Abstract
Signal transducers and activators of transcription (STATs) are intracellular mediators of cytokine receptor signals. Because many early-acting growth factors have been implicated in STAT5 activation, this study sought to investigate whether STAT5 may be a transcriptional regulator of hematopoietic stem cell (HSC) long-term repopulating activity. To test this possibility, bone marrow (BM) and fetal liver (FL) cells from mice containing homozygous deletions of both STAT5a and STAT5b genes (STAT5ab−/−) were characterized for hematopoietic repopulating activities. BM and FL grafts were capable of repopulating lymphoid and myeloid lineages of lethally irradiated primary and secondary hosts, with defects observed primarily in T-lymphocyte engraftment. Because only a fraction of normal HSC function is required to reconstitute hematopoiesis, competitive repopulation assays of adult BM or FL cells were used against wild type adult BM or FL cells to quantitate stem cell function. In these analyses, average 25-, 28-, 45-, and 68-fold decreases in normal repopulating activity were evident in granulocyte (Gr-1+), macrophage (Mac-1+), erythroid progenitor (Ter119+), and B-lymphocyte (B220+) populations, respectively, with T lymphocytes (CD4+) always undetectable from the STAT5ab−/− graft. Consistent with previous reports of divergence between stem cell phenotype and function in cases of perturbed hematopoiesis, the absolute number of cells within Sca-1+c-kit+lin− or lin− Hoechst 33342 side population fractions was not significantly different between wild type and STAT5ab−/−BM or FL cells. These results demonstrate that a significant proportion of the growth factor signals required for multilineage reconstitution potential of HSCs is STAT5 dependent.
Introduction
The functional roles of janus kinases (JAKs) and signal transducers and activators of transcription (STATs) have been characterized in both hematopoietic and nonhematopoietic tissues.1 The JAK/STAT signaling axis allows a diverse set of extracellular signals to result in modification of gene expression patterns in the appropriate target cells. This process is initiated when, on cytokine binding, JAKs come into close proximity and become activated by autophosphorylation on tyrosine residues. Activated JAKs then phosphoryate among many other substrates, the STAT proteins. STATs form homodimers or heterodimers via SH2 domain phosphotyrosine interactions and translocate to the nucleus where they bind to transcriptional elements on DNA.2,3 Activated STAT proteins regulate their target genes often with tissue specificity. In the hematopoietic system, JAK/STAT signaling has been extensively described in a variety of lymphoid and myeloid cell types. Four mammalian JAKs and 7 STAT proteins have been identified and characterized by generation of knockout mice.4 These studies have revealed a large diversity in the role of STATs in hematopoiesis with some STATs showing phenotypes primarily in restricted cytokine pathways (eg, interferons, interleukin [IL]-4, IL-12, and IL-13) that are primarily involved in immune responses (STAT1, STAT2, STAT4, and STAT6). In contrast, the complex phenotypes of STAT3- and STAT5-deficient mice demonstrate a broad cytokine activation and function profile for many individual cell lineages.
STAT5a and STAT5b are 2 very homologous transcription factors with variability between these 2 proteins being primarily in the transactivation domain. Mice deficient in either STAT5a or STAT5b have been generated and characterized. The STAT5a knockout mouse is characterized by defects in responses to granulocyte-macrophage colony-stimulating factor (GM-CSF)5 and mammary gland development.6 The STAT5b knockout mouse is characterized by defects in growth hormone signaling and expression of male-characteristic liver gene expression patterns.7 To study the effects of STAT5 on hematopoiesis and also to eliminate any compensating function between the 2 STAT isoforms, homozygous mutant mice lacking both STAT5a and STAT5b were generated. These female mice were infertile, but heterozygous mice could breed normally and give viable STAT5ab−/− mice, but at a lower than expected frequency because of various phenotypes affecting survival. An important role for STAT5a and STAT5b in T-cell function8,9and terminal myeloid differentiation/apoptosis10 has been shown by using tissues from these STAT5ab−/− mice. Comparable results have also been obtained in cytokine-inducible SH2-containing protein-1 transgenic mice.11 Somewhat surprisingly, the peripheral hematology of the STAT5ab−/−mice was not dramatically altered, although reductions in the numbers of IL-3– and GM-CSF–responsive myeloid progenitors12 and IL-7– responsive B-lymphoid12,13 progenitors have been reported. A requirement for STAT5 in erythropoietin (EPO) signaling was not evident in adult mice, which showed normal numbers of EPO-dependent colonies.12 With age, these mice developed an activated T-cell phenotype and an extensive erythroid infiltration leading to splenomegaly and they have a reduced life span.9 This previous work demonstrated both redundant functions and highly specific functions for STAT5 in committed hematopoietic progenitor cells.
Many of the growth factors reported to activate STAT514-21have been shown to provide a survival signal for primitive hematopoietic cells during ex vivo culture. For example, IL-3, IL-6, stem cell factor (SCF),22,23 Flt3 ligand,24and thrombopoietin (TPO)25-28 have been shown to stimulate primitive hematopoietic cells to proliferate ex vivo when present at the optimal concentrations and combinations. Tyrosine phosphorylation of STAT5 in megakaryocytes and platelets after TPO treatment has been well characterized.29-32 Therefore, given the activity of TPO on multipotential cells and the stem cell–repopulating defect in mice lacking the receptor for TPO (c-mpl),33-36 we were interested in studying the role of STAT5 in the stem cell compartment. Furthermore, TPO is known to be synergistic with other early-acting cytokines such as SCF and Flt3 ligand.27 For applications such as ex vivo retroviral gene transfer using hematopoietic cytokines, STAT5 activation may also be required for the synergistic effects of growth factor stimulation. The requirement for STAT5 as a common signaling intermediate in primitive levels of hematopoiesis had not been previously studied. Therefore, we have tested the hypothesis that a block in signal transduction pathways involving STAT5 could result in decreased stem cell activity in hematopoietic cells from STAT5ab−/− mice. These studies revealed a requirement for STAT5 activation in long-term multilineage reconstitution after hematopoietic cell transplantation.
Materials and methods
Mice and genotyping
The STAT5 mouse colony (C57Bl/6; Ly-5.2) was maintained by crossing heterozygote STAT5ab+/− mice to yield viable STAT5ab−/− pups at a low ratio of only 1:15-20 surviving at 4 weeks of age. Mice were genotyped by polymerase chain reaction (PCR) of tail DNA as described.12 After PCR, the products were separated on a 2% agarose gel and stained with SYBR Gold (Molecular Probes, Eugene, OR) and visualized with the use of a Storm phosphorimager (Molecular Dynamics, Sunnyvale, CA). The congenic mice B6.C-H1b/By (HW80) and B6.SJL-PtprcaPep3b/BoyJ (Ly-5.1) were obtained from The Jackson Laboratory (Bar Harbor, ME).
Mouse peripheral blood hematology
Peripheral blood was obtained after puncture of the retroorbital venous sinus by using a microcapillary tube. Peripheral blood smears were prepared and stained with a HEMA3 Xanthene/Thiazine dye set (Fisher Scientific, Pittsburgh, PA) and analyzed by light microscopy for the differential percentages of granulocytes, lymphocytes, and monocytes. Duplicate microcapillary tubes were spun in a microcentrifuge (Stat-Spin, Norwood, MA) and hematocrits were read manually. For white blood cell counts, cells were diluted in isotonic saline solution and analyzed by using a Coulter counter (Beckman Coulter, Miami, FL) and/or a Serono-Baker 9700 hematology analyzer (Serono-Baker, Allentown, PA).
Bone marrow/fetal liver cell collection and spleen colony-forming unit assays
Bone marrow (BM) was harvested from both hind limbs (tibias and femurs) of either STAT5ab−/− or littermate wild-type mice. BM cells were flushed into phosphate-buffered saline containing 2% fetal bovine serum (Hyclone, Logan UT) and counted using a hemacytometer. Embryonic day 14.5 fetuses were collected after the pregnant females were humanely killed, and the liver cells were removed with a pair of sterile microforceps, dispersed with a 21-gauge needle, and resuspended in phosphate-buffered saline containing 2% fetal bovine serum. A small fraction (1:20) of the fetal liver (FL) cells was then used the same day for STAT5 genotyping by PCR as described above. The total number of BM cells obtained from each mouse was used for calculation of the absolute number of spleen colony-forming units (CFU-Ss) in the BM based on the frequency. CFU-S assays were performed by using BM cells pooled from either 2 wild-type or STAT5ab−/− mice. CFU-Ss were harvested 12 days after injection of standard doses of 5 × 104 or 1 × 105 cells via the lateral tail vein into recipient mice irradiated with 900 rads of gamma radiation 2 to 4 hours earlier (137Cs source; Mark I-68A model irradiator, J. L. Shepherd and Associates, San Fernando, CA). Manual scoring of CFU-S colonies was aided by the use of a StereoZoom 6 Plus dissecting microscope (Leica Microsystems, Buffalo, NY). Preliminary characterization of CFU-S numbers in STAT5ab−/− mice was performed with 1 × 104, 5 × 104, 1 × 105, and 1 × 106 cells to find the linear range. The numbers of CFU-Ss per spleen for the data in Figure1B ranged from 4 to 10 using the 1 × 105 cell dose. A dose dependence on colony formation was observed for all 3 experiments.
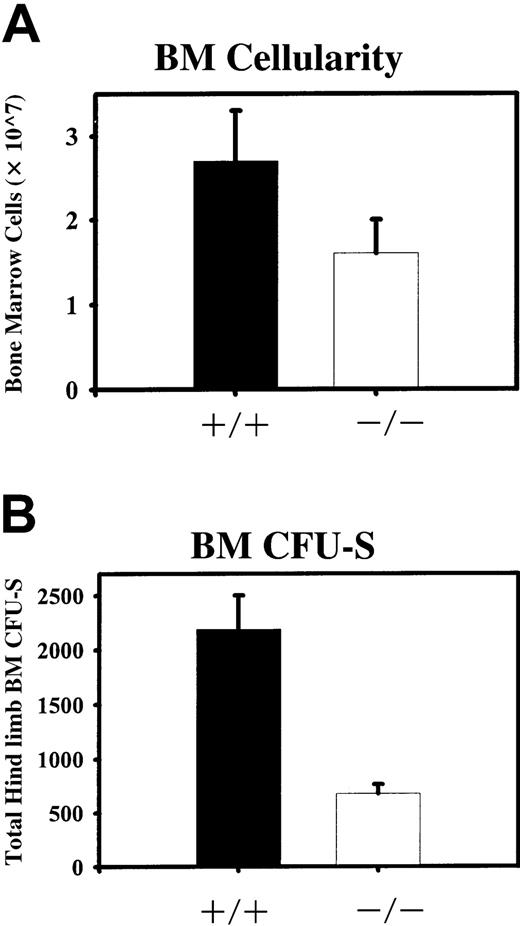
Quantitation of absolute numbers of BM cells and CFU-Ss from both hind limbs of STAT5ab−/− and wild type mice.
(A) BM cellularity was determined after flushing both tibias and femurs from individual mice into phosphate-buffered saline/2% fetal bovine serum. Red blood cells were lysed in 2% acetic acid and nucleated cells were counted with the use of a hemacytometer. Shown is the average BM cellularity from analysis of wild-type (+/+) and STAT5ab−/− (−/−) mice (n = 13). (B) Total BM cells were injected into irradiated mice (900 rads; 137Cs source) at doses of 5 × 104 or 1 × 105 per recipient. Twelve days later the mice were killed and the spleens were collected. The number of CFU-Ss per spleen was determined by manual scoring using a dissecting microscope. The total number of injected cells was then divided by the number of CFU-Ss per spleen to calculate the ratio of CFU-Ss per number of injected cells (CFU-S frequency). The total number of BM cells was then multiplied by the CFU-S frequency to calculate the total number of BM CFU-Ss present in both hind limbs. Shown is analysis of either wild type (+/+) or STAT5ab−/−(−/−) mice groups from 3 separate comparisons.
Quantitation of absolute numbers of BM cells and CFU-Ss from both hind limbs of STAT5ab−/− and wild type mice.
(A) BM cellularity was determined after flushing both tibias and femurs from individual mice into phosphate-buffered saline/2% fetal bovine serum. Red blood cells were lysed in 2% acetic acid and nucleated cells were counted with the use of a hemacytometer. Shown is the average BM cellularity from analysis of wild-type (+/+) and STAT5ab−/− (−/−) mice (n = 13). (B) Total BM cells were injected into irradiated mice (900 rads; 137Cs source) at doses of 5 × 104 or 1 × 105 per recipient. Twelve days later the mice were killed and the spleens were collected. The number of CFU-Ss per spleen was determined by manual scoring using a dissecting microscope. The total number of injected cells was then divided by the number of CFU-Ss per spleen to calculate the ratio of CFU-Ss per number of injected cells (CFU-S frequency). The total number of BM cells was then multiplied by the CFU-S frequency to calculate the total number of BM CFU-Ss present in both hind limbs. Shown is analysis of either wild type (+/+) or STAT5ab−/−(−/−) mice groups from 3 separate comparisons.
Antibody/fluorescent dye staining and flow cytometry
BM cells were flushed from both hind limbs of STAT5ab−/− and wild-type mice. BM cells were stained with a cocktail of antibodies to phycoerythrin (PE)–conjugated lineage markers that included Ly-6G (Gr-1), CD11b (Mac-1), CD45R/B220, CD4 (L3T4), CD8 (Ly-2), NK1.1 (NKR-P1B and NKR-P1C), and Ter119/Ly-76. The cells were also stained with antibodies to fluorescein isothiocyanate (FITC)–conjugated Ly-6A/E (Sca-1) and biotin-conjugated CD117 (c-kit). The biotinylated c-kit antibody was detected by using a secondary streptavidin-PE Cy5 conjugate. FL cells were collected as described above and were stained with the same lineage cocktail except that CD11b (Mac-1) was not included.37 All antibodies for these studies were obtained from PharMingen (San Diego, CA). For flow cytometric analysis, cells were stained with 5 μg/mL Hoechst 33342 as previously reported38 before antibody staining on ice. Cells were then analyzed by using a FACSVantage SE flow cytometer (BD Biosciences, San Jose, CA) equipped with the Enterprise IIC laser, providing both 488 nm and UV (351-364 nm) excitation. The primary beam was tuned to 488 nm at 100 mW for light scatter triggering, and the second beam was tuned to UV at 30 mW for Hoechst 33342 excitation. Fluorescence emission from bound Hoechst 33342 dye was measured at 2 wavelengths, blue fluorescence emission detected with a 424/44 bandpass filter and red fluorescence emission with a 675 longpass filter. Separation of the 2 signals was achieved with a 640-nm longpass dichroic filter. Linear signals from both red and blue fluorescence channels were collected and used to produce typical histograms for identification of side-population (SP) cells. Small debris was excluded from the analysis by electronic gating of the forward versus orthogonal light scatter data. The SP cell gate was defined according to normal C57Bl/6 BM and was similar to that reported38 39The PE-conjugated lineage antibodies described also including Thy1.2 antibody were used for staining peripheral blood leukocyte populations in mice that received transplants. The percentage of Ly-5.2 donor engraftment in Ly-5.1 recipient mice was quantitated by using a FITC-conjugated CD45.2 (anti-Ly-5.2) antibody in combination with the PE-conjugated lineage antibodies.
Competitive repopulation assays
BM cells were freshly harvested from both hind limbs of STAT5ab−/− and littermate wild type mice. FL cells were freshly harvested from embryonic day 14.5 fetuses and were genotyped the same day by PCR. Cells were then mixed thoroughly at either 1:1 or 4:1 donor equivalent ratios with HW80 or Ly-5.1 BM or FL cells collected at the same time. The cell mixtures were then injected via the lateral tail vein into lethally irradiated (1100 rads) recipient mice. The minimum cell dose injected was 1.5 to 2 × 106cells for each competed BM graft and 3 to 5 × 105 cells for each FL graft. The average mixing ratio was 4.7 × 106 wild-type BM cells versus 2.1 × 106 STAT5ab−/− BM cells, because the BM cellularity for the STAT5ab−/− mice was reduced. For FL experiments, the average competed cells for wild- type and STAT5ab−/− was not different because FL cellularity was not reduced (see “Results” section). Beginning at 8 weeks after transplantation, mice were bled from the retroorbital venous plexus. Hemoglobin patterns were analyzed from packed peripheral red blood cells by electrophoresis on cellulose acetate gels. To calculate the relative proportions of single and diffuse donor hemoglobin in peripheral blood from reconstituted mice,40 the hemoglobin gels were digitized by using a ScanJet IIcx/T scanner (Hewlett Packard, Palo Alto, CA). Data files were quantitated by densitometry using ImageQuant software (Molecular Dynamics). For experiments in which donor grafts were competed by using the Ly-5.1/Ly-5.2 system, mice were analyzed by flow cytometry as described above.
Secondary BM transplantations
BM was harvested from primary recipients at times up to 6 months after transplantation and injected via the lateral tail vein into lethally irradiated secondary recipients (1100 rads). Secondary transplanted mice received at least 5 × 106 BM cells each. Hemoglobin electrophoresis patterns were monitored in secondary recipients after reconstitution (4 months). For some analyses, the recipient mice were Ly-5.1 and engraftment was determined by FACS.
Southern blot analyses
Genomic DNA was prepared from peripheral blood, BM, and spleen cells from mice that received transplants by digestion with 0.6 mg/mL proteinase K in 50 mM Tris pH 8.0, 1% sodium dodecyl sulfate, 100 mM NaCl, and 10 mM EDTA pH 8.0. DNA was then extracted with an equal volume of phenol/chloroform/isoamyl alcohol (25:24:1) and precipitated with 2.5 volumes ice-cold ethanol and one-tenth volume sodium acetate. DNA (5-10 μg) was digested overnight with EcoRI and separated on a 0.8% agarose gel by electrophoresis. Gels were blotted overnight onto Hybond N+ nylon membrane (Amersham, Arlington Heights, IL), UV cross-linked, and hybridized with a [32P]-labeled fragment of the mouse β-globin intervening sequence 2. Blots were washed at a final stringency of 0.5 × SSC/0.5% sodium dodecyl sulfate at 65°C and exposed overnight, and autoradiographic images were obtained by using a Molecular Dynamics Storm phosphorimager and x-ray film (Eastman Kodak, Rochester, NY). [32P]-(deoxycytidine triphosphate) dCTP was obtained from Amersham.
Results
STAT5ab−/− mice have reduced absolute numbers of total cells and CFU-Ss in the BM
To perform transplantation studies to assess the role of STAT5 in HSC function, we first backcrossed C57Bl/6 × 129/Sv chimeric STAT5ab+/− mice 8 generations onto the C57Bl/6 background. Heterozygote mice were then crossed to yield homozygous double- knockout mice for analysis. The survival of homozygous mutant mice on the C57Bl/6 background was very low, with 4-week survival of approximately 5% of all mice genotyped. We have used the C57Bl/6 STAT5ab−/− mice to further characterize the hematopoietic defects relative to normal littermate wild-type mice and to determine whether reduced long-term repopulating activity results from loss of STAT5. The peripheral blood hematology of the backcrossed mice is shown in Table 1. The peripheral white blood cell counts were decreased because of a reduced percentage of lymphocytes, and the hematocrits were near normal, except in aged mice (> 3 months old) that developed splenomegaly and were not analyzed. A mild increase in the absolute neutrophil count and absolute monocyte count was also noted. This result is similar to our previous observations in JAK3−/− mice, suggesting a compensatory mechanism for a decreased absolute lymphocyte count. Not shown in Table1, we also determined the absolute red blood cell and platelet counts by using the hematology analyzer for mice that did not receive transplants. The red blood cell numbers were 67% of normal in the STAT5ab−/− mice (wild type [8.2 ± 1.0 × 106/μL] versus STAT5ab−/− [5.5 ± 0.8 × 106/μL] n = 6, P = .004). The platelet numbers were 40% of normal in the STAT5ab−/− mice (wild type [1288 ± 271 × 103/μL] versus STAT5ab−/− [516 ± 107 × 103/μL] n = 6,P < .001).
Steady-state hematology of STAT5ab−/−C57Bl/6 mice
| Hematologic parameter* . | STAT5ab+/+ . | STAT5ab−/− . | % Control . | P . |
|---|---|---|---|---|
| White blood cell/μL | 10 044 ± 1 975 | 4 540 ± 1 606 | 45 | < .005 |
| Absolute lymphocyte count/μL | 8 387 ± 1 656 | 1 087 ± 518 | 13 | < .005 |
| Absolute neutrophil count/μL | 1 163 ± 265 | 2 237 ± 841 | 192 | < .005 |
| Absolute monocyte count/μL | 495 ± 188 | 1 226 ± 874 | 248 | .09 |
| Hematocrit | 46 ± 3 | 35 ± 3 | 76 | < .01 |
| Hematologic parameter* . | STAT5ab+/+ . | STAT5ab−/− . | % Control . | P . |
|---|---|---|---|---|
| White blood cell/μL | 10 044 ± 1 975 | 4 540 ± 1 606 | 45 | < .005 |
| Absolute lymphocyte count/μL | 8 387 ± 1 656 | 1 087 ± 518 | 13 | < .005 |
| Absolute neutrophil count/μL | 1 163 ± 265 | 2 237 ± 841 | 192 | < .005 |
| Absolute monocyte count/μL | 495 ± 188 | 1 226 ± 874 | 248 | .09 |
| Hematocrit | 46 ± 3 | 35 ± 3 | 76 | < .01 |
STAT indicates signal transducers and activators of transcription.
Sample sizes consisted of 5 to 6 mice per group, analyzed between 4 and 6 weeks of age. Mice used for analysis appeared to be healthy and did not show signs of sickness related to splenomegaly disease. The % control is calculated for the average STAT5ab−/− relative to the STAT5ab+/+hematology values. P values were calculated by using the Student 2-tailed t test.
For our studies, BM was collected from young mice (4-6 weeks) to avoid age-related disease and death. Note that, because of the young age of these mice, the BM cellularity from the wild type mice was slightly lower than that typical of older mice. BM was harvested from both hind limbs and, relative to littermate wild type mice, STAT5ab−/− mice showed a 30% to 50% reduction in the total number of BM cells (Figure 1A; n = 13, P < .001). To quantitate a heterogenous in vivo colony-forming population from STAT5ab−/− mice, we scored the absolute number of day 12 CFU-Ss in both hind limbs after transplantation into irradiated mice. The absolute number of CFU-Ss in the BM of STAT5ab−/−mice was significantly reduced by 3.2-fold relative to wild-type mice (Figure 1B; n = 3 experiments, P = .001). These results indicate that BM multipotent progenitor activity is dependent on cytokine signaling pathways that use STAT5.
STAT5ab−/− mouse BM and FL have a normal stem cell phenotype and can repopulate mice that have undergone transplantation
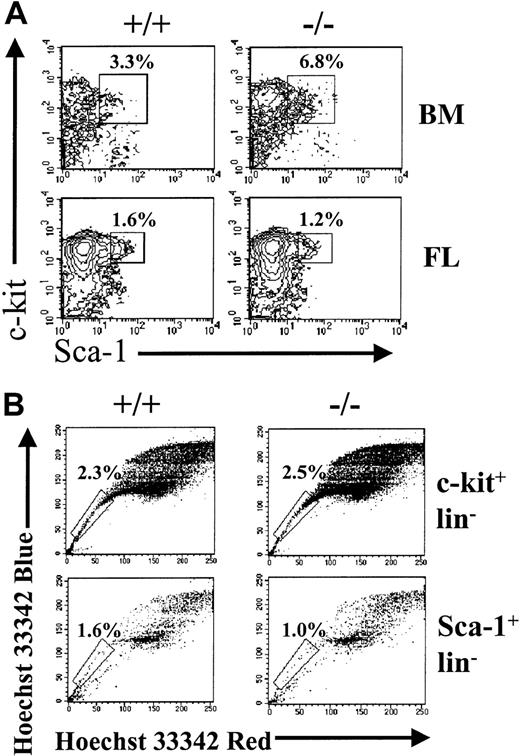
We wanted to determine whether the absolute number of cells expressing phenotypic markers of primitive hematopoietic cells was changed. We analyzed BM and FL populations for the presence of cells that are Sca-1+c-kit+lin− (KLS) (Figure 2A). The absolute number of these cells was not different between wild-type and STAT5ab−/−mice in either the BM or FL. The absolute number of KLS cells in the FL (7218 ± 2234, n = 4) relative to normal littermate FL (9746 ± 2407, n = 5) was unchanged (P = .15). Accordingly, the percentage of FL KLS cells (wild type [0.25 ± 0.05%, n = 5] versus STAT5ab−/−[0.24 ± 0.07%, n = 4]) and the FL cellularity (wild type [2.1 ± 0.74 × 106, n = 11] versus STAT5ab−/− [2.1 ± 0.75 × 106, n = 9]) did not differ between wild-type and STAT5ab−/− mice (P = .81 and 1.0, respectively). The adult BM from these mice also showed a normal absolute number of KLS cells despite a reduction in total BM cellularity (Figure 1). The absolute number of hind limb KLS cells was quantitated at 23 010 ± 19 950 in STAT5ab−/− mice (n = 5) relative to 16 754 ± 12 566 for wild type mice (n = 6), which was not significantly different (P = .54). For the FL samples, a second method was used that was based on Hoechst 33342 dye efflux (Figure 2B). The SP cells reported after dual-emission wavelength analysis has been shown to be enriched for repopulating cells, but they also contain lineage-positive cells such as natural killer cells and erythroid progenitors (Ter119+).41 42 We found that 60% to 70% of BM KLS cells fall within the SP cell gate; therefore, significant overlap occurs between these 2 parameters. The c-kit+lin− SP and Sca-1+lin− SP cell populations were quantitated from both wild-type and STAT5ab−/− FL cells. No significant difference between wild-type and STAT5ab−/− FL SP cell percentage was found by using either positive selection for c-kit or Sca-1. The c-kit+lin− SP number was 15 120 ± 4366 in wild-type mice (n = 4) and 17 640 ± 8250 in STAT5ab−/− mice (n = 4), which was not significant (P = .61). The Sca-1+lin− SP number was much lower and included only 420 ± 148 cells in wild-type mice (n = 5) and 210 ± 75 cells in STAT5ab−/− mice (n = 4, but the difference between wild-type and STAT5ab−/− mice was significant (P = .04). However, because the Sca-1+lin− SP fraction was much lower than the c-kit+lin− SP fraction, the total numbers of STAT5ab−/− SP cells were not reduced.
Flow cytometry analysis of BM or FL cells from STAT5ab−/− and wild- type mice for Sca-1+c-kit+lin− or lin− SP fractions.
Total BM cells were harvested from individual STAT5ab−/−or wild-type mice. FL cells were collected from pregnant females that were mated 14.5 days earlier. The cells were then blocked with normal mouse serum, stained with antibody cocktails, and analyzed by FACS. (A) Shown are representative FACS profiles for lineage-negative BM or FL cells, analyzed for coexpression of Sca-1 (FITC) and c-kit (PECy5). (B) Shown is analysis of FL cells stained first with Hoechst 33342, washed, and next stained with antibodies to cell surface markers. Representative FACS profiles for c-kit+lin−SP and Sca-1+lin−SP cells are shown. The percentages marked represent the c-kit+lin− or Sca-1+lin− cells falling within the SP region (box).
Flow cytometry analysis of BM or FL cells from STAT5ab−/− and wild- type mice for Sca-1+c-kit+lin− or lin− SP fractions.
Total BM cells were harvested from individual STAT5ab−/−or wild-type mice. FL cells were collected from pregnant females that were mated 14.5 days earlier. The cells were then blocked with normal mouse serum, stained with antibody cocktails, and analyzed by FACS. (A) Shown are representative FACS profiles for lineage-negative BM or FL cells, analyzed for coexpression of Sca-1 (FITC) and c-kit (PECy5). (B) Shown is analysis of FL cells stained first with Hoechst 33342, washed, and next stained with antibodies to cell surface markers. Representative FACS profiles for c-kit+lin−SP and Sca-1+lin−SP cells are shown. The percentages marked represent the c-kit+lin− or Sca-1+lin− cells falling within the SP region (box).
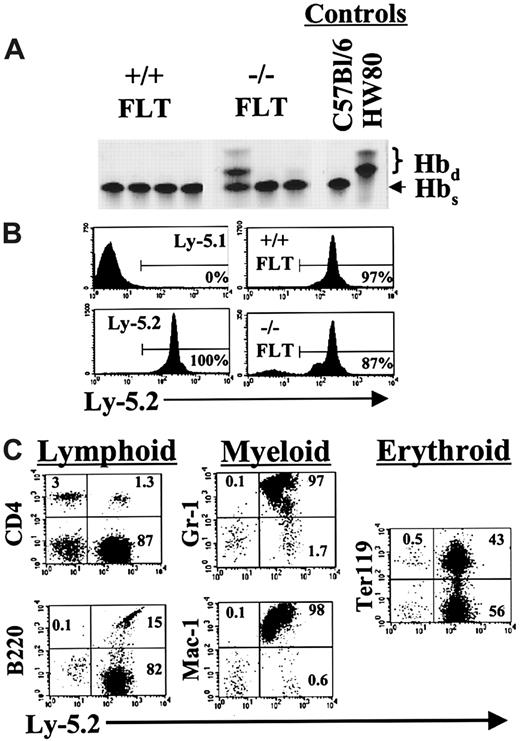
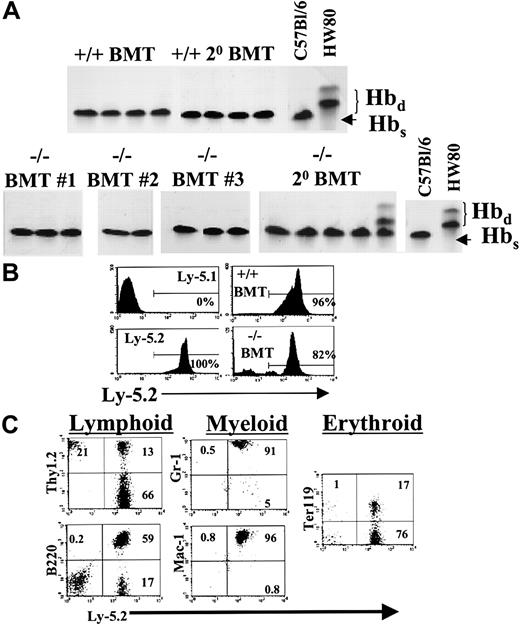
Further studies demonstrated that reconstitution of hematopoiesis could be achieved after direct transplantation of 5 × 106STAT5ab−/− BM or 5 × 105STAT5ab−/− FL cells into lethally irradiated recipient mice (Table 2). Two separate transplantations were performed by using pooled samples from 2 to 3 mice for each BM or FL source injected into 3 to 5 recipient mice. The hematology of the mice that received transplants recapitulated that of primary STAT5ab−/− mice (Table 1). No differences were noted between the HW80 or Ly-5.1 hosts. Both backgrounds were used for the 2 FL hematology experiments. The 2 BM hematology studies were performed in HW80 recipient mice. We did observe rebound overcompensation in the wild-type white blood cell counts after transplantation, which has been commonly observed in a number of our transplantations for unrelated studies and might be related to endogenous cytokine production in response to irradiation. Furthermore, the absolute neutrophil count and absolute monocyte count reconstituted better from the FL source than the BM source for the STAT5ab−/− graft. This difference may possibly indicate an age-dependent decline in myeloid reconstitution potential. For the hematology and reconstitution experiments, BM or FL cells were harvested and STAT5ab−/− or littermate wild-type cells (C57Bl/6 Ly-5.2 background; hemoglobin single [Hbs]) and injected into each of 2 genetically distinguishable lethally irradiated hosts. The HW80 (hemoglobin diffuse [Hbd]) strain was used for hemoglobin electrophoresis analysis of erythroid reconstitution. Complete reconstitution with cells of donor origin was achieved in most mice, but some chimerism was seen as evidenced by the presence of endogenous Hbd bands in one FL transplant recipient and in one secondary recipient from the BM transplantations (Figures 3A, 4A). Both BM and FL cells were also used in the common leukocyte antigen CD45 (Ly-5.1/Ly-5.2)-based system. STAT5ab−/− cells (Ly-5.2 background) were transplanted into C57Bl/6 Ly-5.1 recipient mice and analyzed by FACS 3 to 5 months later for the relative engraftment in multiple lineages. Representative negative control Ly-5.1 mice that did not receive transplants showed less than 1% Ly-5.2+ cells, and positive control mice that received transplants of normal wild-type Ly-5.2 BM cells showed 97% and 96% Ly-5.2+ cells for FL and BM cells, respectively (Figures 3B, 4B). T lymphocytes of BM and FL transplant recipients were markedly mixed with endogenous T cells (59% ± 8%), but reconstitution with the donor graft was more than 99% in cells expressing Gr-1, Mac-1, B220, and Ter119 markers (Figures 3C, 4C). The T-cell reconstitution defect is consistent with the observed reduction in the absolute lymphocyte count in STAT5ab−/− mice and transplant recipients of STAT5ab−/− BM or FL cells. Comparable results were also found in secondary transplant recipients (data not shown). Mice injected with 1 to 2 × 107splenocytes from either wild-type or STAT5ab−/− mice showed no donor reconstitution, ruling out aberrant migration of stem cells to the spleen of STAT5ab−/− mice (data not shown).
Steady-state hematology of mice that received transplants and reconstituted with donor STATab−/− bone marrow or fetal liver cells
| Hematologic parameter* . | STAT5ab+/+ donor . | STAT5ab−/− donor . | % Control . | P . |
|---|---|---|---|---|
| Bone marrow transplantation | ||||
| White blood cells/μL | 15 251 ± 1 849 | 4 145 ± 742 | 27 | < .001 |
| Absolute lymphocyte count/μL | 12 141 ± 2 164 | 2 683 ± 778 | 22 | < .001 |
| Absolute neutrophil count/μL | 2 260 ± 598 | 984 ± 41 | 44 | < .001 |
| Absolute monocyte count/μL | 851 ± 242 | 478 ± 176 | 56 | .01 |
| Hematocrit | 44 ± 1 | 38 ± 1 | 86 | < .001 |
| Fetal liver transplantation | ||||
| White blood cells/μL | 12 679 ± 3 049 | 5 741 ± 1 917 | 45 | < .001 |
| Absolute lymphocyte count/μL | 9 619 ± 3 082 | 3 012 ± 1 764 | 31 | < .001 |
| Absolute neutrophil count/μL | 2 490 ± 705 | 1 686 ± 1 379 | 68 | .19 |
| Absolute monocyte count/μL | 753 ± 384 | 930 ± 355 | 123 | .39 |
| Hematocrit | 44 ± 2 | 38 ± 4 | 86 | .004 |
| Hematologic parameter* . | STAT5ab+/+ donor . | STAT5ab−/− donor . | % Control . | P . |
|---|---|---|---|---|
| Bone marrow transplantation | ||||
| White blood cells/μL | 15 251 ± 1 849 | 4 145 ± 742 | 27 | < .001 |
| Absolute lymphocyte count/μL | 12 141 ± 2 164 | 2 683 ± 778 | 22 | < .001 |
| Absolute neutrophil count/μL | 2 260 ± 598 | 984 ± 41 | 44 | < .001 |
| Absolute monocyte count/μL | 851 ± 242 | 478 ± 176 | 56 | .01 |
| Hematocrit | 44 ± 1 | 38 ± 1 | 86 | < .001 |
| Fetal liver transplantation | ||||
| White blood cells/μL | 12 679 ± 3 049 | 5 741 ± 1 917 | 45 | < .001 |
| Absolute lymphocyte count/μL | 9 619 ± 3 082 | 3 012 ± 1 764 | 31 | < .001 |
| Absolute neutrophil count/μL | 2 490 ± 705 | 1 686 ± 1 379 | 68 | .19 |
| Absolute monocyte count/μL | 753 ± 384 | 930 ± 355 | 123 | .39 |
| Hematocrit | 44 ± 2 | 38 ± 4 | 86 | .004 |
STAT indicates signal transducers and activators of transcription.
Sample sizes consisted of 6 to 8 mice per group analyzed 7 to 11 weeks after transplantation. Mice used for analysis appeared to be healthy and did not show signs of sickness related to splenomegaly disease. The % control is calculated for the average STAT5ab−/− relative to the STAT5ab+/+hematology values. P values were calculated by using the Student 2-tailed t test.
Analysis of hematopoietic reconstitution after transplantation of FL cells into lethally irradiated recipients.
(A) C57Bl/6 FL cells on the hemoglobin single (Hbs) background were collected from STAT5ab−/− and wild-type mice and counted. The cells were then injected into lethally irradiated (1100 rads) C57Bl/6 HW80 recipient mice that differ in the endogenous hemoglobin expression pattern and are hemoglobin diffuse (Hbd). Eight to 10 weeks later the mice were bled, and the packed red blood cells were separated by electrophoresis on cellulose acetate gels. The Hbs and Hbd patterns of control mice that did not receive transplants are shown on the right. (B) In a second experiment, FL cells (Ly-5.2) were injected into lethally irradiated Ly-5.1 recipient mice. Shown are representative examples of mice analyzed for Ly-5.2 expression 11 weeks after transplantation. Ly-5.1 negative control and Ly-5.2 positive controls are shown in the left panels. On the right are mice that received transplants of wild-type FL (upper panel) or STAT5ab−/−FL cells (lower panel). (C) To demonstrate a typical lineage analysis of the STAT5ab−/− FL engraftment, gating based on forward-scatter and side-scatter profiles typical for lymphocytes (B220, CD4, and Ter119), monocytes (Mac-1), and granulocytes (Gr-1) was combined with the lineage antibodies indicated. The percentage of cells falling within the upper quadrants and the lower-right quadrant is shown.
Analysis of hematopoietic reconstitution after transplantation of FL cells into lethally irradiated recipients.
(A) C57Bl/6 FL cells on the hemoglobin single (Hbs) background were collected from STAT5ab−/− and wild-type mice and counted. The cells were then injected into lethally irradiated (1100 rads) C57Bl/6 HW80 recipient mice that differ in the endogenous hemoglobin expression pattern and are hemoglobin diffuse (Hbd). Eight to 10 weeks later the mice were bled, and the packed red blood cells were separated by electrophoresis on cellulose acetate gels. The Hbs and Hbd patterns of control mice that did not receive transplants are shown on the right. (B) In a second experiment, FL cells (Ly-5.2) were injected into lethally irradiated Ly-5.1 recipient mice. Shown are representative examples of mice analyzed for Ly-5.2 expression 11 weeks after transplantation. Ly-5.1 negative control and Ly-5.2 positive controls are shown in the left panels. On the right are mice that received transplants of wild-type FL (upper panel) or STAT5ab−/−FL cells (lower panel). (C) To demonstrate a typical lineage analysis of the STAT5ab−/− FL engraftment, gating based on forward-scatter and side-scatter profiles typical for lymphocytes (B220, CD4, and Ter119), monocytes (Mac-1), and granulocytes (Gr-1) was combined with the lineage antibodies indicated. The percentage of cells falling within the upper quadrants and the lower-right quadrant is shown.
Analysis of hematopoietic reconstitution after transplantation of BM cells into lethally irradiated recipients.
(A) BM cells were harvested from 4- to 6-week-old STAT5ab−/− mice (C57Bl/6 Hbs). The cells were then injected into lethally irradiated (1100 rads) C57Bl/6 HW80 recipient mice (Hbd) that differ in the endogenous hemoglobin expression pattern. Eight to 10 weeks later the mice were bled, and the packed red blood cells were separated by electrophoresis on cellulose acetate gels. The Hbs and Hbdpatterns of control mice that did not receive transplants are shown on the right. Shown are representative examples of mice that received transplants 5 (BMT no. 1), 6 (BMT no. 2), and 3 months (BMT no. 3) earlier of STAT5ab−/− BM cells. Also shown are secondary BM transplanted mice from primary BM transplantation no. 1 analyzed 3 months after transplantation. (B) In a fourth experiment, BM cells (Ly-5.2) were injected into lethally irradiated Ly-5.1 mice, and then peripheral blood leukocytes were stained with antibodies and analyzed by FACS after reconstitution. Shown are representative examples of mice analyzed for Ly-5.2 expression 5 months after transplantation. Ly-5.1 negative control and Ly-5.2 positive controls are shown in the left panels. On the right are mice that received transplants of wild-type BM (upper panel) or STAT5ab−/− BM cells (lower panel). (C) To demonstrate a typical lineage analysis of the STAT5ab−/− BM engraftment, gating based on forward-scatter and side-scatter profiles typical for lymphocytes (B220, Thy1.2, and Ter119), monocytes (Mac-1), and granulocytes (Gr-1) was combined with the lineage antibodies indicated. The percentage of cells falling within the upper quadrants and the lower-right quadrant is shown. Note that for the Thy1.2 analysis cells in the upper-left quadrant were slightly overcompensated, making the percentage in the quadrant appear visually lower than the actual value.
Analysis of hematopoietic reconstitution after transplantation of BM cells into lethally irradiated recipients.
(A) BM cells were harvested from 4- to 6-week-old STAT5ab−/− mice (C57Bl/6 Hbs). The cells were then injected into lethally irradiated (1100 rads) C57Bl/6 HW80 recipient mice (Hbd) that differ in the endogenous hemoglobin expression pattern. Eight to 10 weeks later the mice were bled, and the packed red blood cells were separated by electrophoresis on cellulose acetate gels. The Hbs and Hbdpatterns of control mice that did not receive transplants are shown on the right. Shown are representative examples of mice that received transplants 5 (BMT no. 1), 6 (BMT no. 2), and 3 months (BMT no. 3) earlier of STAT5ab−/− BM cells. Also shown are secondary BM transplanted mice from primary BM transplantation no. 1 analyzed 3 months after transplantation. (B) In a fourth experiment, BM cells (Ly-5.2) were injected into lethally irradiated Ly-5.1 mice, and then peripheral blood leukocytes were stained with antibodies and analyzed by FACS after reconstitution. Shown are representative examples of mice analyzed for Ly-5.2 expression 5 months after transplantation. Ly-5.1 negative control and Ly-5.2 positive controls are shown in the left panels. On the right are mice that received transplants of wild-type BM (upper panel) or STAT5ab−/− BM cells (lower panel). (C) To demonstrate a typical lineage analysis of the STAT5ab−/− BM engraftment, gating based on forward-scatter and side-scatter profiles typical for lymphocytes (B220, Thy1.2, and Ter119), monocytes (Mac-1), and granulocytes (Gr-1) was combined with the lineage antibodies indicated. The percentage of cells falling within the upper quadrants and the lower-right quadrant is shown. Note that for the Thy1.2 analysis cells in the upper-left quadrant were slightly overcompensated, making the percentage in the quadrant appear visually lower than the actual value.
BM from STAT5ab−/− mice does not contribute to hematopoiesis after competitive repopulation against wild type BM
Because hematopoietic cells from these mice did have repopulating ability, we next wanted to compare cells harvested from STAT5ab−/− mice with those from littermate wild-type mice to determine whether differences in relative stem cell activity were evident. For these experiments, BM cells were harvested from either STAT5ab−/− or littermate wild-type mice (C57Bl/6 background; Hbs) and competed at a 1:1 ratio with cells from HW80 mice (Hbd). Mixing based on ratio was important because total BM cellularity does not necessarily correlate with absolute stem cell content. Beginning as early as 7 weeks after transplantation and continued monthly until 6 months before transplantation, mice from BM competitive repopulation experiment no. 1 were analyzed for the relative levels of donor engraftment (Figure5A). The Hbs band was always absent even at the earliest time points for the STAT5ab−/− grafts in all experiments. BM from wild-type mice (nos. 8-10) competed effectively with the competitor marrow, resulting in mice with the expected chimerism (38% ± 6%). In contrast, BM from STAT5ab−/− mice was completely outcompeted by the competitor marrow, indicating a greatly reduced long-term repopulating activity (mice nos. 1-7). At the time the mice from the competitive repopulation experiment (mice nos. 1, 2, 4, 9, and 10) were humanely killed, secondary transplanted mice were generated and followed for 4 months to continue analysis (Figure 5B). The average percentage of Hbs in 7 secondary transplanted mice was not significantly changed from the average found in the primary transplanted mice after wild-type competitive repopulation (32% ± 10%, n = 7, P = .37). As in the primary transplantation, no reconstitution was seen in secondary recipients from the STAT5ab−/− competitive repopulation. The defects in secondary reconstitution rule out a phenotype restricted to short-lived progenitors. Two additional competitive repopulation experiments analyzed at 4 and 3 months after transplantation showed identical results for the STAT5ab−/− BM graft (experiment no. 2, n = 5; experiment no. 3, n = 8) and the wild-type graft (experiment no. 2, 54% ± 3%, n = 3; experiment no. 3, 56%, n = 2). The average percentage of chimerism in the 1:1 mixed wild-type grafts for the 3 separate experiments was 49% ± 10%.
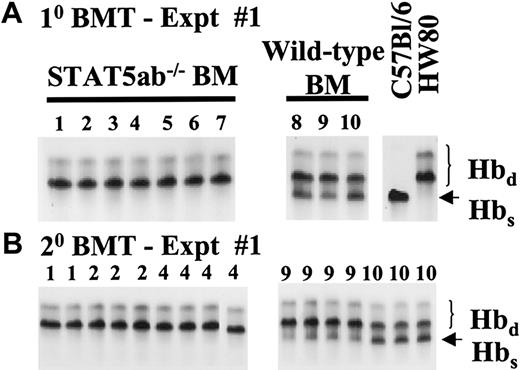
Representative competitive repopulation assay between STAT5ab−/− and wild-type BM grafts.
Total BM cells were collected from either STAT5ab−/− or wild-type mice and mixed at 1:1 donor equivalent ratios. The BM cell mixes were then injected into lethally irradiated recipient mice (1100 rads). (A) Primary transplanted mice from experiment no. 1 were bled from the retroorbital venous plexus 6 months after transplantation and analyzed for the relative ratio of the 2 donor grafts. Hemoglobin electrophoresis of packed red blood cells on cellulose acetate gels was used to analyze the donor engraftment. The Hbs and Hbd patterns of control mice that did not receive transplants are shown on the right. The number above each lane is the individual mouse identification number. Identical experiment no. 2 and experiment no. 3 are not shown. (B) Primary transplanted mice were killed at 6 months, and the BM cells were injected into lethally irradiated (1100 rads) secondary recipient mice. Secondary transplanted mice were analyzed 4 months after transplantation for the relative ratio of the 2 donor grafts. The number above each lane represents the primary donor mouse from which each mouse was derived.
Representative competitive repopulation assay between STAT5ab−/− and wild-type BM grafts.
Total BM cells were collected from either STAT5ab−/− or wild-type mice and mixed at 1:1 donor equivalent ratios. The BM cell mixes were then injected into lethally irradiated recipient mice (1100 rads). (A) Primary transplanted mice from experiment no. 1 were bled from the retroorbital venous plexus 6 months after transplantation and analyzed for the relative ratio of the 2 donor grafts. Hemoglobin electrophoresis of packed red blood cells on cellulose acetate gels was used to analyze the donor engraftment. The Hbs and Hbd patterns of control mice that did not receive transplants are shown on the right. The number above each lane is the individual mouse identification number. Identical experiment no. 2 and experiment no. 3 are not shown. (B) Primary transplanted mice were killed at 6 months, and the BM cells were injected into lethally irradiated (1100 rads) secondary recipient mice. Secondary transplanted mice were analyzed 4 months after transplantation for the relative ratio of the 2 donor grafts. The number above each lane represents the primary donor mouse from which each mouse was derived.
