notch 1 in the HSC library suggests that these proteins may play a role in self-renewal or maintaining identity of HSCs. Although various growth factors can induce stem cells to proliferate in vitro, they result in concomitant differentiation. The function of stem cell factor (SCF) has been known as a hematopoietic stem cell survival factor.56Recently, it has also been shown that the in vitro culture of the HSCs, in the presence of both SCF and thrombopoietin (TPO), induced self-renewal cell division in which only one of the daughter cells had self-renewal potential.57 Asymmetric cell division requires unequal segregation of cell-fate determinants, such as mRNAs and proteins, which are important to maintain self-renewal and repopulating potentials, during mitosis. This type of asymmetric cell division is also observed in germline cell division. DuringDrosophila oogenesis, a germline stem cell divides asymmetrically to produce a daughter stem cell and a cytoblast, which further divides to eventually produce oocytes. This process is controlled by several factors including decapentaplegic (DPP)58 as well as by intrinsic mechanisms involving pumilio, nanos, arrest, and bag-of-marbles. Expression of BMP4, the mammalian homologue of DPP, in HSCs (Table 1) suggests that BMP4 may also play a similar role.
The differential gene expression pattern between Rhloand Rhhi indicated that some of the genes were either HSC- or MPP-specific (Table 2), and many of them were expressed in both populations of cells (Table 3). Some of the MPP highly expressed genes included Activin βC, nuclear molecules (PWP-1, Npm1, and karyopherin), TAX-responsive factor, and inhibitor of CDC42. CDC42 was reported to play a role in controlling HSC shape, adhesion, migration, and mobilization.59 Genes that are preferentially expressed in HSCs include transcription factors, RNA-binding proteins, chromatin modifiers, and protein kinases, many of which are involved in developmental regulation. The HSC preferentially expressed genes might be candidate genes that play a role in stem cell self-renewal.
In summary, cDNA libraries were generated from mouse HSCs with long-term reconstituting potential and from progenitor cells, and the differential gene expression patterns were studied using bioinformatics and array technologies. Although the microarray system has been known for its tendency to lose less abundant mRNAs due to limited resolution, our approach overcomes this tendency by removing cDNAs that show high hybridization signals using macroarray and thereby enriching for cDNAs derived from less-abundant mRNAs. On the other hand, our subtraction technique using cDNA probes derived from mature populations of cells may lead to a loss of molecules that are expressed in multiple developmental stages. The systematic approach used in this study provides an effective and efficient method for identification of genes that are specifically expressed in HSCs with self-renewal capability. This study will therefore provide a fundamental tool for identifying important candidate genes involved in the regulation of self-renewal and expansion of HSCs.
We thank G. van den Engh, K. Allen, and D. Corden for the assistance of cell sorting. We thank E. Hammersmark for the assistance on microarray analysis software. We appreciate V. Ng and X. Tao for their assistance on analysis of Est sequences. We thank A. Banta for the involvement of macroarray work. We are grateful to Drs G. Vassilopoulous, D. Russel, and N. Wolf for the consultation on the bone marrow transplantation and repopulation assay. We appreciate the help of R. Krumlauf, B. Steenhard, and D. Stenger on the manuscript proofreading. We are particularly grateful to X. He, J. Zhang, and C. Niu for their work on the reanalysis of cDNA sequences listed in the tables.
Supported in part by National Institutes of Health grant 1P01 DK53074-02 and Stowers Institute for Medical Research.
I.-K.P. and Y.H. contributed equally to this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Linheng Li, Stowers Institute for Medical Research, 1000 E 50th St, Kansas City, MO 64110; e-mail:lil@stowers-institute.org.

![Fig. 2. Isolation and functional characterization of Rhlo and Rhhi cell populations. / (A) A cartoon showing 2 important populations of cells: LT-HSCs and multipotent progenitors (MPPs). The LT-HSCs can support long-term (up to 6 months) hematopoiesis in competitive repopulation assay. MPP cells can only briefly support (< 4 weeks) hematopoiesis in the same assay. Rhodamine-123 (Rh) was used to separate these 2 populations of cells. (B) Separation of RhloLin−/loSca-1+c-kit+and RhhiLin−/loSca-1+c-kit+cells using flow cytometry. R3: Lin−/loSca-1+c-kit+ cells; R2: Rhlo (15% of cells with the lowest staining); R4: Rhhi (15% of cells with the highest staining). (C) Competitive repopulation assay. Either RhloLin-/loSca-1+c-kit+or RhhiLin-/loSca-1+c-kit+cells (250 cells) derived from the donor Ly5.2 mice were mixed with 4 × 104 Lin−/lo cells from congenic host strain Ly5.1,17 and injected to lethally irradiated (11 Gy [1100 rads]) Ly5.1 mice. Eight mice were used per group. Peripheral blood cells were harvested at different time points after transplantation as indicated. Flow cytometric analyses were performed using different specific cell surface markers (CD3, B220, Mac-1, and Gr-1) together with anti-Ly5.2 antibody. ○ indicates Rhlo lymphoid lineage; ▵, Rhlo myeloid lineage; ●, Rhhi lymphoid lineage; ▴, Rhhi myeloid lineage.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.488/6/m_h80222024002.jpeg?Expires=1767755905&Signature=X8YaxOCuVxaWPKZVNDA3Dwsncre4drcjd7T-laK0FcQk1OTk3QZ56~h4ggWnw~OZDvgtom5n6DHb7V7i4nQDsM3nOoyvxv6KGHgflo3KWVkQNi5Do13rHo~vBdCiwi9d7CY4Vd4zgVcFi-hS1RmC8OdhganY1SPqm8ZwasW2k4Mb05nCISqv0SvPRgpE-6JQxnMHKoIuZqZtc0Eqszbv6YJ3Xo0o8IZR6EcxgJweyxiE47i7fkMrMZljAkSpHnbQbSYnput5Q4x7GxBT9F3YEsIZYy7Vi45rBhHkarwpwCWzUHP7quvUroiZ6XvGqownecGXFtgBK3y7PmuEcyGTEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Isolation and functional characterization of Rhlo and Rhhi cell populations. / (A) A cartoon showing 2 important populations of cells: LT-HSCs and multipotent progenitors (MPPs). The LT-HSCs can support long-term (up to 6 months) hematopoiesis in competitive repopulation assay. MPP cells can only briefly support (< 4 weeks) hematopoiesis in the same assay. Rhodamine-123 (Rh) was used to separate these 2 populations of cells. (B) Separation of RhloLin−/loSca-1+c-kit+and RhhiLin−/loSca-1+c-kit+cells using flow cytometry. R3: Lin−/loSca-1+c-kit+ cells; R2: Rhlo (15% of cells with the lowest staining); R4: Rhhi (15% of cells with the highest staining). (C) Competitive repopulation assay. Either RhloLin-/loSca-1+c-kit+or RhhiLin-/loSca-1+c-kit+cells (250 cells) derived from the donor Ly5.2 mice were mixed with 4 × 104 Lin−/lo cells from congenic host strain Ly5.1,17 and injected to lethally irradiated (11 Gy [1100 rads]) Ly5.1 mice. Eight mice were used per group. Peripheral blood cells were harvested at different time points after transplantation as indicated. Flow cytometric analyses were performed using different specific cell surface markers (CD3, B220, Mac-1, and Gr-1) together with anti-Ly5.2 antibody. ○ indicates Rhlo lymphoid lineage; ▵, Rhlo myeloid lineage; ●, Rhhi lymphoid lineage; ▴, Rhhi myeloid lineage.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.488/6/m_h80222024002.jpeg?Expires=1767861346&Signature=uIwuktL4UZmsfPEu1u-JrEX2YWScUTIKarKJ9TI6fStIBJJIxmcbaShEeR9FlGvH1a8QUNv7CIP6qZPEHmDeXtZfSvZdnh7B~8ssFCxTc~i6oc68AsfSFWCUoVwZlKobM4RfLAB9bdZ2na-J0GwBJ7Trxrx8LUVApY7Srr8dbctQb91JnkAopHJMsnYeY7SU4OLLz24UCdR9QQCXYu29WYHfFVTt~rmPDCOvzLhGuvW-phPIWyM5XMdetppVK55LseDQCWGd0gRYPAlo-cWY1GVE~Eep7rDp1-1PdlG3hoFEonpiVf7Dxi4O2bwtV6L7ozBmixGzRguxZa0u-kdNPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)