Abstract
Tissue engineering requires formation of a de novo stable vascular network. Because of their ability to proliferate, differentiate into endothelial cells, and form new vessels, blood-derived endothelial progenitor cells (EPCs) are attractive source of cells for use in engineering blood vessels. However, the durability and function of EPC-derived vessels implanted in vivo are unclear. To this end, we directly compared formation and functions of tissue-engineered blood vessels generated by peripheral blood– and umbilical cord blood–derived EPCs in a model of in vivo vasculogenesis. We found that adult peripheral blood EPCs form blood vessels that are unstable and regress within 3 weeks. In contrast, umbilical cord blood EPCs form normal-functioning blood vessels that last for more than 4 months. These vessels exhibit normal blood flow, perm-selectivity to macromolecules, and induction of leukocyte-endothelial interactions in response to cytokine activation similar to normal vessels. Thus, umbilical cord blood EPCs hold great therapeutic potential, and their use should be pursued for vascular engineering.
Introduction
The discovery of circulating endothelial progenitor cells (EPCs) in peripheral blood—reported almost a decade ago1 —has generated impetus for using EPCs in applications ranging from alleviation of tissue ischemia to cancer therapy. One such application is vasculogenesis, which is an important objective for tissue engineering. However, in vitro phenotypic studies have demonstrated that cells defined as EPCs consist of a heterogeneous population, containing cells with differential phenotype (ie, endothelial and hematopoietic myeloid cells) and outgrowth potential.2,3 Recent reports have also shown that peripheral blood (PB)– and cord blood (CB)–derived EPCs (also referred to as endothelial colony-forming cells2 ) can form functional blood vessels when implanted in vivo, however the duration and functional performance of these blood vessels have not been defined.2,4,5 In this report, we address 2 critical outstanding questions regarding the use of EPCs for vasculogenesis in tissue engineering. First, can both adult PB- and CB-derived EPCs form stable vessels? Second, if ex vivo expanded EPCs form durable vessels in vivo, how well do these vessels function compared with the preexisting microvasculature? To this end, we used an in vivo engineered vessel model to compare the neovasculature generated by PB- and CB-derived EPCs through vasculogenesis. We assessed the functional performance of the engineered vessels by measuring 3 parameters: red blood cell velocity, vessel permeability to serum albumin, and leukocyte rolling in response to cytokine stimulation
Methods
Adult buffy coat samples were obtained from the blood bank at Massachusetts General Hospital. Umbilical cord blood samples were obtained from the Pediatric Research Institute, University of St Louis, MO, according to guidelines established by the Human Investigation Committee (Institutional Review Board protocols no. 2003-P-000588). All animal procedures were carried out following the Public Health Service Policy on Humane Care of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital.
Statistical analysis
All data were analyzed by ANOVA with the Fisher posthoc test. All data are reported as mean with standard error. Statistical significance was set at P values less than .05.
An expanded Methods section that includes image acquisition information can be found in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results and discussion
To obtain EPC-derived colonies, we cultured PB and CB mononuclear blood cells on type I collagen–coated dish for 14 to 21 days.6 We confirmed the endothelial phenotype of PB- and CB-derived EPCs by immunocytochemistry and flow cytometry. EPCs from both sources expressed multiple endothelial markers, including CD31, VE-cadherin, VWF, VEGFR2, and Tie2; incorporated acetylated low-density lipoprotein (AcLDL); and were negative for the pan hematopoietic marker CD45 and the monocyte marker CD14 (Figures S1,S2).
To determine whether adult PB- and CB-derived EPCs are able to self-assemble into functional blood vessels, we implanted EGFP-labeled EPCs alone or coimplanted them with 10T1/2 cells in a collagen/fibronectin gel onto the pial surface in cranial windows.7 10T1/2 cells, a line of mouse embryonic fibroblasts, have previously been demonstrated to stabilize endothelial cells by functioning as perivascular-like cells and releasing paracrine factors.7-9 Here, we found that both human adult PB- and CB-EPCs were capable of vasculogenesis. However, the density and persistence of the engineered neovasculature were substantially different depending on the source of EPCs.