To confirm the hemoglobin reconstitution analysis results by Southern blot for the single and diffuse alleles, primary transplanted mice were killed at 6 months, and genomic DNA was prepared from leukocytes obtained from peripheral blood, BM, and spleen (SP) of mice nos. 2, 4, 9, and 10. For mouse no. 1 only peripheral blood was obtained for analysis. Southern blot analysis of the DNA from mice in each experimental group was then performed (Figure6). The Southern blots were probed with a fragment of the β-globin intervening sequence 2 and yielded results identical to the hemoglobin electrophoresis. The percentage of Hbs calculated by densitometry of Southern blots, was identical to that obtained by hemoglobin electrophoresis. This result indicates that erythroid reconstitution did not differ from that of the leukocyte populations. For the Hbd control mice, the values obtained for the 1:3 upper-to-lower band ratio were consistent with the hemoglobin electrophoresis data. The average values in peripheral blood, BM, and spleen combined were 34% ± 3% and 32% ± 1% for mouse 9 and mouse 10, respectively. No engraftment with Hbswas detected by Southern blot in mice receiving the STAT5ab−/− competitive repopulation grafts. This result indicates that the engraftment defect in STAT5ab−/− mice also occurs in cells of lymphomyeloid origin. The upper Hbdband in recipients of the wild-type competitive repopulation grafts is weak because of the decrease from the normal 25% of the total hemoglobin because of the contribution from the competitor Hbs graft.
Southern blot analysis of genomic DNA from tissues of competitive repopulation experiment no. 1 mice 6 months after transplantation.
Mice 1, 2, 4, 9, and 10 described in Figure 5 were killed 6 months after BM transplantation, and genomic DNA was prepared from the peripheral blood (PB), bone marrow (BM), and spleen. Southern blot analysis of EcoR1-digested DNA preparations was performed by using a [32P]-labeled probe to the murine β-globin intervening sequence 2. The upper panels show a blot from the wild type competitive repopulation and the lower panels show a blot from the STAT5ab−/− competitive repopulation. Control DNA samples were run on each Southern blot gel from mice with either the Hbs or Hbd and are shown on the right. Shown on the left for each gel is the DNA ladder. The larger arrow marks the correct size for the Hbs fragment on each gel, and the small arrow marks the correct size Hbd fragments on each gel.
Southern blot analysis of genomic DNA from tissues of competitive repopulation experiment no. 1 mice 6 months after transplantation.
Mice 1, 2, 4, 9, and 10 described in Figure 5 were killed 6 months after BM transplantation, and genomic DNA was prepared from the peripheral blood (PB), bone marrow (BM), and spleen. Southern blot analysis of EcoR1-digested DNA preparations was performed by using a [32P]-labeled probe to the murine β-globin intervening sequence 2. The upper panels show a blot from the wild type competitive repopulation and the lower panels show a blot from the STAT5ab−/− competitive repopulation. Control DNA samples were run on each Southern blot gel from mice with either the Hbs or Hbd and are shown on the right. Shown on the left for each gel is the DNA ladder. The larger arrow marks the correct size for the Hbs fragment on each gel, and the small arrow marks the correct size Hbd fragments on each gel.
FL cells harvested from STAT5ab−/− mice show defects in stem cell function when competed against normal wild-type FL cells
To assess whether an age-dependent activated T-cell phenotype associated with STAT5 deficiency could influence the BM stem cell function of STAT5ab−/− mice, we characterized a more primitive stage of hematopoietic cell development in the competitive repopulation assay. FL cells were mixed at 1:1 donor ratios, and, because the FL cellularity was unchanged, the cell doses competed were identical. When equal donor equivalents from pools of 2 wild-type or STAT5ab−/− FL were mixed with HW80 mouse FL cells, the reconstitution from the STAT5ab−/− graft was completely outcompeted at all times measured up to 20 weeks after transplantation (Figure 7A). This result was identical to that obtained with adult BM, indicating that autoreactive T cells, which are absent at this developmental stage, are not responsible for the dysfunction in hematopoietic reconstitution on BM or FL transplantation. A second experiment was performed in the hemoglobin system with identical results (STAT5ab−/− n = 5, wild type n = 3, data not shown). A third experiment was also performed using the CD45 marker after mixing of FL cells from 2 separate donor mice with Ly-5.1 FL cells at either a 1:1 or 4:1 ratio. A representative example of both wild-type and STAT5ab−/−competitions is shown (Figure 7B) from a total of 5 recipients of 1:1 mixed cells and 3 recipients of 4:1 mixed cells for both the wild-type and STAT5ab−/− groups. This approach complements the hemoglobin electrophoresis results and provides quantitative multilineage characterization of the repopulating defects. We have mixed STAT5ab−/− (Ly-5.2) or wild-type E14.5 FL cells harvested from the same pregnant female (Ly-5.2) at donor equivalent ratios of 1:1 or 4:1 versus wild-type E14.5 FL cells from a Ly-5.1 pregnant female. Multilineage engraftment was determined by costaining for Gr-1, Mac-1, B220, CD4, or Ter119 markers and for Ly-5.2 at 10 and 17 weeks after transplantation, with no changes observed between the 2 time points. Wild-type livers competed with values consistent with the input 50% (28%-59% range) or 80% (61%-93% range) mixing ratio. Even with the 4:1 mixing ratio, the STAT5ab−/− graft only contributed from undetectable to 3.7%. The levels of engraftment of CD4+ T cells were always undetectable for the STAT5ab−/− graft and in rank order were B220+B cells (1.3%-1.6%), Ter119+ erythroid progenitors (1.6%-1.7%), Gr-1+ myeloid cells (3.3%-4.6%), and Mac-1+ myeloid cells (3.7%-4.7%). The net overall repopulating deficiency was thus greatest in T cells (because undetectable) and could be quantitated at 25- to 28-fold for granulocytes (Gr-1) and macrophage (Mac-1), 45-fold for erythroid progenitors (Ter119), and 68-fold for B lymphocytes. This result formally demonstrates the lymphomyeloid nature, the severity, and the generalizability of the repopulating defect in hematopoietic cells from STAT5ab−/− mice.
Competitive repopulation assay between STAT5ab−/− and wild-type FL grafts.
(A) C57Bl/6 FL cells (Hbs and Ly-5.2) were obtained from embryonic day 14.5 fetuses from matings of STAT5ab+/− or wild-type HW80 mice (Hbd background). The STAT5ab+/− cross FL cells were genotyped by PCR the same day, and then either wild-type or knockout cells were mixed with an equal donor equivalent of wild- type HW80 FL cells. The cells were transplanted into lethally irradiated adult C57Bl/6 recipient mice and analyzed 20 weeks later for the relative contribution of donor engraftment. A second experiment analyzed at 16 weeks is not shown. (B) For a third experiment, C57Bl/6 FL cells (Ly-5.2) were obtained as described above but were competed at either a 1:1 or a 4:1 ratio against wild-type Ly-5.1 FL cells. The cells were transplanted into lethally irradiated adult C57Bl/6 Ly-5.1 recipient mice and analyzed 10 weeks later for Ly-5.2 cells costaining for Gr-1, Mac-1, CD4, B220, or Ter119.
Competitive repopulation assay between STAT5ab−/− and wild-type FL grafts.
(A) C57Bl/6 FL cells (Hbs and Ly-5.2) were obtained from embryonic day 14.5 fetuses from matings of STAT5ab+/− or wild-type HW80 mice (Hbd background). The STAT5ab+/− cross FL cells were genotyped by PCR the same day, and then either wild-type or knockout cells were mixed with an equal donor equivalent of wild- type HW80 FL cells. The cells were transplanted into lethally irradiated adult C57Bl/6 recipient mice and analyzed 20 weeks later for the relative contribution of donor engraftment. A second experiment analyzed at 16 weeks is not shown. (B) For a third experiment, C57Bl/6 FL cells (Ly-5.2) were obtained as described above but were competed at either a 1:1 or a 4:1 ratio against wild-type Ly-5.1 FL cells. The cells were transplanted into lethally irradiated adult C57Bl/6 Ly-5.1 recipient mice and analyzed 10 weeks later for Ly-5.2 cells costaining for Gr-1, Mac-1, CD4, B220, or Ter119.
Discussion
Many of the growth factors that have characterized biologic activities on primitive hematopoietic cells have been shown to induce phosphorylation of STAT5 via the JAKs. Although this indirect evidence suggested that STAT5 activation might be important for cytokine stimulation of HSCs, no functional studies using STAT5-deficient mice had been performed. In addition, other signal transduction pathways apart from the JAK-STAT pathway could serve redundant functions, making the role of JAKs and STATs in HSCs uncertain. For example, both TPO and SCF can activate other downstream molecules such as SHC, Raf-1/MAP kinase, and phosphatidylinositol-3′ kinase43-49 in various cell types. The requirement for any of these pathways in the HSC has not been directly studied. To address the role of STAT5 signaling in the earliest stages of hematopoietic differentiation, we have initiated studies using a STAT5ab−/− mouse transplantation model.
As previously reported, we found that STAT5ab−/− mice had relatively mild defects in the number of peripheral blood hematopoietic cells.12 Reductions in lymphocytes were most severe.12,13 STAT5 has been implicated in responses to a variety of cytokines as determined by in vitro assays using immortalized or transformed cell lines. Evidence for cytokines that require STAT5 activation in primary cells supports a role in pathways triggered by IL-2,50 TPO,30GM-CSF,51 IL-3,52 and cytokines such as IL-4 that use the common gamma chain.53 Evidence for activation of STAT5 by granulocyte colony-stimulating factor54 and EPO16 has not been strongly supported in STAT5ab−/− mice. A modest role for STAT5 in EPO signaling during fetal development has been reported,55 but this issue remains controversial because adult STAT5ab−/− mice show no marked defects and many attain a near-normal hematocrit. However, experiments under stress erythropoiesis have not yet been performed. The T-cell defects in these mice appear to be responsible for the splenomegaly disease, because crossing the mixed background mice onto a RAG2−/− or JAK3−/− background eliminates this phenotype but does not correct the repopulating defect (data not shown). We also found in this study that mice reconstituted with STAT5ab−/− BM or FL cells survived long-term and did not develop splenomegaly disease, possibly because of the reduced T-lymphocyte engraftment. The numbers of total BM cells and CFU-Ss were reduced, perhaps reflecting a requirement for STAT5 at the level of the common lymphoid56 or common myeloid57 progenitor cell. During embryonic development, STAT5 expression is turned on during differentiation of embryonic stem cells,58 allowing speculation that the appearance of STAT5 expression correlates with the need for growth factor responsiveness of HSCs that could be critical for long-term repopulating function in vivo.
We have hypothesized that STAT5 activation plays a significant role in HSC function, in addition to its functions important for progenitor cell proliferation. Competitive repopulation experiments presented here revealed a marked decrease in the long-term repopulating activity of STAT5ab−/− mice. This defect was evident in peripheral blood, BM, and spleen tissues that are comprised of varying ratios of lymphoid and myeloid cells. This finding suggests that multilineage repopulating activity is deficient in STAT5ab−/− mice. Further confirmation of the lymphomyeloid repopulating defect was obtained by using the FACS-based Ly-5.1/Ly-5.2 detection system. In competitive repopulation experiments the decreased repopulating activity was quantitated to be minimally 25-fold in myeloid cells and 68-fold in lymphoid progeny. These numbers may underestimate the true repopulating defect because higher than 4:1 mixing ratios were not tested, and the increase from a 1:1 to a 4:1 ratio did not increase the contribution from the STAT5ab−/− graft, in contrast to the marked increase seen with the wild-type graft. The possibility exists that STAT5 could also be required for the differentiation of cells reconstituting these tissues. However, committed progenitors and lineage-positive cells lack short- or long-term repopulating activity in the transplantation assay used. The hemoglobin electrophoresis analysis is also likely representative of the engraftment at the stem cell level, because we have shown here that reconstitution of hematocrit to pretransplantation levels can be achieved in reconstituted mice (Tables 1 and 2). Therefore, the correlation between the hemoglobin electrophoresis results, the DNA analysis of lymphoid and myeloid tissues, and the FACS analyses strongly support defects at the HSC level.
Consistent with the results of our study, challenge with 80 mg/kg carboplatin and 750 rads Cs137 irradiation has revealed a markedly slower recovery of platelets, hemoglobin, and white blood cells (Carl W. Jackson and Tamari I. Pestina, St Jude Children's Research Hospital, personal communication, November 2, 2000) and a lower survival rate59 even when given a normally rescuing dose of pegylated recombinant murine megakaryocyte growth and development factor. In our study here we show that during conditions of stress, such as after BM transplantation, defects in STAT5 activation may be more easily observed than during steady-state hematopoiesis. Because our transplantation model using mutant BM and FL cells is one with perturbed hematopoiesis, the lack of correlation between KLS number and function is not unexpected. Studies in which the HSC phenotype fails to correlate with function60,61 have been described as well as the demonstration that CD34 expression can be reversibly modulated62 in HSCs. Practical limitations in the numbers of viable mice and KLS cells that could be obtained from the C57Bl/6 colony did not allow scale up to perform limiting dilution studies with sorted cells. Therefore, we cannot conclude whether the marked repopulating defect in the mutant mice is due to a reduced quality or to a reduced quantity of stem cells.
The specific growth factors that are relevant for the reduced in vivo reconstitution ability of STAT5ab−/− stem cells that have been transplanted remains unclear. The defective repopulation might be greater than previously reported for c-mpl−/−mice34 because of requirements for STAT5 activation downstream of c-kit18 or Flt3.63 Currently it is unclear whether STAT5 activation by specific factors occurs directly or indirectly through induction of immediate early target genes that cross-talk with several signal transduction pathways. STAT5 is a transcription factor known to activate a number of downstream targets. These targets include cell cycle regulatory genes and antiapoptosis genes. Those genes regulated by STAT5 transcriptional activity in early stages of hematopoiesis that are important mediators of stem cell function remain to be determined. Characterization of these mice for the growth factor responsiveness and cell cycle activation profile of STAT5ab−/− HSCs may provide valuable insights into transcriptional regulation of HSC function.
In summary, we have demonstrated that STAT5 activity is essential for normal long-term repopulation of lethally irradiated hosts. Therefore, in addition to a role in progenitor cell function, STAT5 plays an important role in the earliest stages of differentiation from the lymphomyeloid repopulating HSCs. This finding has implications for BM transplantation, suggesting that use of STAT5-activating cytokines may be beneficial for promoting stable long-term engraftment.
We thank David Bodine (Genetics and Molecular Biology Branch, Hematopoiesis Section, National Human Genome Research Institute, National Institutes of Health) for providing the murine β-globin intervening sequence 2 DNA probe and Cheng-Kui Qu (American Red Cross Holland Laboratory, Hematopoiesis Department) for advice on isolation of fetal liver cells.
Supported by the American Lebanese Syrian Associated Charities, the Howard Hughes Medical Institute, and the American Red Cross.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kevin D. Bunting, Hematopoiesis Dept, American Red Cross Holland Laboratory, 15601 Crabbs Branch Way, Rockville, MD; e-mail: buntingk@usa.redcross.org.

![Fig. 6. Southern blot analysis of genomic DNA from tissues of competitive repopulation experiment no. 1 mice 6 months after transplantation. / Mice 1, 2, 4, 9, and 10 described in Figure 5 were killed 6 months after BM transplantation, and genomic DNA was prepared from the peripheral blood (PB), bone marrow (BM), and spleen. Southern blot analysis of EcoR1-digested DNA preparations was performed by using a [32P]-labeled probe to the murine β-globin intervening sequence 2. The upper panels show a blot from the wild type competitive repopulation and the lower panels show a blot from the STAT5ab−/− competitive repopulation. Control DNA samples were run on each Southern blot gel from mice with either the Hbs or Hbd and are shown on the right. Shown on the left for each gel is the DNA ladder. The larger arrow marks the correct size for the Hbs fragment on each gel, and the small arrow marks the correct size Hbd fragments on each gel.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/2/10.1182_blood.v99.2.479/6/m_h80222015006.jpeg?Expires=1769180530&Signature=1gC5NOhGn1INWhzoYuZ014yr5a403Ij3q6Iws9moNK8FN6sRpwI7nfu1qsds2CoRw7iQrJyKEsZ-j6vylXkYI~v6ydBRLkyvYVDqatYHYcZTTV~Zik1QUnFu0NiP27p2hjUmudecDHiugh42K2x38cTWR9KQCY6SrrZYv~TJujtsqDCHMP9M8Yd3NjJmeAflkg6NOD5uhgcY8vnHg2xV0qPwBl-8hzr0j-PEDQMro9tIHddeYC4h2mWQQSF-MGGYcwYptGNbHS~fWANL91MYDX-2E6E5WGJ0Y-C8AwV5venxhT4T8XLuxglLQIgDX0MRc509KsJ7kfsUVyjlvRQMFw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal