In utero transplantation (IUT) is becoming a viable option for the treatment of various immune and metabolic disorders diagnosed early in gestation. In this study, donor fetal liver cells had a 10-fold competitive engraftment advantage relative to adult bone marrow in allogeneic fetal severe combined immunodeficient (SCID) recipients compared with adult recipients. In contrast, adult bone marrow cells engrafted slightly better than fetal liver cells in allogeneic adult SCID transplant recipients. By using different ratios of fetal and adult cell mixtures, fetal liver cells repopulated 8.2 times better than adult bone marrow cells in fetal recipients, but only 0.8 times as well in adult recipients. Fetal SCID recipients were more permissive to an allogeneic donor graft than adult recipients. These data indicate that the recipient microenvironment may regulate the engraftment efficiency of a given stem cell source and suggest that the use of cord blood should be tested in clinical IUT.
Introduction
In utero transplantation (IUT) offers a therapeutic option for the treatment of hemoglobinopathies and immune or metabolic disorders diagnosed early in gestation.1-8 The preimmune fetal recipient may be more tolerant than an adult recipient to an unmatched donor stem cell source in the absence of potential toxicities associated with conventional transplantation-conditioning regimes.6,9-11 Importantly, early successful establishment of donor stem cell engraftment prenatally may prevent or reduce the pathology associated with the underlying disorder. Fetal liver (FL), cord blood, and adult bone marrow (BM) are potential stem cell sources for transplantation. The relative engraftment efficiency of each source may depend on intrinsic differences in cytokine requirements as well as extrinsic signals that differ in the fetal versus the adult microenvironment.11 12 In this study, we compare the relative engraftment capacity of allogeneic FL versus adult BM in both fetal and adult severe combined immunodeficient (SCID) recipients.
Study design
Mice
BALB/c SCID mice (H2d) (National Institutes of Health [NIH], Bethesda, MD) were used as recipients for IUT and adult transplantation. Timed pregnancies were performed as previously described.1 BM was obtained from C57BL/6 CD45.2 mice (NIH) and were T-cell depleted as previously described.13 FL was obtained from C57BL/6 CD45.1 mice (NIH) at 15 to 16 days of gestation.
IUT and adult transplantation
Single-cell suspensions of FL and adult BM were combined at the indicated cell dosages and ratios and were infused either intraperitoneally into BALB/c SCID fetuses at day 15/16 of gestation2 or intravenously into unconditioned adult BALB/c SCID mice. Live birth rate in IUT mice was 20% to 30%. Intraperitoneal injection of adult recipients did not result in detectable donor chimerism in any mice.
Flow cytometry
Peripheral blood leukocytes (PBLs) were stained with fluorescein isothiocyanate–, phycoerythrin-, or biotin-conjugated antibodies to CD4, CD8, MAC-1 (CD11b), CD19, CD45.1, CD45.2, H2b, and H2d with SA-peridinin chlorophyll protein (PharMingen, San Diego, CA) and analyzed on a FACScalibur by using CellQuest software (Becton Dickinson, San Jose, CA).
Results and discussion
To determine the relative engraftment capacity of FL versus T-cell–depleted adult BM in fetal recipients, BALB/c SCID fetuses were injected intraperitoneally on day 15/16 of gestation with equal numbers of C57BL/6 CD45.2 adult T-cell–depleted BM and C57BL/6 CD45.1 FL cells. PBLs were typed for donor H2Kb and CD45.1 and CD45.2 at 2 months after birth. Thirty-four (94%) of 36 IUT recipients had evidence of donor engraftment (≥ 2% donor cells). FL engrafted approximately 10-fold better than adult BM (Table1). Nearly 79% of PBLs was from donor FL compared with 8.6% from donor adult BM. Mice retyped at 123 to 130 days after birth had similar values with 72% of PBLs originating from donor FL and 7.8% from donor adult BM. Engraftment was stable, as a cohort of mice followed over a year had 74% and 11% donor cells from FL and adult BM origin, respectively. Engraftment was determined to be multilineage with a greater representation of donor cells from FL versus BM origin in T cells, B cells, and myeloid cells. Adoptive transfer of BM from engrafted chimeras (C57BL/6 → BALB/c SCID) reconstituted lethally irradiated adult C57BL/6 recipients with both C57BL/6 CD45.1 (donor FL origin) and CD45.2 (donor BM origin) cells. In these secondary recipients, as in the original IUT recipients, there were 8 to 10 times more cells from donor CD45.1 FL than from adult BM origin (data not shown). Collectively, these data indicate that fetal hematopoietic cells may be preferable to adult hematopoietic cells for IUT.
Although long-term reconstituting stem cells have been reported to be approximately 7 times more frequent in FL at 12 to 15 days of gestation than in adult BM, the frequency has been reported to decline approximately 3-fold by 15.5 days of gestation.14 15Because FL was obtained at 15 to 16 days of gestation, we hypothesized that the striking competitive engraftment advantage of FL as compared with adult BM in fetal recipients may not be due solely to an increased stem cell content in FL but may be due also to a favorable fetal microenvironment.
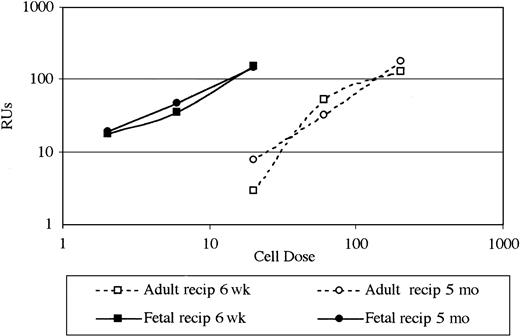
To compare engraftment of FL cells to adult BM in fetal versus unconditioned adult SCID recipients, ratios of 1:1, 0.3:1, and 0.1:1 FL to adult BM were administered (Table 2). Fetal and adult transplant recipients received an approximately equivalent cell dose on a per-weight basis (range, 120-1200 × 106 cells/kg depending on FL/BM ratio). These formal dose response studies indicated FL had a substantial competitive engraftment advantage over adult BM in IUT recipients. Mice that underwent transplantation as fetuses with a 1:1 ratio of FL to adult BM had 81% of PBLs originating from donor FL and 10% originating from donor adult BM. Reducing the ratio of FL to adult BM to 0.1:1 resulted in 42% and 46% of cells originating from donor FL and donor adult BM, respectively. Repopulating units (RUs) were calculated for FL, where 1 RU was defined as equal to the repopulating ability of 105 adult BM cells.16 FL produced RU numbers directly proportional to FL numbers used over a 10-fold range (Figure 1) and averaged 8.2 RU per 105 FL; thus, FL repopulated 8.2 times as well as BM in allogeneic fetal SCID recipients.
Fetal liver repopulated 8.2 times as well as adult bone marrow in allogeneic fetal SCID recipients compared with 0.8 in nonirradiated allogeneic adult SCID recipients.
Donor fetal cell dose (× 105) is shown on the x-axis. RUs in FL are shown on the y-axis. RUs are defined as the repopulating ability of 105 BM cells, so RU per 105 BM cells is always 1.0. RUs thus give a quantitative functional measure relative to the constant BM dose. The numbers of RU in FL over a range of FL doses are illustrated in fetal and adult recipients (recip) 6 weeks and 5 months after transplantation by using data from Table 2. RU in FL: RU FL/% FL = RU BM/% BM.
Fetal liver repopulated 8.2 times as well as adult bone marrow in allogeneic fetal SCID recipients compared with 0.8 in nonirradiated allogeneic adult SCID recipients.
Donor fetal cell dose (× 105) is shown on the x-axis. RUs in FL are shown on the y-axis. RUs are defined as the repopulating ability of 105 BM cells, so RU per 105 BM cells is always 1.0. RUs thus give a quantitative functional measure relative to the constant BM dose. The numbers of RU in FL over a range of FL doses are illustrated in fetal and adult recipients (recip) 6 weeks and 5 months after transplantation by using data from Table 2. RU in FL: RU FL/% FL = RU BM/% BM.
In contrast to these data in IUT recipients, FL and adult BM had an approximate equivalent capacity to reconstitute an adult recipient. Five months after transplantation, adult transplant recipients of a 1:1 ratio of FL to adult BM had 36% and 39% of PBLs originating from donor FL and donor adult BM, respectively (Table 2). Reducing the ratio of FL to adult BM to 0.1:1 in adult recipients resulted in only 1% to 3% of cells originating from the donor FL stem cell source. FL produced RU values proportional to FL numbers used (Figure 1) but only averaged 0.8 RU per 105 FL; thus, FL repopulated only 0.8 times as well as BM in allogeneic adult SCID recipients.
These results in adult mice contrast with earlier studies in which fetal cells had a 3.5- to 7.0-fold long-term engraftment advantage as compared with adult cells in adult recipients.17 However, those studies used lethally irradiated syngeneic adult recipients rather than nonirradiated allogeneic SCID recipients used in the current study, suggesting that the host conditioning regime or allogenicity of the donor may be important factors. Furthermore, no previous study has compared fetal and adult cells in both fetal and adult recipients.
Additionally, these data indicate that fetal SCID recipients are more readily engraftable with an allogeneic donor graft than are adult SCID recipients. In contrast to the high engraftment rate (90%) in fetal IUT recipients, only 10 (53%) of 19 adult transplant recipients had evidence of donor engraftment when typed at 6 to 7 weeks after transplantation. Mice that underwent transplantation as fetuses also had higher levels of donor engraftment than adult transplant recipients.
Collectively, these data indicate that fetal recipients are more permissive to an allogeneic donor graft than are adult recipients and further that the recipient microenvironment may regulate the proliferative responses of a given stem cell source. FL had an 8.2-fold competitive engraftment advantage as compared with adult BM in fetal recipients. In contrast, FL engrafted only 0.8 times as well as the adult BM in adult transplant recipients. Our data may have important clinical implications and suggest that fetal hematopoietic cells (eg, cord blood cells) may be preferable to adult BM for IUT in humans.
Supported by grants R01 HL49997 and R01 HL52952 (B.R.B.) as well as R01 HL63230 and R01 HL58820 (D.E.H.) from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce R. Blazar, MMC 109, University of Minnesota Hospital, Minneapolis, MN 55455; e-mail: blaza001@tc.umn.edu.