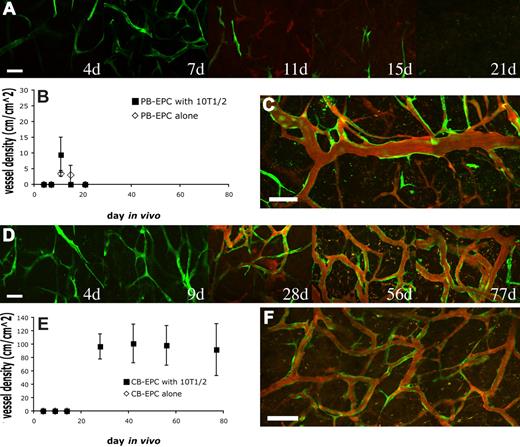
In the case of PB-EPCs coimplanted with 10T1/2, the EPCs began to elongate and to connect to one another resulting in a meshlike network 7 days after implantation (Figure 1A; Figure S3A). By day 11, some of the EPCs had joined together to form patent vascular tubes that were connected to the host circulation (Figure 1A). However, the number of these functional engineered vessels was low and they were distributed haphazardly within the collagen gel. Coimplantation of PB-EPCs with 10T1/2 cells did not significantly increase either perfused or nonperfused vessel densities compared with PB-EPC implantation alone (Figure 1B; Figure S3A). This is in contrast with in vitro data where 10T1/2 cells promote cordlike formation by the EPCs (Figure S4). With time, the PB-EPC–derived blood vessels began to regress and disappeared almost completely 1 month after implantation, regardless of the absence or presence of 10T1/2 cells (Figure 1B). Occasionally, a few of the blood vessels remained 27 days after implantation (Figure 1C).
Vasculogenic potential of peripheral blood (PB)– versus cord blood (CB)–derived endothelial progenitor cells (EPCs). PB-EPCs and CB-EPCs were mixed with 10T1/2 cells in a collagen gel, and implanted into cranial windows in severe combined immunodeficient (SCID) mice. Images were taken at periodic time points with multiphoton laser scanning microscope for in vivo dynamics of vascularization by the implanted endothelial cells. PB-EPCs formed vascular-like structure 4 days after implantation and some of them became perfused at day 11. The PB-EPC–derived blood vessels were transient and almost completely disappeared by day 21 (A). There was no significant difference in the mean (± SEM) density of functional vessels derived from PB-EPCs between groups implanted with PB-EPCs only and PB-EPCs with 10T1/2 cells (B) (n = 4 for each group and experiments were performed with 3 different batches of adult peripheral blood). In some animals, there were still some sparse but functional blood vessels 27 days after implantation (C). In contrast, CB-EPCs formed a uniformly dense network of functional blood vessels (D). Implantation of CB-EPCs alone led to a rapid regression of the implanted cells, while coimplantation of CB-EPCs and 10T1/2 cells resulted in a stable and functional vasculature (E) (n = 4 for each group and experiments were performed with 3 different batches of human umbilical cord blood). The CB-EPC–derived vascular network was stable and functional for more than 119 days in vivo (F). Green indicates PB- or CB-derived endothelial cell expressing enhanced green fluorescent protein (EGFP); red, functional blood vessels contrast-enhanced by rhodamine-dextran. Scale bars represent (A,D) 50 μm; (C,F) 100 μm.
Vasculogenic potential of peripheral blood (PB)– versus cord blood (CB)–derived endothelial progenitor cells (EPCs). PB-EPCs and CB-EPCs were mixed with 10T1/2 cells in a collagen gel, and implanted into cranial windows in severe combined immunodeficient (SCID) mice. Images were taken at periodic time points with multiphoton laser scanning microscope for in vivo dynamics of vascularization by the implanted endothelial cells. PB-EPCs formed vascular-like structure 4 days after implantation and some of them became perfused at day 11. The PB-EPC–derived blood vessels were transient and almost completely disappeared by day 21 (A). There was no significant difference in the mean (± SEM) density of functional vessels derived from PB-EPCs between groups implanted with PB-EPCs only and PB-EPCs with 10T1/2 cells (B) (n = 4 for each group and experiments were performed with 3 different batches of adult peripheral blood). In some animals, there were still some sparse but functional blood vessels 27 days after implantation (C). In contrast, CB-EPCs formed a uniformly dense network of functional blood vessels (D). Implantation of CB-EPCs alone led to a rapid regression of the implanted cells, while coimplantation of CB-EPCs and 10T1/2 cells resulted in a stable and functional vasculature (E) (n = 4 for each group and experiments were performed with 3 different batches of human umbilical cord blood). The CB-EPC–derived vascular network was stable and functional for more than 119 days in vivo (F). Green indicates PB- or CB-derived endothelial cell expressing enhanced green fluorescent protein (EGFP); red, functional blood vessels contrast-enhanced by rhodamine-dextran. Scale bars represent (A,D) 50 μm; (C,F) 100 μm.
In contrast, CB-EPCs were able to produce a dense network of blood vessels that were distributed uniformly throughout the collagen gel (Figure 1D). We quantified the vascular density of the same region of collagen gel over time. In the group with CB-EPCs implanted alone, the density of engineered blood vessels was low and they quickly regressed before they could become perfused (Figure 1E; Figure S3B). However, we found that in the group coimplanted with 10T1/2 cells, CB-EPCs formed a network of microvessels that remained stable and patent for more than 119 days (Figure 1E,F). We observed some host vessels infiltration into the gel, however the majority of the blood vessels were CB-EPC derived. The collagen gel was excised and whole-mount staining with human specific antibodies showed the expression of the endothelial markers, CD31 and VE-cadherin, on the EGFP-positive EPC-derived vascular endothelial cells (Figure 2A; Figure S5). Collagen type IV staining revealed that the engineered blood vessels had an intact basement membrane (Figure 2B). The 10T1/2 cells persisted and functioned as perivascular cells in vivo (Figure S6).
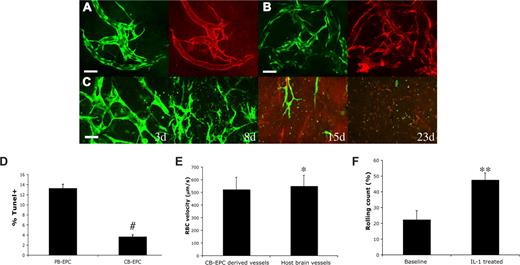
Characterization of CB-EPC–derived vascular networks in vivo. Whole mount staining of the implanted collagen gel revealed that the CB-EPCs (EGFP+) at day 87 after implantation maintained the expression of CD31 (A) and had intact basement membrane of collagen type IV (B) in vivo (EGFP, green; CD31 and collagen type IV, red). CB-EPCs implanted alone at high cell density (5 million cells/mL at high density vs 1 million cells/mL at normal density) were not able to form long-lasting blood vessels (C). The blood-derived endothelial cells were exposed to serum-free media for 48 hours, and the cells were then stained and quantified for TUNEL, a marker for apoptotic cells (D). CB-EPCs exhibited a higher resistance to serum-free medium–induced apoptosis. The vascular permeability of the CB-EPC–derived blood vessels to albumin was measured (Table 1). CB-EPC–derived blood vessels had low vascular permeability with values similar to those of normal mouse pial blood vessels. Blood flow rate as measured by red blood cell (RBC) velocity was comparable between the CB-EPC–derived vessels and mouse brain vessels (E). The number of rolling leukocytes on CB-EPC–derived blood vessels was increased after induction of systemic inflammation with intraperitoneal injection of 100 ng IL-1β for 4 hours (F). #P < .001; *NS; **P < .05. Scale bars (A,B) represent 50 μm.
Characterization of CB-EPC–derived vascular networks in vivo. Whole mount staining of the implanted collagen gel revealed that the CB-EPCs (EGFP+) at day 87 after implantation maintained the expression of CD31 (A) and had intact basement membrane of collagen type IV (B) in vivo (EGFP, green; CD31 and collagen type IV, red). CB-EPCs implanted alone at high cell density (5 million cells/mL at high density vs 1 million cells/mL at normal density) were not able to form long-lasting blood vessels (C). The blood-derived endothelial cells were exposed to serum-free media for 48 hours, and the cells were then stained and quantified for TUNEL, a marker for apoptotic cells (D). CB-EPCs exhibited a higher resistance to serum-free medium–induced apoptosis. The vascular permeability of the CB-EPC–derived blood vessels to albumin was measured (Table 1). CB-EPC–derived blood vessels had low vascular permeability with values similar to those of normal mouse pial blood vessels. Blood flow rate as measured by red blood cell (RBC) velocity was comparable between the CB-EPC–derived vessels and mouse brain vessels (E). The number of rolling leukocytes on CB-EPC–derived blood vessels was increased after induction of systemic inflammation with intraperitoneal injection of 100 ng IL-1β for 4 hours (F). #P < .001; *NS; **P < .05. Scale bars (A,B) represent 50 μm.
We next investigated whether implanting CB-EPCs at a higher density could obviate the need for 10T1/2 pericyte precursors. CB-EPCs were implanted alone at a 5-fold increase in cell density (5 million cells/mL vs 1 million cells/mL). Some of the implanted CB-EPCs aligned into functional blood vessels, however these vessels were only transiently perfused and disappeared by 23 days (Figure 2C). Implantation of 10T1/2 cells alone led to a minimal angiogenic response (data not shown). These findings reaffirm that coimplantation of endothelial and perivascular cells is critical for vasculogenic vessel remodeling and sustenance.
The long-lasting functional blood vessels might be linked to a higher proliferative capacity of CB-EPCs. Indeed, Ingram et al showed that CB-EPCs have a higher level of telomerase activity compared with PB-EPCs.6 We found a significantly higher percentage of CB-EPCs were positive for the Ki-67 proliferation marker compared with PB-EPCs (65.9% for CB-EPCs vs 38.3% for PB-EPCs, P < .01) (Figure S7), confirming a proliferative advantage for CB-EPCs.
In addition, PB-EPCs might have a competitive disadvantage compared with CB-EPCs due to poorer survival.10 Cell transplantation is often inefficient. For example, it has been estimated that less than 1% of implanted cells survived 4 days after implantation into a mouse heart.11 We tested in vitro the EPCs' resistance to stress-induced apoptosis by exposing them to serum-free medium for 48 hours. After 48 hours of exposure to serum-free media, only 3.7% of CB-EPCs were apoptotic (ie, positive for terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling [TUNEL]) versus 13.3% of the PB-EPCs (Figure 2D; Figure S8). These findings suggest that CB-EPCs are more resistant to stress-induced apoptosis, in addition to having a proliferative advantage compared with PB-EPCs.
Finally, the function of the engineered blood vessels is critical, since functional abnormalities can have serious pathological consequences.12 For example, 2 major problems with immature blood vessels are the irregular flow and the increase in permeability. This leads to leakiness of plasma proteins or even hemorrhage, similar to blood vessels inside tumors.13 We measured the permeability to serum albumin of the CB-EPC–derived blood vessels at 2 months after implantation. We found that CB-EPC–derived vessels have low vascular permeability, with values close to those of the neighboring brain capillaries (Table 1). We have not detected any hemorrhagic episode during the period of observation. The average blood flow rate was comparable between the CB-EPC–derived vessels and host pial vessels (Figure 2E; Figure S9).
Vascular permeability
| CB-EPC + 10T 1/2 . | Normal braincapillaries7 . | Tumors7 . | Type 1 collagengel with VEGF7 . |
|---|---|---|---|
| 0.73 ± 0.21* | 0.3 ∼ 0.6* | 2.9 ∼ 3.9* | 2.5 ∼ 4.9* |
| CB-EPC + 10T 1/2 . | Normal braincapillaries7 . | Tumors7 . | Type 1 collagengel with VEGF7 . |
|---|---|---|---|
| 0.73 ± 0.21* | 0.3 ∼ 0.6* | 2.9 ∼ 3.9* | 2.5 ∼ 4.9* |
×10−7 cm/s.
Besides serving as a conduit for blood, vascular endothelium should also have the ability to become activated to recruit leukocytes in the setting of homeostasis, infection, or tissue injury. To test this in CB-EPC–derived endothelium, we induced systemic inflammation by injection of IL1β in mice with CB-EPC–engineered vessels.14 We measured the number of rolling leukocytes at baseline and 4 hours after injection of IL1β in selected engineered blood vessels that had characteristics of postcapillary venules (based on morphology, diameter, and rate of blood flow). In these engineered vessels, we found a significant increase in the percentage of rolling leukocytes after induction of systemic inflammation, suggesting that CB-EPC–derived endothelium undergoes proper cytokine activation (Figure 2F).
In summary, we found that CB-EPCs have enhanced vasculogenic ability in vivo compared with adult PB-EPCs. Moreover, CB-EPC coimplantation with pericyte precursors led to the formation of long-lasting and functionally normal blood vessels, providing an attractive cellular therapy platform for tissue engineering.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was partially supported by an American Heart Association Predoctoral Fellowship (P.A.); National Institutes of Health grants P01CA80124 (R.K.J., D.F.), R01CA96915 (D.F.), R01CA115767 (R.K.J.), and R01CA85140 (R.K.J.); American Association for Cancer Research–Genentech BioOncology (D.G.D.); Department of Defense Breast Cancer Research Program Predoctoral Traineeship Award (R.M.L.); and the Harvard Stem Cell Institute (D.T.S.).
Authorship
Contribution: P.A. designed, performed, and analyzed research, and wrote the paper; L.M.D. and K.S.C. designed, performed, and analyzed research; D.G.D., D.F., and R.K.J. designed and analyzed research and wrote the paper; J.A.T. and R.L. analyzed research; and D.T.S. designed and analyzed research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rakesh K. Jain, Edwin L. Steele Laboratory, Department of Radiation Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114; e-mail: jain@steele.mgh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal