Abstract
Invariant natural killer T cells (iNKT cells) are a small subset of immunoregulatory T cells highly conserved in humans and mice. On activation by glycolipids presented by the MHC-like molecule CD1d, iNKT cells promptly secrete T helper 1 and 2 (Th1/2) cytokines but also cytokines with hematopoietic potential such as GM-CSF. Here, we show that the myeloid clonogenic potential of human hematopoietic progenitors is increased in the presence of glycolipid-activated, GM-CSF–secreting NKT cells; conversely, short- and long-term progenitor activity is decreased in the absence of NKT cells, implying regulation of hematopoiesis in both the presence and the absence of immune activation. In accordance with these findings, iNKT-cell–deficient mice display impaired hematopoiesis characterized by peripheral-blood cytopenias, reduced marrow cellularity, lower frequency of hematopoietic stem cells (HSCs), and reduced early and late hematopoietic progenitors. We also show that CD1d is expressed on human HSCs. CD1d-expressing HSCs display short- and long-term clonogenic potential and can present the glycolipid α-galactosylceramide to iNKT cells. Thus, iNKT cells emerge as the first subset of regulatory T cells that are required for effective hematopoiesis in both steady-state conditions and under conditions of immune activation.
Introduction
Self-renewal and differentiation of hematopoietic stem cells (HSCs) and progenitor cells depends on integration of signals from the hematopoietic cells themselves and from the diverse cellular components of the microenvironment, including stromal and T cells.1 The role of T cells in hematopoiesis is complex and not fully understood.2-8
T cells activated in the context of the immune response regulate the activity of hematopoietic cells and their lineage-committed progenitors through production of a variety of cytokines such as IL-3, GM-CSF, and oncostatin M.2,3,7,8
Recent murine data suggest that T cells are also required for the maintenance of normal, steady-state hematopoiesis.2,3 Monteiro et al3 showed that T-cell–deficient mice exhibited defective maturation of myeloid progenitors and that this could be restored by reconstitution with CD4+ cells. This supports observations in a STAT4–/– T helper 1 (Th1)–deficient mouse model in which the numbers of cycling progenitors were reduced.2 T-cell–depleted grafts in humans as well as in mice are associated with increased graft failure rates, implying that the role of T cells in regulating hematopoiesis extends beyond the committed progenitor compartment and includes primitive HSCs with potential for long-term engraftment.9
Although there is evidence of regulation of hematopoiesis by T cells and especially CD4+ T cells, little is known about the role of other T-cell subsets and in particular regulatory T cells in this process. Similarly, the exact mechanisms through which T cells regulate the function of primitive HSCs remain to be determined.
Natural killer T (NKT) cells are a small subset of immunoregulatory T cells10,11 that modulate adaptive and innate immune responses by promptly secreting Th1/2 polarizing cytokines when activated.10,12,13 Two lines of evidence suggest that NKT cells may play an important role in regulating hematopoiesis. First, when activated, murine NKT cells secrete cytokines with hematopoietic potential, including GM-CSF and IL-3,10,12,13 and second, mice treated with the NKT-cell ligand alpha-galactosylceramide (αGalCer) develop increased committed hematopoietic progenitor activity in the bone marrow and spleen associated with production of NKT-cell–derived GM-CSF and IL-3.14 NKT cells possess a characteristic T-cell receptor (TCR) that is highly conserved between humans and mice. It consists of an invariant TCR α chain (Vα24Jα18 in humans15,16 and its homolog Vα14Jα18 in mice16,17 ), paired with a biased set of β chains (preferentially Vβ11 in humans). NKT cells are restricted by CD1d,10,11 a MHC-like, glycolipid-presenting molecule known to be expressed on professional antigen-presenting cells, thymocytes, monocytes, B cells, and a variety of epithelial tissues, including keratinocytes and enterocytes.18-21 However, whether CD1d is expressed on HSCs and could therefore mediate direct NKT-cell–HSC interactions, is unknown.
These observations led us to postulate that NKT cells regulate hematopoiesis when activated in the course of the immune response and are also necessary for the maintenance of normal steady-state hematopoiesis. Secretion of hematopoietic cytokine and/or specific interaction between the invariant TCR and CD1d on hematopoietic stem/progenitor cells might be necessary for these effects. To test these hypotheses we investigated the effects of addition and depletion of NKT cells on both committed progenitor-cell cultures and long-term initiating cells; we assessed CD1d expression and function in purified CD34+ cells and their subsets; and we used a NKT-cell–deficient mouse model to evaluate the role of NKT cells in steady-state hematopoiesis.
Materials and methods
Mice
Seven- to 10-week-old female C57BL/6 mice and mice devoid of Vα14 NKT cells as a result of specific deletion of the TCR Jα18 gene segment22 and backcrossed to C57BL/6 mice 9 times23 were used in the study. All mice were maintained in the Biological Services Unit at the John Radcliffe Hospital, Oxford, United Kingdom, and used according to established institutional guidelines. The study was approved by the Ethical Review Committee of the University of Oxford.
Selection of NKT cells and NKT-cell depletion of cord-blood mononuclear cells (CBMNCs)
Cord blood was obtained from the North London Blood Transfusion Centre (London, United Kingdom). For expansion of CB NKT cells, CBMNCs were obtained after density centrifugation over Ficoll. Fresh NKT cells were obtained by staining CBMNCs with anti–TCR Vα24 PE and anti-Vβ11 FITC antibodies (Serotec, Oxford, United Kingdom) and flow sorted on a FACSDiva (fluorescence-activated cell sorting; Becton Dickinson, Oxford, United Kingdom), achieving sorting purity of 95% or greater. For NKT-cell expansion, CBMNCs were plated at 1 to 2 × 106 cells per well in 24-well plates and cultured in RPMI 1640 supplemented with 5% human serum, 1% l-glutamine, 1% penicillin and streptomycin, α-galactosylceramide24 (αGalCer) at 100 ng/mL, and IL-2 (20 IU/mL) every 3 days. After 10 to 12 days in culture, expanded NKT cells were sorted as described for fresh NKT cells. CBMNCs were rigorously depleted of NKT cells after staining with anti–TCR Vα24 PE and anti-Vβ11 FITC antibodies and flow sorting. In parallel, NKT-cell–replete cells were obtained by sorting the same anti–TCR Vα24–Vβ11-stained CBMNCs but flow-sorted only on the basis of their FSC and SSC characteristics.
Immunomagnetic-bead cell separation
CD34+ cells were isolated from CBMNCs either by positive or negative selection. For positive selection, the cells were labeled with anti-CD34–conjugated immunomagnetic beads and isolated according to the manufacturer's instructions (magnetic cell sorting [MACS]; Miltenyi Biotec, Surrey, United Kindgom). For negative selection of CD34+ cells, lineage+ cells were removed through negative selection using a cocktail for the following markers: CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, CD41, and glycophorin A (Stem Cell Technologies, Vancouver, BC). CD1d+Lin–CD34+ cells were sorted on a FACSDiva flow sorter (Becton Dickinson) and then used for in vitro clonogenic assays (see “Human CBMNC hematopoietic clonogenic assays”).
Immunophenotypic analysis
CD34+ cells were MACS-selected from CBMNCs and subsequently stained with the following mouse antihuman antibodies: CD1d-biotin (clone CD1d42), streptavidin PE, CD34 PerCP, CD38-APC, CD33-FITC, CD2-FITC, and CD19-FITC, all from BD-Pharmingen (Mississauga, ON); CD117-APC, CD90-FITC, CD7-APC, and CD71-FITC from Caltag (Burlingame, CA); and CD133-FITC from R&D Systems (Abingdon, United Kingdom); as controls, appropriate isotypes were used. Data acquisition and analysis were performed on a FACSCalibur (Becton Dickinson) equipped with 2 lasers and CellQuest Pro software.
For immunophenotypic analysis of murine BM cells, cells were flushed from femurs and tibias into Iscove MDM medium supplemented with 2% fetal bovine serum (Stem Cell Technologies). After red-cell lysis with ammonium chloride solution (Stem Cell Technologies) and counting, cells were blocked for 10 minutes with rat anti–mouse CD16/32 and subsequently stained with the following antimouse monoclonal antibodies: CD117-FITC (clone 2B8) and isotype control from BD-Pharmingen; Sca-1–Biotin (clone CT-6A.2), coupled with streptavidin-APC, from Caltag. The murine lineage cocktail consisted of PE-conjugated CD3, CD4, CD5, CD8a, CD11b, NK1.1, B220 (RA3-6B2), Gr-1, and TER-119, all from Caltag; a cocktail of the corresponding isotype controls was used as the negative control. After dead-cell exclusion with propidium iodide, acquisition and analysis were performed as described for the human cells.
Hematologic analysis of murine peripheral blood and bone marrow
Complete blood counts were performed in mouse peripheral blood using a Sysmex XE2100 counter (Norderstedt, Germany). Differential counts on peripheral-blood smears were performed under an optical microscope after staining with hematoxylin and eosin.
Human CBMNC hematopoietic clonogenic assays
To study the effect of activated NKT cells on hematopoiesis, 0.5 to 5 × 103 CBMNC flow-sorted, purified-activated NKT cells were cocultured with MACS-selected CB CD34+ cells at indicated ratios for 18 to 24 hours in U-bottom 96-well plates and 0.5 to 5 × 103 were then plated for colony-forming unit (CFU) assays in methylcellulose without exogenous cytokines. In some experiments, cultures were treated with varying doses of an anti–GM-CSF neutralizing polyclonal Ab (R&D Systems) or isotype Ig, followed by plating in methylcellulose. In a separate set of experiments, 3 × 105 CBMNC flow-sorted, purified-activated NKT cells were cocultured with 3 × 104 MACS-selected CB CD34+ cells. After 18 to 24 hours, supernatants were collected for GM-SCF and IL-3 enzyme-linked immunosorbent assays (ELISAs), whereas the cells were stained for intracellular IFNγ (see “ELISA assays”).
For cytokine-replete CFU assays, cells were plated in Methocult 4435 “complete” 1% methylcellulose medium, supplemented with 30% fetal bovine serum, 1% bovine serum albumin, 3 U/mL rh-erythropoietin, 10–4 M 2-mercaptoethanol, 2 mM L-glutamine, and the following cytokines: 50 ng/mL rh stem-cell factor, 20 ng/mL rh GM-CSF, 20 ng/mL rh IL-3, 20 ng/mL rh IL-6, and 20 ng/mL rh G-CSF (Stem Cell Technologies). All cultures were performed in duplicates, and the numbers of CFUs were scored after 14 days. Cells were also plated for long-term culture-initiating cell (LTC-IC) assay established on mouse fibroblasts (M2-10B4) as stroma feeders. Mouse fibroblasts were irradiated at 8000 cGy and plated in 96-well collagen-coated plates (12 500 cells/well). Cells were seeded by serial dilutions (4 dilutions with 10 replicates each) and cultured in long-term culture medium (MyeloCult H5100; Stem Cell Technologies) supplemented with hydrocortisone 21–hemi-succinate (10–6 M). After 5 weeks, nonadherent and adherent cells were trypsinized and plated in cytokine-supplemented methylcellulose (Methocult 4435; Stem Cell Technologies) and scored after an additional 18 to 21 days of culture. Frequency of LTC-ICs was performed with L-Calc software (Stem Cell Technologies).
Murine bone marrow (BM) cell clonogenic assays
For CFU clonogenic assays, BM cells were plated in cytokine-replete methylcellulose medium (Methocult 3434; Stem Cell Technologies) in duplicates, and the numbers of the colonies were scored after 12 days. For the LTC-IC assays BM cells were added by using limiting dilution (4 doses with 12 replicates each) to feeder layers established with primary murine cells irradiated with 1500 cGy, after an initial 2-week culture period. Test cells were cultured in long-term culture medium (MyeloCult H5300; Stem Cell Technologies) supplemented with hydrocortisone 21–hemi-succinate (10–6 M; Stem Cell Technologies). Cultures were maintained at 33°C with weekly half-media exchanges of Myelocult. Four weeks later, nonadherent and adherent cells were removed and plated for CFU assays. The LTC-IC frequency was calculated by using L-Calc software (Stem Cell Technologies).
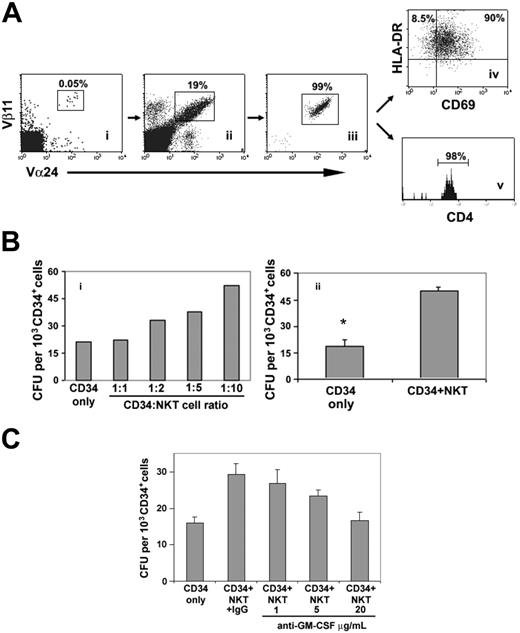
Activated NKT cells enhance in vitro short-term clonogenic hematopoietic activity through production of GM-CSF. (A) Human cord-blood NKT cells (i) were expanded in vitro (ii), in the presence of αGalCer. Flow-sorted cells (iii) were fully activated as determined by CD69 and HLA-DR expression (iv), and almost all were CD4+ (v). (B) CD34+ cells were cocultured with activated NKT cells for 18 to 24 hours at different CD34/NKT cell ratios and plated in methylcellulose in the absence of exogenous cytokines (i). The clonogenic capacity of CD34+ cells was enhanced at CD34/NKT cell ratios of 1:2 or greater. Only myeloid colonies were formed (representative of 2 independent experiments). The enhancing effect of NKT cells on myelopoiesis was confirmed in an extended panel of CFU assays performed in the absence of exogenous cytokines (ii). Values are the mean and SEM of 5 independent experiments (*P < .05 by paired Student t test). (C) Effect of an anti–GM-CSF neutralizing antibody on the clonogenic capacity of CD34+ cells cocultured with activated CB NKT cells at 1:10 ratio. The clonogenic capacity was inhibited by anti–GM-CSF in a dose-dependent manner. For each condition, values are the mean and SEM of quadruplicate assays. Representative of 2 independent experiments.
Activated NKT cells enhance in vitro short-term clonogenic hematopoietic activity through production of GM-CSF. (A) Human cord-blood NKT cells (i) were expanded in vitro (ii), in the presence of αGalCer. Flow-sorted cells (iii) were fully activated as determined by CD69 and HLA-DR expression (iv), and almost all were CD4+ (v). (B) CD34+ cells were cocultured with activated NKT cells for 18 to 24 hours at different CD34/NKT cell ratios and plated in methylcellulose in the absence of exogenous cytokines (i). The clonogenic capacity of CD34+ cells was enhanced at CD34/NKT cell ratios of 1:2 or greater. Only myeloid colonies were formed (representative of 2 independent experiments). The enhancing effect of NKT cells on myelopoiesis was confirmed in an extended panel of CFU assays performed in the absence of exogenous cytokines (ii). Values are the mean and SEM of 5 independent experiments (*P < .05 by paired Student t test). (C) Effect of an anti–GM-CSF neutralizing antibody on the clonogenic capacity of CD34+ cells cocultured with activated CB NKT cells at 1:10 ratio. The clonogenic capacity was inhibited by anti–GM-CSF in a dose-dependent manner. For each condition, values are the mean and SEM of quadruplicate assays. Representative of 2 independent experiments.
CD34+CD1d+ cell–mediated antigen presentation
Positive (MACS) selected CB CD34+ cells were stained with CD34-FITC (BD Biosciences, Heidelberg, Germany) and CD1d-biotin (clone CD1d42) coupled with streptavidin PE and then sorted into total CD34+ and CD1d–CD34+ subpopulations (purity ≥ 98%). Resting autologous NKT cells were isolated from the flow-through by sorting after staining with anti–TCR Vα24 and -Vβ11 mAbs. Fifty thousand purified total CD34+ or CD1d–CD34+ cells and 2000 NKT cells were cocultured in lymphocyte medium supplemented with low-dose IL-2 (5 IU/mL) and either αGalCer (100 ng/mL) or vehicle. Twenty-four hours later cells were stained with anti-CD69 FITC (Caltag), anti–TCR Vα24Jα18-PE (Pharmingen, Oxford, United Kingdom), and HLA-DR–APC and analyzed using a FACSCalibur. In a separate set of experiments, activation of NKT cells was assessed by intracellular staining for IFNγ (see “Intracellular staining”).
ELISA assays
IL-3 and GM-CSF were quantified by a Quantikine immunoassay (R&D Systems) according to the instructions of the manufacturer. Samples were tested in duplicate.
Intracellular staining
For intracellular staining of αGalCer-expanded NKT cells, cells were treated with PMA/ionomycin (2.5 ng/mL and 500 ng/mL, respectively;
Sigma, St Louis, MO) and brefeldin (5 μg/mL) for 4 hours and then stained with PE anti–TCR Vα24Jα18, followed by fixation and permeabilization (Fix&Perm Kit; Caltag) and staining with FITC-IFNγ (Pharmingen) and analysis on a FACSCalibur.
For intracellular staining of freshly isolated NKT cells cocultured with CD34+ and CD34+CD1d– cells, cells were treated with brefeldin 5 μg/mL but not PMA/ionomycin for the last 4 hours of the coculture and subsequently stained with surface anti–TCR Vα24Jα18 and anti-CD4 and intracellular anti-IFNγ.
Statistical analysis
All analyses were performed using SPSS 12.0 software (SPSS Science, Chicago, IL). Data are presented as mean ± SEM. The significance of the differences was assessed by paired or unpaired, one-sided Student t test as appropriate.
Results
Activated human NKT cells enhance myelopoiesis
To test whether αGalCer-activated human NKT cells enhance hematopoiesis, MACS-selected CB CD34+ cells were set up in liquid culture (10% FCS in DMEM) in the presence or absence of activated NKT cells at various NKT/CD34 cell ratios (NKT cells were 99% TCR Vα24+/Vβ11+, 98% CD4+, 98% HLA-DR+, 90% CD69+; Figure 1A). After 24 hours the cells were plated in clonogenic assays in semisolid medium without exogenous cytokines. Addition of NKT cells in a ratio of 2:1 to 10:1 caused a dose-dependent increase in myeloid colonies compared with CD34+ cells plated in the absence of NKT cells, with a maximal effect at an NKT/CD34+ cell ratio of 10:1 (Figure 1B). This was confirmed by testing 5 further CB samples at a NKT/CD34 cell ratio of 10:1: clonogenic capacity increased by 2.7-fold (P < .05; Figure 1B). NKT cells cultured alone generated no colonies (not shown). GM-CSF but not IL-3 was detected (Table 1) in the NKT-CD34+ cell coculture supernatants and not in supernatants from CD34+ cells cultured alone (not shown). Intracellular staining revealed that, as well as GM-CSF, activated NKT cells also secreted IFNγ (Table 1). These results suggest that NKT cells, despite their secretion of the myelosuppressive cytokine IFNγ, exerted a stimulatory effect on myeloid colony growth through their secretion of GM-CSF (Figure 1C). This was further supported by the dose-dependent decrease in the number of myeloid colonies in the presence of a neutralizing anti–GM-CSF Ab (Figure 1C).
Cytokine levels in supernatants from NKT-cell–CD34+-cell coculture
IL-3* was not detected in any of the experiments.
Levels of the cytokines in supernatants of coculture of 3 × 105 preactivated CB NKT cells plus 3 × 104 CD34+ cells.
Percentage of NKT cells secreting IFNγ as determined by intracellular staining and flow-cytometry analysis.
Cord-blood MNCs give rise to reduced numbers of hematopoietic progenitors in the absence of NKT cells
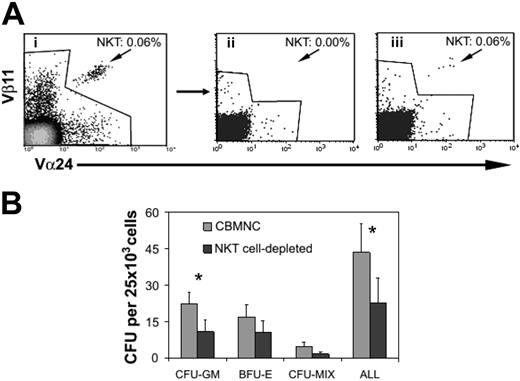
To investigate whether NKT cells play a role in steady-state hematopoiesis (ie, in conditions where there is no preactivation of NKT cells), we compared the clonogenic capacity of NKT-cell– replete CBMNCs and CBMNCs which had undergone rigorous NKT-cell depletion (Figure 2A). CBMNCs were cultured in cytokine-supplemented semisolid medium to determine whether NKT-cell depletion had a selective effect on myeloid lineage colonies or also affected the growth of erythroid and multipotent colony-forming progenitors. Depletion of NKT cells caused a significant reduction (by 48%) in CFU-GM (n = 6; P < .05) compared with NKT-cell–replete cultures (Figure 2B). A statistically insignificant reduction in BFU-E and CFU-mix was also observed (n = 6; P = .07 for both colony assays; Figure 2B). In a further set of 4 experiments we also tested whether NKT-cell depletion altered the numbers of LTC-ICs. As shown in Table 2, CBMNCs depleted of NKT cells produced fewer LTC-ICs in each of the 4 samples tested (mean reduction, 44% ± 18%).
LTC-IC frequency (per 106 cells) in total and NKT cell–depleted CBMNC
Experiment no. . | Total CBMNCs . | NKT-cell—depleted CBMNCs . |
|---|---|---|
| 1 | 1539 | 628 |
| 2 | 526 | 327 |
| 3 | 343 | 286 |
| 4 | 1191 | 600 |
| Mean ± SD | 900 ± 644 | 460 ± 186 |
Experiment no. . | Total CBMNCs . | NKT-cell—depleted CBMNCs . |
|---|---|---|
| 1 | 1539 | 628 |
| 2 | 526 | 327 |
| 3 | 343 | 286 |
| 4 | 1191 | 600 |
| Mean ± SD | 900 ± 644 | 460 ± 186 |
NKT-cell depletion of CBMNCs reduces CFU activity. (A) CBMNCs (i) were depleted of NKT cells (ii) or not depleted (iii) by flow sorting. (B) The short-term clonogenic capacity of NKT-cell–replete and –depleted CBMNCs were compared in CFU assays performed in the presence of cytokines. Values are the mean and SEM of 6 independent experiments (*P < .05 by paired Student t test).
NKT-cell depletion of CBMNCs reduces CFU activity. (A) CBMNCs (i) were depleted of NKT cells (ii) or not depleted (iii) by flow sorting. (B) The short-term clonogenic capacity of NKT-cell–replete and –depleted CBMNCs were compared in CFU assays performed in the presence of cytokines. Values are the mean and SEM of 6 independent experiments (*P < .05 by paired Student t test).
Human HSCs express CD1d and display short- and long-term clonogenic activity
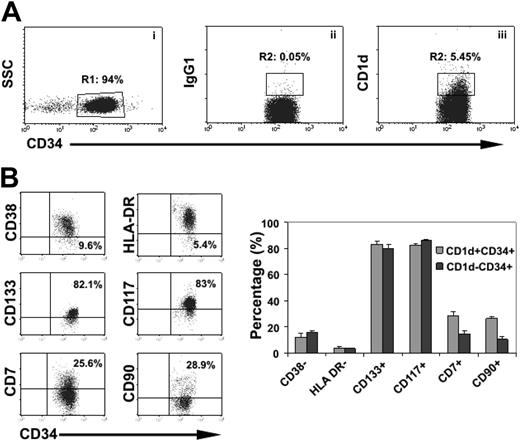
Because the immunoregulatory functions of NKT cells depend on interaction of their invariant TCRs with CD1d expression on target cells, we postulated that if NKT cells directly regulated hematopoiesis, CD1d must be expressed on HSCs. Because whether CD1d is expressed by HSCs is unknown, we studied the expression of CD1d in different subsets of hematopoietic stem/progenitor cells as defined by surface marker expression. We found that 5.2% ± 2.1% of MACS-selected cord-blood CD34+ cells express CD1d (n = 6; Figure 3A). Assessment of markers associated with more primitive hematopoietic cells showed that similar proportions of CD34+CD1d+ and CD34+CD1d– cells were CD117+, CD133+, HLA-DR–, and CD38–. However, interestingly, the frequencies of CD7 and CD90 were 2- and 3-fold higher, respectively, in CD34+CD1d+ cells as compared with CD34+CD1d– cells (Figure 3B), indicating that CD1d also marks subsets enriched in lymphoid progenitors.25-27 Expression of markers associated with more mature, committed progenitors (CD33, CD71, and CD2) was the same on CD34+CD1d+ cells as on CD34+CD1d– cells (not shown) apart from CD19 which was expressed on a much higher percentage of CD34+CD1d+ cells compared with CD34+CD1d– cells (12.8% ± 4.9% versus 0.8% ± 0.3%, respectively). To confirm that CD1d is expressed on early and late clonogenic hematopoietic progenitors we studied the clonogenic capacity of flow-sorted lineage-depleted CD34+CD1d+ and CD34+CD1d– cells. In 3 independent experiments, we found that purified CD34+CD1d+ cells contained substantial CFU and LTC-IC activity, although at a frequency slightly lower than CD34+CD1d– cells (Table 3). Specifically, in 3 independent experiments, CD34+CD1d+ cells gave rise to CFU-GM, BFU-E, and CFU-mix with a total of 112 ± 16 CFU per 103 cells, whereas CD34+CD1d– cells gave rise to 240.4 ± 26 CFU per 103 cells. CD34+CD1d+ cells also contained LTC-IC activity, although at a slightly lower frequency as compared with CD34+CD1d– cells (24.8 ± 7.1 LTC-IC per 103 cells versus 37 ± 11 LTC-IC per 103 cells, respectively; Table 3). These data indicate that CD1d is expressed on early as well as committed hematopoietic progenitors.
CFU and LTC-IC frequency (per 103 cells) in cord-blood CD34+ CD1d+ and CD34+ CD1d– cells
. | CFU-GM . | . | BFU-E . | . | CFU-mix . | . | Total CFU . | . | LTC-IC . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | CD1d+ . | CD1d- . | CD1d+ . | CD1d- . | CD1d+ . | CD1d- . | CD1d+ . | CD1d- . | CD1d+ . | CD1d- . | |||||
| Experiment no. | |||||||||||||||
| 1 | 46.6 | 126.7 | 33.0 | 86.6 | 20 | 50 | 99.0 | 263.0 | 28.5 | 34.5 | |||||
| 2 | 51.4 | 173.0 | 38.5 | 23.0 | 40 | 51 | 130.0 | 247.0 | 16.6 | 27.8 | |||||
| 3 | 57.0 | 123.0 | 31.0 | 65.0 | 22 | 23 | 110.0 | 211.0 | 29.4 | 50.0 | |||||
| Mean ± SD | 51.6 ± 5.2 | 140 ± 27 | 34.2 ± 3.8 | 58.2 ± 32.3 | 27 ± 11 | 41 ± 15 | 113.2 ± 15 | 240.4 ± 26 | 24.8 ± 7.1 | 37 ± 11 | |||||
. | CFU-GM . | . | BFU-E . | . | CFU-mix . | . | Total CFU . | . | LTC-IC . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | CD1d+ . | CD1d- . | CD1d+ . | CD1d- . | CD1d+ . | CD1d- . | CD1d+ . | CD1d- . | CD1d+ . | CD1d- . | |||||
| Experiment no. | |||||||||||||||
| 1 | 46.6 | 126.7 | 33.0 | 86.6 | 20 | 50 | 99.0 | 263.0 | 28.5 | 34.5 | |||||
| 2 | 51.4 | 173.0 | 38.5 | 23.0 | 40 | 51 | 130.0 | 247.0 | 16.6 | 27.8 | |||||
| 3 | 57.0 | 123.0 | 31.0 | 65.0 | 22 | 23 | 110.0 | 211.0 | 29.4 | 50.0 | |||||
| Mean ± SD | 51.6 ± 5.2 | 140 ± 27 | 34.2 ± 3.8 | 58.2 ± 32.3 | 27 ± 11 | 41 ± 15 | 113.2 ± 15 | 240.4 ± 26 | 24.8 ± 7.1 | 37 ± 11 | |||||
CD1d expression in CB HSCs. (A) MACS-selected live cord-blood CD34+ cells gated in R1 (i) were analyzed for expression of CD1d (iii) or isotypic control (ii). (B). Expression of primitive markers on CD1d+CD34+ and CD1d+CD34– cells. Plots are gated in the combination of R1 + R2 gates in panel A. Representative plots are shown on the left and cumulative results on the right. Values and bars represent the mean and SEM respectively, n = 4.
CD1d expression in CB HSCs. (A) MACS-selected live cord-blood CD34+ cells gated in R1 (i) were analyzed for expression of CD1d (iii) or isotypic control (ii). (B). Expression of primitive markers on CD1d+CD34+ and CD1d+CD34– cells. Plots are gated in the combination of R1 + R2 gates in panel A. Representative plots are shown on the left and cumulative results on the right. Values and bars represent the mean and SEM respectively, n = 4.
CD1d-expressing HSCs can activate NKT cells
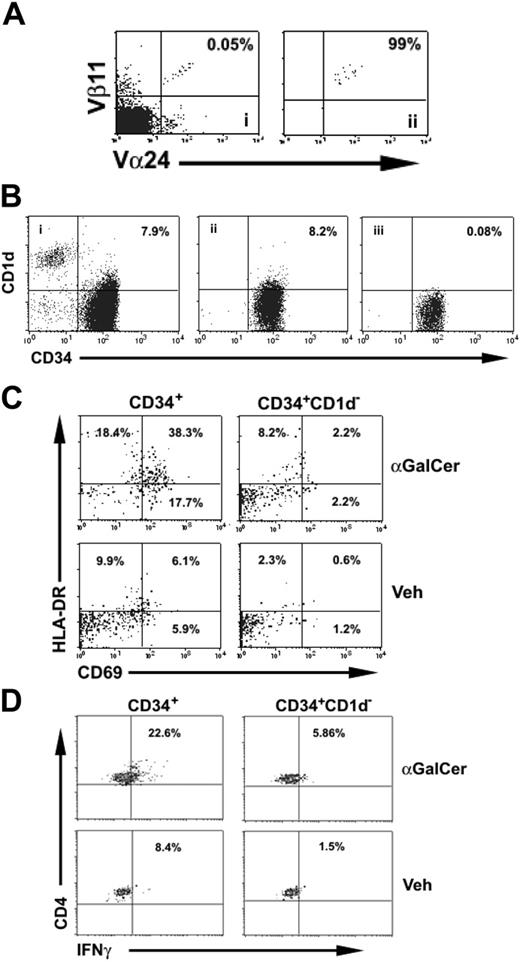
Finally, we tested whether interaction of the invariant TCR with CD1d expressed on HSCs was functional and could trigger activation of NKT cells. For this purpose, NKT cells were sorted to a purity of greater than 95% (Figure 4A). At the same time, autologous, MACS-selected CD34+ cells were sorted into 2 populations: a population of “total” CD34+ cells (ie, both CD34+CD1d+ and CD34+CD1d– cells) and a population of CD34+ CD1d– cells (each with a purity of 98%; Figure 4B).
Each of these 2 populations of CD34+ cells was then cocultured with NKT cells in the presence of either αGalCer or vehicle, and the activation status of the NKT cells was determined by coexpression of CD69 and HLA-DR (Figure 4C). In this system, only the total CD34+ cell population contained CD1d-expressing cells, and the only cells expressing CD1d were the CD34+ cells because other CD1d+ cells (eg, monocytes and B cells) had been removed by the rigorous flow-sorting procedure. In the presence of αGalCer and after 24 hours of coculture with total CD34+ cells, 38% of NKT cells were CD69+HLA-DR+, whereas only 2.2% of NKT cells cocultured with CD1d–CD34+ cells were CD69+HLA-DR+. NKT cells cocultured with total CD34+ cells in the presence of vehicle were not activated (6.1% CD69+HLA-DR+) and neither were NKT cells cocultured with CD1d–CD34+ cells (0.6% CD69+HLA-DR+). Similarly, in a separate set of experiments, a markedly increased proportion of NKT cells cocultured with CD34+ cells and αGalCer produced IFNγ as determined by intracellular staining than under in any of the other 3 conditions (Figure 4D). Thus, 2 separate lines of evidence suggest that CD1d expressed on CD34+ cells can present antigen to NKT cells.
CD34+CD1d+ cells present antigen to NKT cells. (A) Fresh NKT cells from cord-blood samples (i) were purified by flow sorting (ii) after staining with anti–TCR Vα24 and -Vβ11 mAbs. (B) MACS-selected CB CD34+ cells (i) were further flow-sorted into total CD34+ (ii) and CD1d–CD34+ (iii) fractions and cocultured with highly purified fresh NKT cells. (C) Analysis of surface activation markers HLA-DR and CD69 after 24 hours revealed that, in the presence of αGalCer, NKT cells cocultured with total CD34+ cells were highly activated compared with NKT cells cocultured with CD1d–CD34+ cells. In the presence of vehicle, NKT cells cultured either with total CD34+ or CD1d–CD34+ cells showed little evidence of activation. Plots are gated in NKT cells identified by staining with the NKT-cell–specific mAb Vα24Jα18 after dead-cell exclusion with propidium iodide. Data are representative of 2 independent experiments. (D) Activation of NKT cells cocultured with CD34+ or CD34+ CD1d– cells with or without αGalCer as determined by intracellular staining for IFNγ. As with surface activation markers, NKT-cell production of IFNγ was highest when they were cocultured with total CD34+ cells and in the presence of αGalCer. Data are representative of 2 independent experiments.
CD34+CD1d+ cells present antigen to NKT cells. (A) Fresh NKT cells from cord-blood samples (i) were purified by flow sorting (ii) after staining with anti–TCR Vα24 and -Vβ11 mAbs. (B) MACS-selected CB CD34+ cells (i) were further flow-sorted into total CD34+ (ii) and CD1d–CD34+ (iii) fractions and cocultured with highly purified fresh NKT cells. (C) Analysis of surface activation markers HLA-DR and CD69 after 24 hours revealed that, in the presence of αGalCer, NKT cells cocultured with total CD34+ cells were highly activated compared with NKT cells cocultured with CD1d–CD34+ cells. In the presence of vehicle, NKT cells cultured either with total CD34+ or CD1d–CD34+ cells showed little evidence of activation. Plots are gated in NKT cells identified by staining with the NKT-cell–specific mAb Vα24Jα18 after dead-cell exclusion with propidium iodide. Data are representative of 2 independent experiments. (D) Activation of NKT cells cocultured with CD34+ or CD34+ CD1d– cells with or without αGalCer as determined by intracellular staining for IFNγ. As with surface activation markers, NKT-cell production of IFNγ was highest when they were cocultured with total CD34+ cells and in the presence of αGalCer. Data are representative of 2 independent experiments.
NKT-cell regulation of hematopoiesis in vivo
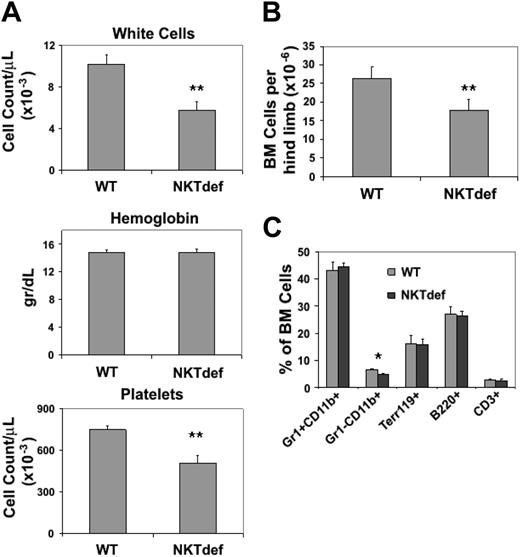
To look at the role of NKT cells in regulating hematopoiesis in vivo we went on to evaluate hematopoiesis in mice rendered NKT-cell deficient by knocking out the Jα18 gene.22 Jα18–/– (NKTdef) mice are unable to form the invariant TCR Vα14Jα18 chain and therefore have a selective loss of the invariant NKT cells. Their hematopoiesis has not been investigated in detail. Peripheral-blood analysis showed that compared with wild-type mice, white-cell counts were decreased in NKTdef mice by 43% (n = 10, P < .001) and platelet counts were decreased by 32% (n = 10, P < .001); hemoglobin concentrations were normal (Figure 5A), but there was no significant difference in the peripheral-blood differential counts in wild-type and NKTdef mice (data not shown). BM cellularity was reduced by 35% in NKTdef mice compared with wild-type mice (n = 13, P < .001; Figure 5B), suggesting that the leucothrombocytopenia was due to reduced marrow production. Flow cytometric analysis of bone marrow cells indicated that there was global impairment of hematopoiesis because, with the exception of Gr1–CD11b+ cells (ie, monocytic cells), the relative frequencies of myeloid, erythroid, and lymphoid lineages in NKTdef mice were not significantly different compared with WT mice (Figure 5C).
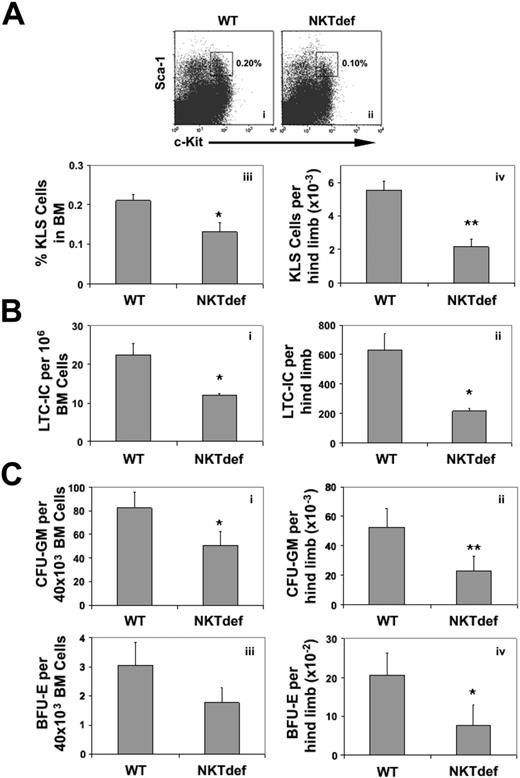
We assessed the numbers of c-Kit+Lineage–Sca-1+ cells, a subset of HSCs enriched in LTC-ICs and in vivo long-term repopulating activity.1 Compared with wild-type animals, the frequency and absolute numbers per hind limb of c-Kit+Lineage–Sca-1+ cells were reduced in NKTdef mice by 40% (n = 8, P < .05) and 61% (n = 8, P < .001), respectively (Figure 6A). Consistent with this, the relative frequency and the absolute numbers of LTC-ICs in NKTdef mice were decreased by 50% (n = 4, P < .05) and 68% (n = 4, P < .05), respectively (Figure 6B). Similarly, the frequency and total number of CFU-GMs per hind limb were reduced in NKTdef mice compared with WT mice by 40% and 57%, respectively (n = 8, P < .05; Figure 6C). Interestingly, despite the normal Hb levels in the NKTdef mice, the frequency and total number of BFU-Es were both reduced by 43% (n = 8, P = .07) and 62% (n = 8, P < .05), respectively. Because this could be due to compensatory enhancement of erythropoiesis in the spleen, we assessed the frequency of splenic erythroid progenitor and precursor cells by BFU-E assays and staining for the erythroid marker Ter-119. These showed that erythropoiesis was increased approximately 3-fold in NKTdef as compared with WT mice (data not shown). Thus, absence of NKT cells is associated with decreased early and late hematopoietic clonogenic capacity in mice in vivo as well as in humans in vitro.
Hematologic analysis of NKT-cell–deficient mice. (A) Complete blood counts in WT and NKTdef mice are shown (n = 10). (B) Bone marrow cellularity in WT and NKTdef mice (n = 13) as assessed by the number of nucleated cells flushed from hind limbs. (C) Relative frequency of bone marrow precursors in WT and NKTdef mice (n = 13) as assessed by staining with lineage-specific mAb and flow cytometry (*P < .05; **P < .001 by unpaired Student t test).
Hematologic analysis of NKT-cell–deficient mice. (A) Complete blood counts in WT and NKTdef mice are shown (n = 10). (B) Bone marrow cellularity in WT and NKTdef mice (n = 13) as assessed by the number of nucleated cells flushed from hind limbs. (C) Relative frequency of bone marrow precursors in WT and NKTdef mice (n = 13) as assessed by staining with lineage-specific mAb and flow cytometry (*P < .05; **P < .001 by unpaired Student t test).
Discussion
T cells regulate the function of committed hematopoietic, especially myelomonocytic, progenitors under conditions of immune activation.3,7,28 Recent data from murine models indicate that T cells are also required for homeostatic control of committed progenitors.2,3 We show here that NKT cells, a small subset of immunoregulatory T cells, regulate hematopoiesis in steady-state conditions and in conditions of immune activation.
Decreased HSC pool size, committed and primitive progenitor activity in NKT-cell–deficient mice. (A) c-Kit+Lineage–Sca-1+ (KLS) HSCs in WT (i) and NKTdef (ii) mice. Dot blots gated on Lin– propidium iodide– cells show c-Kit+Lin–Sca-1+ cells. The frequency (iii) and absolute number (iv) of c-Kit+Lineage–Sca-1+ per hind limb in WT and NKTdef mice are shown (n = 8). (B) Frequency (i) and absolute number (ii) of LTC-IC per hind limb in WT and NKTdef mice are shown (n = 4). (C) The frequency (i,iii) and absolute number (ii,iv) of CFU-GM and BFU-E per hind limb, respectively, in WT and NKTdef mice (n = 8) (*P < .05; **P < .001 by unpaired Student t test).
Decreased HSC pool size, committed and primitive progenitor activity in NKT-cell–deficient mice. (A) c-Kit+Lineage–Sca-1+ (KLS) HSCs in WT (i) and NKTdef (ii) mice. Dot blots gated on Lin– propidium iodide– cells show c-Kit+Lin–Sca-1+ cells. The frequency (iii) and absolute number (iv) of c-Kit+Lineage–Sca-1+ per hind limb in WT and NKTdef mice are shown (n = 8). (B) Frequency (i) and absolute number (ii) of LTC-IC per hind limb in WT and NKTdef mice are shown (n = 4). (C) The frequency (i,iii) and absolute number (ii,iv) of CFU-GM and BFU-E per hind limb, respectively, in WT and NKTdef mice (n = 8) (*P < .05; **P < .001 by unpaired Student t test).
We found that human NKT cells activated with αGalCer secrete GM-CSF and when cocultured with CD34+ cells in the absence of exogenous cytokines have an enhancing effect on short-term hematopoiesis, mainly myelomonocytic activity. Neutralization experiments suggested that a substantial part of this enhancing effect is GM-CSF dependent. These findings would be broadly in line with an earlier report showing that in vivo activation of murine NKT cells by systemic administration of αGalCer, a powerful NKT-cell ligand, resulted in secretion of IL-3 and GM-CSF and increased myeloid as well as erythroid CFU activity in the spleen and peripheral blood.14 This effect was not observed in BM, although that may have related to the timing of sampling.14 In contrast to the murine data we did not detect any IL-3, and we saw only a modest (and not significant) effect of activated NKT cells on erythroid progenitor formation. Because IL-3 synergizes with Epo to stimulate erythroid progenitor activity,29 lack of IL-3 secretion by NKT cells and the absence of exogenous Epo could also explain the paucity of BFU-E activity in our system. Alternatively, the lack of erythroid activity may indicate that erythroid progenitors are more sensitive than their myeloid counterparts to the effects of IFNγ and TNFα, 2 myelosuppressive cytokines28,30,31 also secreted by activated CB NKT cells (this work, Kadowaki et al,32 and D'Andrea et al33 ).
Human NKT cells are not a homogeneous population; they are CD4+ or CD4–CD8αβ–.34,35 These subsets in adults comprise about equal proportions of NKT cells and differ in the type of cytokines they secrete.12,13 GM-CSF is secreted almost exclusively by CD4+ NKT cells, indicating that this subset may be responsible for the enhancing effect on myelopoiesis.12,13 In line with this we show that, as previously reported, the majority of cord-blood NKT cells are CD4+36 and secrete GM-CSF on activation.
The role of NKT cells and especially of their CD4+ and CD4– NKT subsets in the regulation of adult human hematopoiesis remains to be determined. Our preliminary results suggest that CD1d is expressed in adult CD34+ cells and that adult NKT cells affect hematopoiesis in a way similar to CB NKT cells (data not shown).
Whether NKT cells are required for steady-state hematopoiesis is more difficult to determine. Using human in vitro clonogenic assays, we provide evidence supporting the notion that NKT cells are important in this setting. Specifically, rigorous depletion of NKT cells resulted in approximately 50% reduction of CBMNC CFU as well as LTC-IC activity, indicating that early multipotential as well as committed progenitor cells are subject to regulation of their function by NKT cells. Consistent with the human in vitro data, we also show impaired in vivo hematopoiesis in NKTdef mice consisting of peripheral-blood cytopenia, reduced bone marrow cellularity, reduced size of the HSC pool, and reduced activity of early and late hematopoietic progenitors.
NKT cells may modulate steady-state hematopoiesis either indirectly, for example, by contributing to the activation of CD4+ T cells and their production of hematopoietic cytokines as proposed by Monteiro et al,3 or directly through cell contact with HSCs at different levels of the hematopoietic hierarchy. In support of the latter, we show for the first time that CD1d, the restricting element of NKT cells, is expressed in subsets of human HSCs that have been previously shown to mark lineage-committed and uncommitted progenitors with myeloid and lymphoid potential and that CD1d marks HSCs with early and late hematopoietic progenitor activity. The higher expression of the lymphoid progenitor marker CD7 on CD34+CD1d+ cells suggests that this subset might be enriched in lymphoid progenitors,27 explaining thus the lower number of myeloid and erythroid progenitors in CD34+CD1d+ cells as compared with CD34+CD1d– cells. In addition, we demonstrate that CD1d on HSCs can specifically activate NKT cells in the presence of a NKT-cell ligand. As well as establishing CD1d as a novel marker on HSCs, these data suggest that the NKT-cell–instructed regulation of hematopoiesis might operate through cell contact of NKT cells with HSCs and interaction of the invariant TCR with CD1d on HSCs.
In conclusion, we have shown that NKT cells when activated enhance the clonogenic capacity of myeloid progenitors and they are also required for maintenance of normal hematopoiesis in the absence of immune activation. Our findings offer further evidence for a concerted regulation of the immune and hematopoietic systems and the potential for new therapeutic approaches for the manipulation of hematopoiesis.
Prepublished online as Blood First Edition Paper, December 22, 2005; DOI 10.1182/blood-2005-07-2804.
Supported by the Leukaemia Research Fund and Leuka (I.K.), the Cancer Research UK (C399-A2291) (V.C.), the US Cancer Research Institute (V.C.), and the UK Medical Research Council (V.C.). A.K. is a Leukaemia Research Fund Bennett Senior Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal