Abstract
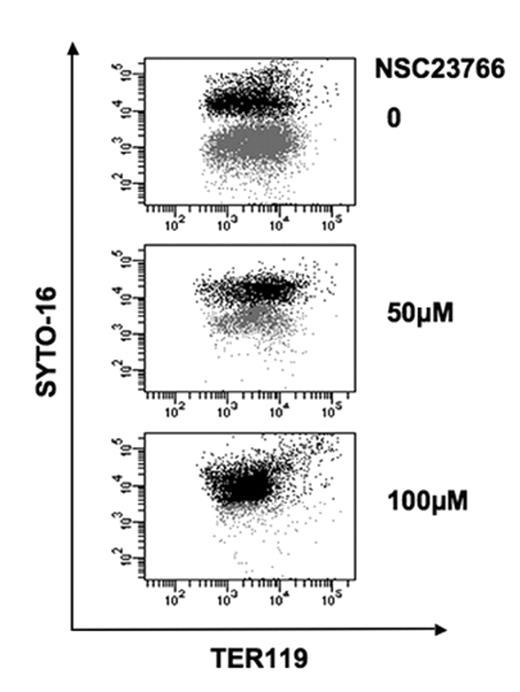
Rac GTPases (i.e. Rac1, Rac2 and Rac3), a subfamily of Rho GTPases, control actin organization and have overlapping as well as distinct roles in cell survival, proliferation, and differentiation in various hematopoietic cell lineages (Gu et al, Science 2003, Cancelas et al, Nature Med 2005). Using conditional gene-targeting in mice, we have previously demonstrated that Rac1 and Rac2 deficiency causes anemia with abnormal erythrocyte cytoskeleton and decreased deformability (Kalfa et al, Blood 2006). In the present studies, we found by colony assays that although bone marrow (BM) BFU-E activity was unaltered from that of the wild type (WT) mice, Rac1−/−;Rac2−/− erythroid bursts had a strikingly different morphology appearing as round, small, dense colonies, likely a manifestation of motility defects associated with Rac GTPase deficiency. Total CFU-Es recovered from Rac1−/−;Rac2−/− BM were as low as 25% of that in WT mice (p<0.05). To further assess erythroblast differentiation, BM cells were immunostained with fluorescent label-conjugated anti-CD71 and anti-Ter119, as previously described (Socolovski et al. Blood 2001). Flow cytometry analysis revealed that proerythroblasts and basophilic erythroblasts in the BM were significantly decreased in Rac1−/−;Rac2−/− (∼30–50% of WT content) while the terminal differentiation to orthochromatic erythroblasts was comparable. In vivo BrdU labeling and flow cytometry with 7-AAD and annexin-V in combination with staining for CD71 and Ter119 revealed no difference in proliferation or survival between WT and Rac1−/−;Rac2−/− erythroid cells after the proerythroblast stage. These data suggest that deficiency of Rac1 and Rac2 GTPases affect erythropoiesis mainly at the early stages of BFU-E and CFU-E formation but not during terminal differentiation to orthochromatic erythroblasts. Given the prominent role of Rac GTPases in regulating actin structure, we next evaluated the possible involvement of Rac GTPases in enucleation, the terminal step of erythropoiesis that likely requires significant actin remodeling. We performed quantitative analysis in ex vivo erythropoiesis cultures, by flow cytometry, using SYTO16, a cell-permeable nucleic acid-staining dye. The frequency of enucleated red cells (SYTO16-low, Ter119-positive population) was similar in the WT and the Rac1−/−;Rac2−/− erythroid cultures. However, application of a Rac GTPase inhibitor, NSC23766, to the WT or the Rac1−/−;Rac2−/− erythroid cultures during the enucleation phase resulted in an inhibition of enucleation up to 80% dose-dependently (figure 1). Rac1 and Rac2 deficiency led to a compensatory elevation of Rac3 activity that was effectively suppressed by NSC23766, as demonstrated by immunoblotting in the Rac1−/−;Rac2−/− erythroblasts and effector-domain pull-down studies. Moreover, NSC23766 inhibited Rac1, Rac2, and Rac3 activities as well as actin polymerization of the erythroblasts. Thus, Rac1, Rac2, and Rac3 have redundant but essential roles in supporting actin dynamics necessary for the nucleus extrusion during the enucleation process.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal