Abstract
Hereditary systemic amyloidosis caused by fibrinogen Aα-chain gene mutations is an autosomal dominant condition with variable penetrance, usually of late onset, and typically presents with nephropathy leading to renal failure. Amyloid deposits often develop rapidly in transplanted kidneys, and concomitant orthotopic liver transplantation has lately been performed in several patients with the hope of halting amyloid deposition. Fibrinogen is produced in vitro by hepatocytes but also by other human cell types, and although the liver is the source of plasma fibrinogen in vivo in rats, this is not known in humans. Transplantation of livers expressing wild-type fibrinogen into patients with variant fibrinogen amyloidosis provides a unique opportunity to establish the source of human plasma fibrinogen. We therefore characterized plasma fibrinogen Aα-chain allotypes by electrospray ionization mass spectrometry mapping of tryptic digests before and after liver transplantation. Before liver transplantation, fibrinogen amyloidosis patients with the Glu526Val Aα-chain variant had approximately equal proportions of peptide with the wild-type sequence TFPGFFSPMLGEFVSETESR, and with the amyloidogenic variant sequence TFPGFFSPMLGEFVSVTESR, as expected for individuals heterozygous for the mutation. After transplantation, only the wild-type sequence was detected, and the liver is thus the source of at least 98% of the circulation fibrinogen.
Introduction
Fibrinogen1 circulates in the plasma at a concentration of 2 g/L to 4 g/L with a half-life of approximately 4 days and is a modest acute-phase reactant, increasing in concentration in response to most forms of tissue injury, infection, or inflammation. The native 340-kDa molecule is a disulfide-bonded homodimer, each protomer composed of 3 nonidentical disulfide cross-linked polypeptide chains (Aα, Bβ, and γ) that are synthesized under the control of 3 genes. Fibrinogen is produced by cultured hepatocytes in vitro, and plasma fibrinogen has been demonstrated in vivo to come from the liver in rats.2,3 However, in response to inflammatory mediators, expression of mRNA for individual polypeptide chains and their synthesis and/or secretion in vitro has also been described in a variety of nonhepatic cells, including epithelial cells from various different human tissues, granulosa cells, cervical carcinoma cells, and trophoblasts.1 Extra-hepatic fibrinogen synthesis may be important for tissue repair at local sites of injury, and/or may have a pathogenetic role, but it is not known whether it contributes significantly to either the normal plasma fibrinogen concentration or to increased concentrations in the acute-phase response.
Six mutations encoding sequence variation in the C-terminus of the fibrinogen Aα-chain gene are known to cause autosomal dominant hereditary systemic amyloidosis, in which the amyloid fibrils are composed of fragments of the variant polypeptide.4–13 Although there may be widespread visceral and occasional neural involvement, the kidneys are affected first, leading inexorably to end-stage renal failure. There is a high incidence of fibrinogen amyloid deposition in renal allografts as well as progression of amyloid deposition elsewhere.8,10,11 On the presumption that the liver is the source of the amyloidogenic variant fibrinogen, a combined liver and kidney transplant was first performed in 1995, in a patient with amyloidotic renal failure caused by the mutation encoding Glu526Val substitution in the mature Aα-chain.14 Zeldenrust et al14 reported this case in 2003, but meanwhile in 1996 we had undertaken combined liver and kidney transplantation in a patient with the same mutation, whose previous renal transplant had been destroyed by amyloid deposition and who had developed progressive hepatic amyloidosis leading to liver failure.10 These patients, and others who received liver allografts, have shown remarkable clinical benefit associated with cessation of amyloid deposition and regression of existing amyloid deposits.15,16
Liver transplantation for treatment of hereditary amyloidosis caused by an amyloidogenic mutation in a hepatically expressed gene was first performed in familial amyloid polyneuropathy caused by transthyretin gene mutations.17 This approach, which we have called surgical gene therapy,18 results in almost complete replacement of amyloidogenic variant transthyretin by normal wild-type protein in the plasma. Patients with the most common transthyretin mutation, Met30, usually show amyloid regression and clinical benefit after liver transplantation but in patients with other mutations, amyloid deposition continues, probably because wild-type transthyretin can also deposit as amyloid and because transthyretin is also significantly synthesized and secreted by the choroid plexus.17 In contrast, there is no evidence that wild-type fibrinogen can be amyloidogenic, but disappearance of the amyloidogenic fibrinogen variant from the plasma following liver transplantation, which is the rationale for the procedure, has not previously been investigated. We therefore examined plasma fibrinogen in patients with hereditary fibrinogen amyloidosis caused by the Aα-chain Glu526Val mutation, before and after liver transplantation. Here we show that variant fibrinogen is eliminated from the blood, validating liver transplantation as an appropriate therapy, and demonstrating for the first time that plasma fibrinogen is apparently derived entirely from the liver in humans.
Patients, materials, and methods
Patients and plasma
Two unrelated British males (cases 1 and 2) with hereditary systemic amyloidosis caused by the Glu526Val variant of fibrinogen Aα-chain, underwent combined heptorenal transplantation in 2004 in the United Kingdom.15 Plasma samples from a female patient (case 3) with the same condition, taken 7 years after hepatorenal transplantation,10 and from 2 other such patients with chronic renal failure awaiting transplantation (one male [case 4], one female [case 5]) were also analyzed. These individuals are unrelated, ages 54 to 67 years, and of British/English origin as far as is known. Plasma from healthy, age-matched control subjects was used for comparison. The novel medical care described here was performed with informed consent from each patient in accordance with the Declaration of Helsinki, and the study was performed with institutional review board approval by the ethics committees of the Royal Free Hospital and King's College Hospital.
Plasma was separated by centrifugation (3000g, 20 minutes, +4°C) within 4 to 6 hours of collecting citrate-anticoagulated venous blood from patients before and after transplantation. Aliquots (3 mL) were immediately shell-frozen in acetone-dry CO2, lyophilized, and stored at 4°C under vacuum-desiccation until required. Plasma was reconstituted with pure H2O, and thrombin clotting time (TCT) and total functional fibrinogen levels were measured by turbidimetric assay (ACL Futura; Beckman Coulter NZ Pty, Auckland, New Zealand). C-reactive protein (CRP) and serum amyloid A protein (SAA) were quantified by particle-enhanced immunonephelometric assays.19
Isolation and characterization of total fibrinogen and polypeptide chains
Reconstituted plasma (300 μL) was vortexed with saturated (NH4)2SO4 (85 μL) and centrifuged (5000g, 1 minute). Pellets containing purified fibrinogen were washed twice with 500 μL of a 1:4 dilution of saturated (NH4)2SO4, and aliquots were analyzed by 4% (nonreducing) and 7.5% (reducing) sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).20 Purified protein was redissolved at 4 mg/mL to 5 mg/mL (according to Clauss fibrinogen concentration) in 300 μL of 10 mM Tris-HCl buffer pH 8.0 containing 8 M urea and 30 mM DTT, and incubated for 18 hours at 37°C under N2. Aliquots (50 μL-60 μL) of reduced protein were then injected onto a C4 silica column (Jupiter300, 4.6 × 250 mm, 5 μm; Phenomenex, Torrance, CA), connected to a Waters 680 HPLC system (Waters, Milford, MA), and equilibrated with 64% solvent B (0.05% trifluoroacetic acid in 60% acetonitrile) in solvent A (0.05% trifluoroacetic acid). Individual Aα, Bβ, and γ chains were eluted at 0.75 mL/min in 200-μL fractions, monitored at 215 nm, with a linear gradient to 85% solvent B over 20 minutes, and dried under N2 at 55°C.21
Mass spectrometry and peptide mapping
Isolated Aα-chains (approximately 0.150 mg) were redissolved in 50 mM NH4HCO3 (50 μL) and incubated with TPCK-treated bovine trypsin (1.5 μg; Worthington Biochemical, Lakewood, NJ) for 16 hours at 37°C. After drying under vacuum with P2O5, the digests were redissolved in 60 μL of 0.1% HCOOH in 50% acetonitrile and analyzed by electrospray ionization mass spectrometry (ESIMS) on a VG Platform II instrument (single quadrupole; Waters-Micromass, Manchester, United Kingdom). Samples (20 μL) were introduced into the source at 5 μL/min and the mass-charge ratio (m/z) range 900 to 1200 was scanned every 2 seconds with a cone voltage ramp of 45 V to 75 V. The probe was maintained at 60°C and +3000 V. Positive ion spectra were acquired using Mass-Lynx software (Waters-Micromass).
Results
Fibrinogen concentration and TCT were within the normal range in all plasma samples other than one taken when the patient was mounting an acute-phase response (Table 1)due to postoperative sepsis. The Glu526Val variant Aα-chain evidently does not affect the clearance or clotting function of fibrinogen.5 In addition to the raised fibrinogen concentration, the plasma sample obtained from the patient with sepsis contained greatly increased concentrations of both CRP (Table 1) and SAA (560 mg/L, upper limit of normal 5 mg/L), indicating that a major acute-phase response was in progress.
Plasma fibrinogen and C-reactive protein (CRP) concentrations, and thrombin clotting times
| Case no., time from tp . | Fibrinogen level, g/L . | CRP level, mg/L . | TCT, s . |
|---|---|---|---|
| 1 | |||
| 29 d before tp | 3.9 | < 0.8 | 22 |
| 46 d after tp | 4.4 | < 0.8 | 26 |
| 137 d after tp | 4.0 | 3.0 | 20 |
| 2 | |||
| 0 d before tp* | 3.5 | < 0.8 | 21 |
| 92 d after tp | 6.1 | 81.8 | 19 |
| 3 | |||
| 7 y after tp | 3.9 | 6.2 | 22 |
| 4 | |||
| Awaiting tp | 3.9 | 10.0 | 23 |
| 5 | |||
| Awaiting tp | 3.9 | 4.8 | 22 |
| Case no., time from tp . | Fibrinogen level, g/L . | CRP level, mg/L . | TCT, s . |
|---|---|---|---|
| 1 | |||
| 29 d before tp | 3.9 | < 0.8 | 22 |
| 46 d after tp | 4.4 | < 0.8 | 26 |
| 137 d after tp | 4.0 | 3.0 | 20 |
| 2 | |||
| 0 d before tp* | 3.5 | < 0.8 | 21 |
| 92 d after tp | 6.1 | 81.8 | 19 |
| 3 | |||
| 7 y after tp | 3.9 | 6.2 | 22 |
| 4 | |||
| Awaiting tp | 3.9 | 10.0 | 23 |
| 5 | |||
| Awaiting tp | 3.9 | 4.8 | 22 |
Normal ranges are as follows: fibrinogen level, 1.5-4 g/L; CRP level, less than 10 mg/L; and TCT, 18-25 s. Fibrinogen was determined by the Clauss (functional) method.
tp indicates transplantation.
Patient also had a splenectomy at the time of transplantation.
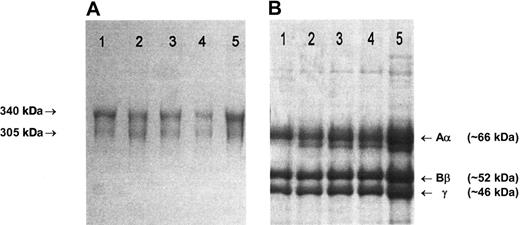
Fibrinogen preparations isolated from plasma of patients and healthy controls all migrated normally as two 340 kDa and 305 kDa bands in nonreduced SDS-PAGE (Figure 1A), and 3 major bands, representing individual Aα, Bβ and γ chains, were present in SDS-PAGE under reducing conditions (Figure 1B).21 Fractionation of each preparation to yield pure Aα, Bβ, and γ chains gave identical elution profiles.21 In ESIMS analysis in positive ion mode, the [M+2H] ion of peptide T-62 (TFPGFFSPMLGEFVSETESR), a tryptic fragment that incorporates Glu526, can be clearly seen at its predicted m/z position of 1133 in the peptide map from a healthy control individual (Figure 2A). The mass decrease of 30 Da associated with the Glu526 → Val substitution predicts the appearance of a novel [M+2H] ion at 1118 m/z in individuals heterozygous for the mutation. This was seen in the pretransplantation samples from 2 individuals shown (Figure 2B,E), and could be detected at an abundance of 1% to 2% of total fibrinogen based on the signal-baseline ratio of the 1118 m/z peak relative to the healthy control. In all pretransplantation samples examined, the ratio of variant to normal peptide was approximately 1:1, indicating normal synthesis and secretion of variant molecules and a normal half-life in the circulation. In contrast, only the wild-type Aα526Glu sequence (1133 m/z) was detected in samples from individuals at 1 to 4 months after transplantation, including, importantly, the 92-day sample taken from patient 2 when mounting a major acute-phase response (Figure 2F). Encouragingly, no variant sequence was detected in the plasma of a patient obtained 7 years after transplantation.
SDS-PAGE of purified fibrinogen. (A) A 4% nonreducing SDS-PAGE. The arrows indicate the migration positions of the major (340 kDa) and minor (305 kDa) circulating forms of fibrinogen, that have, respectively, fully intact chains or one proteolytically cleaved Aα-chain.22 (B) A 7.5% reducing SDS-PAGE. (A-B) Lane 1: unrelated healthy individual; lane 2: case 1, 29 days before transplantation; lane 3: case 1, 46 days after transplantation; lane 4: case 2, immediately before transplantation; lane 5: case 2, 92 days after transplantation. Gels were stained with Brilliant blue R250. Fibrinogen preparations from cases 3 to 5 migrated in an identical manner to those shown here.
SDS-PAGE of purified fibrinogen. (A) A 4% nonreducing SDS-PAGE. The arrows indicate the migration positions of the major (340 kDa) and minor (305 kDa) circulating forms of fibrinogen, that have, respectively, fully intact chains or one proteolytically cleaved Aα-chain.22 (B) A 7.5% reducing SDS-PAGE. (A-B) Lane 1: unrelated healthy individual; lane 2: case 1, 29 days before transplantation; lane 3: case 1, 46 days after transplantation; lane 4: case 2, immediately before transplantation; lane 5: case 2, 92 days after transplantation. Gels were stained with Brilliant blue R250. Fibrinogen preparations from cases 3 to 5 migrated in an identical manner to those shown here.
Representative ESIMS tryptic maps of purified Aα chains recorded in the m/z range 1060-1150. (A) Unrelated healthy individual. (B-D) Case 1, 29 days before (B), 46 days after (C), and 137 days after (D) transplantation. (E- F) Case 2, immediately before (E) and 92 days after (F) transplantation. The normal and variant forms of the [M+2H] ion of peptide T-62, that matches a fragment with a sequence that includes amino acid residue 526, are indicated at their predicted positions of 1133 and 1118 m/z. Before liver transplantation, equal amounts of wild-type Glu526 and variant Val526 Aα-chains were detected in the plasma fibrinogen of the fibrinogen amyloidosis patients. After liver transplantation only wild-type Glu526 chains were detected.
Representative ESIMS tryptic maps of purified Aα chains recorded in the m/z range 1060-1150. (A) Unrelated healthy individual. (B-D) Case 1, 29 days before (B), 46 days after (C), and 137 days after (D) transplantation. (E- F) Case 2, immediately before (E) and 92 days after (F) transplantation. The normal and variant forms of the [M+2H] ion of peptide T-62, that matches a fragment with a sequence that includes amino acid residue 526, are indicated at their predicted positions of 1133 and 1118 m/z. Before liver transplantation, equal amounts of wild-type Glu526 and variant Val526 Aα-chains were detected in the plasma fibrinogen of the fibrinogen amyloidosis patients. After liver transplantation only wild-type Glu526 chains were detected.
Discussion
Previous studies of plasma protein allotypes in patients undergoing liver transplantation have established that the liver is the only or the major source of many circulating plasma proteins including complement C3,23 C6,24 C8, and factor B, α1-acid glycoprotein, α1-antitrypsin, transferrin,25 α2-HS-glycoprotein, Gc-globulin, haptoglobin, inter-α trypsin inhibitor,26 and plasminogen.27 On the other hand, a small proportion of plasma C3 is derived from nonhepatic cells and most C728 does not come from the liver. Although all the patients studied here had undergone renal as well as liver transplantation, there is no evidence to suggest that fibrinogen is synthesized or secreted by the kidney. The complete absence of detectable variant fibrinogen Aα526Val chains in postoperative plasma indicates that any potential extra-hepatic contribution to plasma fibrinogen synthesis must be very low. Our findings thus establish that the liver is effectively the sole site of synthesis of human plasma fibrinogen, including during an acute-phase response in the one patient studied in these circumstances. Currently, the only effective therapy for any form of systemic amyloidosis is reduction in abundance of the protein precursor of the amyloid fibrils,29 and our present results thus formally validate the rationale for use of liver transplantation in treatment of hereditary fibrinogen amyloidosis.
Authorship
Contribution: G.A.T. and S.O.B. performed research and analyzed data; A.J.S., J.O'G., and P.N.H. managed the patients, provided clinical data and samples; M.B.P. initiated and directed the study; and M.B.P. and G.A.T. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: G. A. Tennent, Centre for Amyloidosis and Acute Phase Proteins, Department of Medicine, Royal Free and University College Medical School, Rowland Hill St, London NW3 2PF, United Kingdom; e-mail: g.tennent@medsch.ucl.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the staff of the Institute of Liver Studies and Liver Transplant Services, King's College Hospital, and the United Kingdom National Health Service (NHS) National Amyloidosis Centre for their care of the patients; Ruth Gallimore for CRP and SAA measurements, and Beth Jones for preparation of the manuscript.
This work was supported by Medical Research Council (MRC) Programme Grant G97 900 510 (M.B.P. and P.N.H.), and by NHS Research and Development Funds.

![Figure 2. Representative ESIMS tryptic maps of purified Aα chains recorded in the m/z range 1060-1150. (A) Unrelated healthy individual. (B-D) Case 1, 29 days before (B), 46 days after (C), and 137 days after (D) transplantation. (E- F) Case 2, immediately before (E) and 92 days after (F) transplantation. The normal and variant forms of the [M+2H] ion of peptide T-62, that matches a fragment with a sequence that includes amino acid residue 526, are indicated at their predicted positions of 1133 and 1118 m/z. Before liver transplantation, equal amounts of wild-type Glu526 and variant Val526 Aα-chains were detected in the plasma fibrinogen of the fibrinogen amyloidosis patients. After liver transplantation only wild-type Glu526 chains were detected.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/109/5/10.1182_blood-2006-08-040956/4/m_zh80050708690002.jpeg?Expires=1767730173&Signature=Jj3YWGRhXxv0L88IsVp-IS4Qw~wM00i344AWKTEX2h4usNYb2jZLvKgi~8Er0wGyq2981Iw5CAMBu08gwgxYm3NaFOM3ovnZScnX7yEWX8Cpar7X7CiD9qMZ-8Kkai8jtcjJThmBrDgFgreU2nJIdlst9plBZ1FTglw2ec3bAjQ54lOd6POTVP~ND1KcLv58y0fLK8NeH~xsWk2DLh8C7-NAxm2AA101ImyqnvmIiz3KKFHfuZyaJYTiHmaz71zbDCQmCXWJZ7sGe6WAm2v046BNJufTeRx5oEDbKzKwid~Qcps0mXHmC7kf6PS6-io78cIF1~KT~7jSGWs6HsqGeQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal