Abstract
Multiple myeloma (MM) and plasmacytomas are cancers of antibody-secreting cells (ASCs). PRDM1/BLIMP1 is an essential regulator of ASC development. Histologic evidence shows that 100% of MM expresses PRDM1/BLIMP1, indicating that PRDM1/BLIMP1 is important for the development or persistence of MM. In contrast, some diffuse large B-cell lymphomas (DLBCLs) lose PRDM1 expression, suggesting that PRDM1 may act as a tumor suppressor in DLBCL. Thus, the role of PRDM1/BLIMP1 in transformation of mature B cells is unclear. We have used a plasmacytoma-prone transgenic mouse model to study the effect of Blimp1 loss on plasmacytoma prevalence, latency, and phenotype. Two possible outcomes could be envisaged: loss of Blimp1 might decrease plasmacytoma prevalence, through reduction of plasma cells, and so the number of susceptible transformation targets. Alternatively, Blimp1 may participate in the transformation process itself. Our results support the latter scenario, showing that decreasing Blimp1 dosage does not change plasma cell number in nontransgenic mice in vivo, but it significantly reduces plasmacytoma prevalence in transgenic mice. Loss of functional Blimp1 completely prevents plasmacytoma formation in this tumor model. These observations suggest that Blimp1 is limiting for plasma cell transformation and thus has potential as a target for new therapies to combat MM.
Introduction
Multiple myeloma (MM) and plasmacytomas (PCTs) are cancers of antibody-secreting plasma cells (ASCs), the effector cells of the humoral immune system. MM is the second most common blood cancer, representing approximately 1% of all cancers and 2% of all cancer deaths.1 It is an incurable disease, with current treatments designed to delay progression and to relieve symptoms. The average 5-year survival is approximately 30%, and 10-year survival averages only approximately 3%. MM usually occurs in the elderly, although recent figures show that it is becoming more common and occurring in younger adults.2 PCTs and MM share many pathologic and molecular features, including abnormal accumulation of plasma cells and the presence of clonal immunoglobulin (Ig) in serum.3 Here, we explore the contribution of the Blimp1/Prdm1 gene (PRDM1 in humans) to tumor development in a transgenic mouse model of PCT
Blimp1 is called the “master regulator” of normal plasma cell development. Work in our laboratory and in others has shown that all ASCs express Blimp1, and no mature plasma cells can form without it.4-6 Blimp1, a transcriptional repressor, controls the activities of genes, such as Pax5 and Bcl6, that specify the mature B and germinal center B-cell fates, respectively.5,7 Extinction of the gene expression programs that characterize these developmental stages is required for the initiation of plasma cell differentiation, and further gene expression changes enable the cell to specialize, focusing its activities on the production and secretion of large amounts of antibody. Another putative Blimp1 target gene is c-myc.8 By repressing c-myc, Blimp1 is postulated to halt cell division, a characteristic change that occurs during the terminal differentiation of long-lived plasma cells, a nondividing population in the marrow and spleen.5,9
With the use of a mouse that bears a GFP cassette incorporated into the Blimp1 locus,5 plasma cell ontogeny can be followed by monitoring Blimp1/GFP levels in vivo. Short-lived, Blimp1low plasmablasts first appear in the spleen and in the blood. Blimp1high cells are found predominantly in the bone marrow (BM) and are thought to arise from Blimp1low precursors.5,10 These long-lived BM plasma cells require Blimp1 for their persistence.11 Interestingly, our recent genetic studies suggest that initiation of plasma cell differentiation is Blimp1 independent, because phenotypically and functionally immature Ig-secreting cells, “pre-plasmablasts,” are produced in Blimp1-deficient animals.12
Circumstantial evidence suggests that PRDM1/Blimp1 might be important for the development or persistence of MM. A recent, large immunohistologic survey of 679 B- and T-cell lymphomas showed that 100% of primary MM or plasmablastic tumors were PRDM1 positive, whereas tumors representing less-mature B cells, or T cells, were negative, or only sporadically positive for PRDM1 staining.13 Other studies have shown similar results.14,15 The universal expression of PRDM1 in MM implies that this factor is required for neoplasms of plasma cells to form or for malignant plasma cells to persist. In line with this, a recent study showed that continued expression of PRDM1/BLIMP1 is essential for MM cell survival and that reducing PRDM1 levels caused both an increase in expression of the proapoptotic protein Bim and a decrease in the prosurvival factor Mcl1.16
In the large study by Garcia et al, approximately 43% of diffuse large B-cell lymphomas (DLBCLs), which phenotypically resemble activated or germinal center B cells, expressed PRDM1 protein, indicating that terminal differentiation had been initiated in some cells within such tumors.13 Patients with PRDM1-positive DLBCL had a shorter overall survival rate than patients with PRDM1-negative DLBCL. In 2 smaller surveys, subsets of DLBCL (8 of 92 and 8 of 35, respectively) were found to have PRDM1 mutations or loss of expression.17,18 Both groups hypothesized that PRDM1 acts as a tumor suppressor in DLBCL, with its loss promoting transformation by prohibiting terminal differentiation. The correlation was not functionally tested in either study, so whether loss of PRDM1 in DLBCL is necessary for transformation, or is simply inconsequential at this cell stage, is not yet known. Similarly, although studies on MM suggest that PRDM1 is required for the persistence of plasma cell tumors, it is not known whether PRDM1 is needed for tumor initiation or development. Thus, the role played by PRDM1/BLIMP1 in transformation of B cells at late stages of differentiation is unclear. Here, we have used an established PCT-prone mouse model to ask these questions.
The transgenic mice used here (called CVA40 mice) bear an Eμ-v-abl transgene that drives expression of the v-Abl protein tyrosine kinase in the B-cell lineage. The mice are prone to PCT development, but they do not develop other B-cell malignancies. This is despite early expression of the transgene19 and the capacity for v-Abl to transform pre-B cells.20 Disease in this transgenic model is highly influenced by sex (more common in males, as is MM in humans2 ) and by mouse strain. In the PCTs, the germline c-myc gene is commonly rearranged through chromosome translocation to the IgH locus, an event that is likely to occur in mature B cells through an error during isotype switching.21-23 c-myc dysregulation is a strong accelerator of transformation in this model, because the combination of Eμ-v-abl with an Eμ-myc transgene causes extremely rapid PCTs in all animals.19 We have used the CVA40 mouse model to study the effect of the loss of Blimp1 function on PCT prevalence, latency, and phenotype. Two potential effects of Blimp1 deficiency could be envisaged. First, loss of Blimp1 might decrease PCT prevalence through loss of plasmablast or plasma cells, and so the number of target cells for transformation. However, we show that decreasing Blimp1 gene dosage by half in mice does not change plasma cell number or serum Ig levels in vivo. Alternatively, reduction of Blimp1 levels could promote tumor development, perhaps by releasing expression of important target genes such as c-myc. Our results support this latter scenario, showing that decreasing Blimp1 gene dosage by half significantly reduces PCT prevalence. They suggest that Blimp1 may somehow participate in the transformation process itself in plasma cells. Complete loss of functional Blimp1 prevents plasma cell tumor formation in this mouse model. These observations indicate that Blimp1 is limiting for PCT formation, strengthening the case for PRDM1/BLIMP1 as a potential target for new therapies to combat MM.
Methods
Mice
Because disease in v-abl transgenic mice is influenced by the parental source of the transgene, we mated female v-abl transgenic mice on a Balb/c background24 to male Blimp1gfp/+ C57BL/6 mice.5 All F1 progeny bearing a copy of the v-abl transgene were monitored regularly for signs of ill health. Sick mice presented with the same panel of symptoms originally reported for CVA40 mice,19,24 with gut-associated tumors in most cases. Animals were killed and autopsied when a palpable tumor was detected or when any other sign of distress or ill health was noted. This was the time used for each animal in calculations of tumor incidence over time.
For the fetal liver transplants, a male F1 (v-abl+, Blimp1gfp/+) mouse was mated with several F1 nontransgenic Blimp1gfp/+ females. Fetal livers were harvested at E13, genotyped (for v-abl, Blimp1gfp and sex with the use of male-specific Sry primers), and cell suspensions were injected intravenously into lethally irradiated (2 × 5.5 Gy[550 rad]) F1 (C57BL/6 × Balb/c)male recipients. Regardless of Blimp1 genotype, all v-abl+ fetal livers were injected at a 1:5 ratio with E13 fetal liver cells from a wild-type littermate to prevent the T-cell–mediated pathology of mice reconstituted solely with Blimp1gfp/gfp cells.25 After 8 weeks of reconstitution, donor cell contribution in peripheral blood was measured by quantitative genomic polymerase chain reaction (PCR; see “PCR”). Only those mice showing at least 10% donor representation were included in the study. These mice were monitored as mentioned earlier for 365 days.
Five mice reconstituted with Blimp1gfp/gfp fetal liver were excluded from the study because they developed thymomas (CD4+/CD8+) of host cell origin, as determined by tumor cell genotype. These presumably arose as a consequence of irradiation.26 One mouse reconstituted with Blimp1gfp/+ cells had both a PCT and a thymoma when killed at 288 days. Because the thymomas occurred after a long latency (mean, 231 ± 57 days), they were rarely observed in Blimp1+/+or Blimp1gfp/+ reconstituted mice, many of which had already succumbed to PCTs (with mean latencies of 177 and 263 days, respectively; Table 3). Approval for the use of mice in this study was obtained from the Animal Ethics Committee of The Walter and Elisa Hall Institute.
Flow cytometry and antibodies
Cell suspensions were prepared from tumors, spleen, or bone marrow and stained as described with antibodies against CD138/Syndecan-1 (clone 281-2), B220 (RA3-6B2; both from BD PharMingen, San Diego, CA) or CD19 (1D3), IgM (331.12), Mac1 (M1/70), CD4 (GK1.5), TCRβ (H57-597), and Gr1 (RB6-8C5), all prepared in our laboratory.
PCR
Quantitative real-time PCR was performed with total RNA prepared from sorted populations from tumors (for Blimp1gfp/+ plasmacytomas, the B220−, GFP+ populations were collected; for Blimp1gfp/gfp tumors, all GFP+ cells were collected) or normal plasma cells (Syndecan1+, B220−, and GFP+ where relevant) ex vivo or from in vitro cultures,27 with the use of the following primers: Blimp1 (detects all “α isoform” transcripts from the wt allele), 5′ GACGGGGGTACTTCTGTTCA and 3′ GGCATTCTTGGGAACTGTGT; Blimp1 (β isoform-specific), 5′ GAAATGGAAAGATCTATTCCAGAG and 3′ GGCATTCTTGGGAACTGTGT; Blimp 1 (wt allele-specific), 5′ CCACATGAGTGCCAGGTCTGCCACAAG and 3′ CAGTCGCTTGTGCAGCTTCAGGTGCAC; Blimp 1 (ko/gfp allele-specific), 5′ AACACGTGGTACAACCCAAAG and 3′ GCGGAATTCATTTAATCACCCA; v-abl, 5′ GGGTGGAGGCCAGTACGGGGAGGTG and 3′ CCCTAGCAGCTGCACCAGGTTAGGG; Bcl6, 5′ GCCGGCTCAATAATCTCGTGAACAGGTCC and 3′ CCAGCAGTATGGAGGCACATCTCTGTATGC; Igk, 5′ GGGCTGATGCTGCACCAAC and 3′ TCTTGCTGGGTGGTTGTG; Mta3, 5′ GAAGCCTGCCTATTCGAAGAA and 3′ GCGCTTCTGCTGCACGTATCTGTCAGTAG; Sry, 5′ GGTGTGGTCCCGTGGTGAGAGG and 3′ TGTTGCTGCTGGTGGTGGTTATGG. Other primer sequences used here have been published.27-29 Expression levels were normalized to the hmbs housekeeping gene.
Quantitative genomic DNA PCR of red cell–depleted peripheral blood cells28 from F1 recipient mice was used to measure relative reconstitution of hosts with donor-derived cells. v-abl gene levels (donor cell–specific) were normalized to oct2 gene levels (present in all cells). The results were made relative to blood cells from CVA40 mice (100% of cells positive for v-abl DNA).
Southern analysis
Genomic DNA from tumors was digested with XbaI, electrophoresed, and transferred with the use of standard methods. Liver DNA was used as a control. The filter was probed with a DNA fragment corresponding to positions −466 to −79 bp from the start of transcription of the mouse c-myc gene,30 amplified with the following primers: 5′ GCGGGGGCTCCTAGATAACTCATT and 3′ CCTCTCGCTTCCCCGCCCTCTG.
Paraprotein gels, enzyme-linked immunoabsorbent assay, and EliSpot assays
For paraprotein gels, 0.5 μL serum was used as described.19 For the enzyme-linked immunoabsorbent assays (ELISAs) used to detect hyper-Ig in tumor-bearing animals and to determine the isotype, serum was diluted 1/100 000, a dilution at which normal serum gives no signal in the assay. For the ELISAs performed to measure serum Igκ in nontransgenic animals (Figure 1G), serum was diluted 1/500, then serially 1 in 2. Igκ levels were quantified with the use of a purified Igκ/IgM antibody standard. In all cases, the capturing reagent was sheep anti–mouse Ig (Silenus Laboratories, Melbourne, Australia), and detection used horseradish peroxidase–coupled antibodies specific for mouse Igκ, IgM, IgG1, or IgA (Southern Biotech, Birmingham, AL), as previously described27
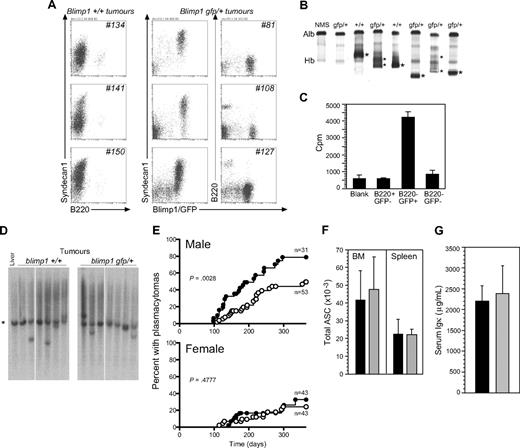
Phenotype and latency of PCTs in v-abl transgenic mice bearing 1 or 2 functional Blimp1 alleles. (A) Surface phenotypes of cells in mesenteric tumors from 3 representative Blimp1+/+ (nos. 134, 141, and 150) and 3 Blimp1gfp/+/v-abl transgenic mice (nos. 81, 108, and 127). Tumors were predominantly composed of Syndecan1+ (and GFP+, when the Blimp1/GFP reporter allele was present), B220− cells, the phenotype of mature plasma cells. (B) Native gel electrophoresis shows clonal Ig or paraprotein (*) in the serum of PCT-bearing mice. Alb indicates albumin; and Hb, hemoglobin. (C) Tritiated-thymidine incorporation during a 6-hour culture of cell populations sorted from a Blimp1gfp/+ tumor. (D) Genomic Southern assay showing c-myc gene rearrangements in the vicinity of the promoter. *The 3.6-kb germline band. (E) Kaplan-Meier plot of PCT incidence, by sex and genotype (●, Blimp1+/+; ○, Blimp1gfp/+). See “Methods” for statistical analyses. (F) Total number of ASCs in BMs (of 1 femur) and spleens. Values are means ± SDs of 4 mice (■, Blimp1+/+; ▩, Blimp1gfp/+). (G) Serum Igκ levels in naive, adult male B6 (Blimp1+/+) and Blimp1gfp/+. Values are means (± SDs) of 4 mice (■, Blimp1+/+; ▩, Blimp1gfp/+).
Phenotype and latency of PCTs in v-abl transgenic mice bearing 1 or 2 functional Blimp1 alleles. (A) Surface phenotypes of cells in mesenteric tumors from 3 representative Blimp1+/+ (nos. 134, 141, and 150) and 3 Blimp1gfp/+/v-abl transgenic mice (nos. 81, 108, and 127). Tumors were predominantly composed of Syndecan1+ (and GFP+, when the Blimp1/GFP reporter allele was present), B220− cells, the phenotype of mature plasma cells. (B) Native gel electrophoresis shows clonal Ig or paraprotein (*) in the serum of PCT-bearing mice. Alb indicates albumin; and Hb, hemoglobin. (C) Tritiated-thymidine incorporation during a 6-hour culture of cell populations sorted from a Blimp1gfp/+ tumor. (D) Genomic Southern assay showing c-myc gene rearrangements in the vicinity of the promoter. *The 3.6-kb germline band. (E) Kaplan-Meier plot of PCT incidence, by sex and genotype (●, Blimp1+/+; ○, Blimp1gfp/+). See “Methods” for statistical analyses. (F) Total number of ASCs in BMs (of 1 femur) and spleens. Values are means ± SDs of 4 mice (■, Blimp1+/+; ▩, Blimp1gfp/+). (G) Serum Igκ levels in naive, adult male B6 (Blimp1+/+) and Blimp1gfp/+. Values are means (± SDs) of 4 mice (■, Blimp1+/+; ▩, Blimp1gfp/+).
To enumerate ASC numbers in the mice, total, unmanipulated cell suspensions were prepared from spleens and BM (1 femur per animal) from 4 age-matched adult male nontransgenic Blimp1+/+ and Blimp1gfp/+mice (age, ∼ 7 months). The cell suspensions were diluted to a starting concentration of 105 live cells/mL and further diluted serially 3-fold down the plate. Samples were assayed in triplicate by EliSpot for ASCs, using total sheep anti–mouse Ig as both plate coat (at 2 μg/mL) and detector (horseradish peroxidase–conjugated sheep anti–mouse Ig; Silenus Laboratories). Spots were counted with an AID EliSpot Reader HR (Strassberg, Germany), and numbers were converted to indicate total ASCs in the entire tissue sample.
Statistical analysis
Survival curves were compared with the log-rank31 and Cox proportional hazard32 tests. Mice with no tumors were censored at 365 days. In the experiment of Figure 2 comprising reconstituted mice, several mice were censored because of death from causes unrelated to tumor development (early death [< 30 days]) after irradiation without reconstitution [3 animals], eye infection [1 animal], host cell–derived thymoma [5 animals], or a failure to reconstitute with donor cells, as described above). There was no relationship between these events and the Blimp1 genotype of transplanted cells.
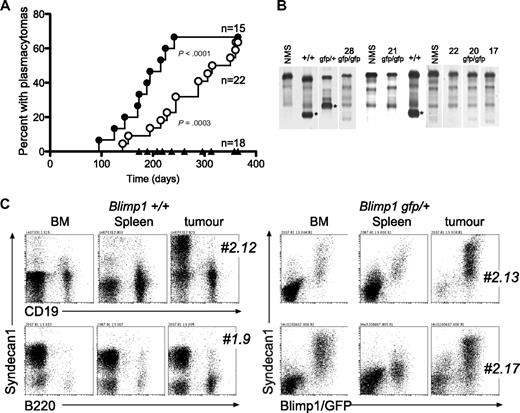
Tumor phenotype and prevalence in mice reconstituted with v-abl transgenic fetal liver as a function of Blimp1 gene dose. (A) Kaplan-Meier plot of PCT incidence by Blimp1 genotype (●, Blimp1+/+; ○, Blimp1gfp/+, ▴, Blimp1gfp/gfp). See “Methods” for statistical analyses. (B) Native gel electrophoresis to reveal paraprotein (*) in the serum of PCT-bearing mice. Vertical lines (white) have been inserted to indicate gel lanes repositioned to juxtapose relevant samples; sera were run in batches on different days, but the NMS control included on each gel is shown in the figure. (C) Flow cytometric analyses of cells from tumors, BM, and spleens of mice reconstituted with Blimp1+/+ (2 mice) and Blimp1gfp/+ fetal liver stem cells (2 mice), stained as indicated. In the latter 2 mice, GFP+ cells were B220− (data not shown). # indicates mouse designation number.
Tumor phenotype and prevalence in mice reconstituted with v-abl transgenic fetal liver as a function of Blimp1 gene dose. (A) Kaplan-Meier plot of PCT incidence by Blimp1 genotype (●, Blimp1+/+; ○, Blimp1gfp/+, ▴, Blimp1gfp/gfp). See “Methods” for statistical analyses. (B) Native gel electrophoresis to reveal paraprotein (*) in the serum of PCT-bearing mice. Vertical lines (white) have been inserted to indicate gel lanes repositioned to juxtapose relevant samples; sera were run in batches on different days, but the NMS control included on each gel is shown in the figure. (C) Flow cytometric analyses of cells from tumors, BM, and spleens of mice reconstituted with Blimp1+/+ (2 mice) and Blimp1gfp/+ fetal liver stem cells (2 mice), stained as indicated. In the latter 2 mice, GFP+ cells were B220− (data not shown). # indicates mouse designation number.
Results
Plasmacytoma incidence is decreased by the loss of one functional Blimp1 allele
Two genetically modified mouse lines were combined for these studies. The Blimp1-GFP reporter mouse was generated by targeting the green fluorescent protein (GFP) gene to the endogenous Blimp1 locus using embryonic stems cells from the C57BL/6 strain.5 All antibody-secreting plasma cells in this mouse express GFP. The reporter allele encodes a nonfunctional truncated Blimp1 protein.5,6 In vitro–derived Blimp1gfp/+ ASCs express less wild-type Blimp1 mRNA than those from control mice, whether generated in TD or TI conditions (Figure S3C, available on the Blood website; see the Supplemental Materials link at the top of the online article), and they have roughly one-half the amount of Blimp1 protein compared with Blimp1+/+ ASCs (Figure S3B; Kallies et al5 ).
The Eμ-v-abl transgenic mouse is prone to PCT development.19 The disease is highly dependent on strain and sex, with Balb/c being susceptible and C57BL/6 relatively resistant; F1 mice develop PCTs with an intermediate prevalence.24 Males are more susceptible to disease, and the parental source of the transgene influences disease incidence.19,24 The disease manifestations are consistent with those of several mouse PCT models: high levels of clonal Ig in the serum, a tumor mass, most frequently gut-associated, comprising cells secreting Ig of switched isotypes (most frequently IgG and IgA) and commonly bearing rearrangements of the c-myc gene.3
To ask whether Blimp1 levels influence disease in this model, we crossed Blimp1gfp/+ mice to CVA40 mice, which carry the Eμ-v-abl transgene on a Balb/c genetic background, to generate an F1 generation. We used exclusively female CVA40 mice, mating them to male Blimp1gfp/+ mice, and selected F1 progeny carrying the v-abl transgene, either wild type or heterozygous for Blimp1, for long-term observation (170 mice distributed across the genotypes and sexes).
All mice that succumbed to tumors within the 365-day observation period had PCTs. Tumors were composed of cells resembling transformed mature plasma cells (B220−, Blimp1/GFP+, and/or Syndecan1/CD138int/hi; Figure 1A). Plasmacytomas in this model consistently had this surface phenotype (Figures 1, S2). They varied in the proportion of such cells in a given tumor mass, and the levels of Syndecan1 varied between tumors. We have shown that Blimp1/GFP is a reliable marker of ASCs, unlike Syndecan1, because Syndecan1−, Blimp/GFP+ ASCs are generated under some culture conditions27 (see Figure S2A). We sorted cells from one Blimp1gfp/+ tumor into B220+/GFP−, B220−/GFP+, and double-negative cells and cultured 105 cells of each population for 6 hours in the presence of 3[H]-thymidine to assess the proliferative status of cells in the tumor. The B220−/GFP+ ASC fraction of the tumor was the only proliferating fraction (Figure 1C). Most normal plasma cells in vivo are nondividing cells.5,9
We confirmed the presence of clonal Ig “paraprotein” in the serum of PCT-bearing mice by native gel electrophoresis (Figure 1B). We found that the abnormally high serum level of Ig (up to 1000-fold) of a single isotype could also be reliably detected by ELISA (Table 1; Figure S1). Blimp1+/+ and Blimp1gfp/+ tumors displayed c-myc gene rearrangements at a similar frequency (∼ 55%-65%). This used the Southern assay of Figure 1D, and a probe identifying a 3.6-kb fragment at the 5′ end of the c-myc gene, including the transcriptional start site.30 If Blimp1 loss contributed to malignancy through derepression of c-myc, Blimp1gfp/+ tumors would be predicted to display a reduced frequency of c-myc rearrangements compared with Blimp1+/+tumors, but this was not seen in the sample examined. Thus, the tumors that appeared in both groups of mice were indistinguishable and matched the published description of the disease in CVA40 mice.19
Frequency of paraprotein or hypergammaglobulinemia in serum of tumor-bearing animals (male), according to Blimp1 genotype
| Genotype . | N . | Paraprotein or hyper-Ig, n (%) . | Isotype, n (%)* . |
|---|---|---|---|
| +/+ | 21 | 16 (76) | 4 IgA+ (25) |
| 2 IgG1+ | |||
| 3 IgM+ | |||
| 7 Igk/IgH† | |||
| +/gfp | 20 | 19 (95) | 8 IgA+ (42) |
| 1 IgG1+ | |||
| 2 IgM+/A+ | |||
| 8 Igk/IgH† |
| Genotype . | N . | Paraprotein or hyper-Ig, n (%) . | Isotype, n (%)* . |
|---|---|---|---|
| +/+ | 21 | 16 (76) | 4 IgA+ (25) |
| 2 IgG1+ | |||
| 3 IgM+ | |||
| 7 Igk/IgH† | |||
| +/gfp | 20 | 19 (95) | 8 IgA+ (42) |
| 1 IgG1+ | |||
| 2 IgM+/A+ | |||
| 8 Igk/IgH† |
Determined as described in “Methods.”
Isotype not determined.
Strikingly, a Kaplan-Meier analysis of disease incidence showed that the loss of one functional copy of the Blimp1 gene delayed the onset and decreased the incidence of PCTs considerably. This was most evident in male mice (P = .003 with the log-rank test31 ; Figure 1E; Table 2), which are more susceptible to the disease. Twenty-two (71%) of 31 Blimp1+/+ transgenic males succumbed to PCTs, with a median latency of 164.5 days, compared with 22 (41%) tumors in 53 Blimp1gfp/+ transgenic males, with a median latency of 204 days. As expected, female F1 mice developed PCTs later than males.24 Because the numbers of tumors that developed were lower, the difference between Blimp1+/+ and Blimp1gfp/+ females did not reach significance over the 1-year duration of the experiment (P = .477; Figure 1E). Nevertheless, for those mice that did develop tumors, the prevalence was lower when one functional allele of Blimp1 was lost (28% for Blimp1+/+ compared with 21% for Blimp1gfp/+ mice, and median latency was delayed by 44 days; Table 2). We can infer from the fact that the Blimp1gfp/+ tumors secrete similar amounts of clonal antibodies compared with Blimp1+/+ tumors, and express Syndecan1, that they have not lost the functional Blimp1 allele.
PCT prevalence in mice bearing the v-abl transgene and 1 or 2 functional copies of the Blimp1 gene, by sex
| Sex and genotype of tumor-bearing animal . | Tumor latency,* (median), d . |
|---|---|
| Female +/+ (n = 12) | 221.1 ± 82.2 (168.5) |
| Female +/gfp (n = 9) | 205.47 ± 72.2 (212) |
| Male +/+ (n = 22) | 174.0 ± 63.5 (164.5) |
| Male +/gfp (n = 22) | 201.0 ± 69.2 (204) |
| Total +/+ (n = 34) | 190.6 ± 73.1 (167.5) |
| Total +/gfp (n = 31) | 202.3 ± 68.9 (212) |
| Sex and genotype of tumor-bearing animal . | Tumor latency,* (median), d . |
|---|---|
| Female +/+ (n = 12) | 221.1 ± 82.2 (168.5) |
| Female +/gfp (n = 9) | 205.47 ± 72.2 (212) |
| Male +/+ (n = 22) | 174.0 ± 63.5 (164.5) |
| Male +/gfp (n = 22) | 201.0 ± 69.2 (204) |
| Total +/+ (n = 34) | 190.6 ± 73.1 (167.5) |
| Total +/gfp (n = 31) | 202.3 ± 68.9 (212) |
Mean ± SD.
To address the question whether the reduction in PCT prevalence in Blimp1gfp/+ mice would simply reflect a reduction in ASC numbers in mice with only one functional copy of the Blimp1 gene, we directly measured ASC numbers in spleen and BM of nontransgenic Blimp1+/+ and Blimp1gfp/+ mice, using EliSpot assays. We used nontransgenic mice for this assessment, to avoid possible effects of premalignant changes in cell numbers that might skew the comparison, because transformation latency is different in transgenic Blimp1+/+ and Blimp1gfp/+ mice. We found that heterozygous mice have equivalent numbers of ASCs in spleen and BM to wild-type mice (Figure 1F), and expect that ASC numbers would also be comparable between v-abl transgenic Blimp1+/+ and Blimp1gfp/+ mice before malignant changes. Consistent with this, serum Ig in naive mice (measured as total Igκ) is equivalent in Blimp1+/+ and Blimp1gfp/+ mice (Figure 1G). Thus, Blimp1 is a limiting factor for plasma cell transformation in this model, with loss of one functional copy of the gene both delaying the disease and decreasing its prevalence.
Plasmacytomas fail to form in the absence of functional Blimp1
We wanted to test whether PCTs require Blimp1 for their formation. We have shown that, although mature plasma cells cannot form in the absence of Blimp1, initiation of plasma cell differentiation is Blimp1 independent, and phenotypically and functionally immature “pre-plasmablasts” secreting low amounts of Ig are easily detected in Blimp1-deficient animals.12 It was therefore possible that pre-plasmablast tumors would be generated in v-abl transgenic Blimp1gfp/gfp mice.
The Blimp1 mutation causes embryonic lethality in homozygous animals.5 Therefore, to generate mice that were transgenic for v-abl and homozygous for the mutant Blimp1gfp allele, we generated an F2 generation. F1 males that were Blimp1gfp/+ and v-abl+ were crossed to F1 Blimp1gfp/+ nontransgenic females. C57BL/6 × Balb/c F2 generation mice have a slightly reduced susceptibility to PCT in this disease model compared with F1 mice.24 To avoid impractically long latency periods and observation times, we used male transgenic mice in the breeding strategy, because passage of the Eμ-v-abl transgene through the male line accelerates the onset of disease.24 Similarly, male embryos were used as donors (genotyping of fetuses included a test for the male-specific Sry gene), and male F1 recipients were used as hosts for fetal liver reconstitution, because the male factor, cell intrinsic or extrinsic, that influences disease prevalence in this model has not been determined. Because mice reconstituted with homozygous Blimp1gfp/gfp cells frequently succumb to an aggressive T cell–mediated autoimmune syndrome unless protected by cotransferred Blimp1+/+cells,25 all of the fetal liver transfers done here included a small number of wild-type cells from control littermates. Eight weeks after reconstitution, quantitative PCR was used to determine the relative contribution of donor cells to peripheral blood cells of the chimeric mice (see “PCR”). Only successfully reconstituted mice were included in the study.
Recipient mice were monitored for a 1-year period. As shown in Figure 2A and Table 3, PCT prevalence in the reconstituted mice closely resembled that in intact mice, as long as they possessed at least one functional Blimp1 allele. Specifically, mice reconstituted with Blimp1+/+ or Blimp1gfp/+ cells developed plasmacytomas with the identical presentation (gut-associated tumors containing an abundance of B220−/GFP+/Syndecan1+ cells [Figure 2C], clonal Ig in the serum [Figure 2B], and frequent c-myc rearrangements; data not shown) to intact mice. Again, the disease was delayed and less frequent in mice that had only one functional Blimp1 allele. Interestingly, although PCTs were primarily found in association with the gut, abnormally high percentages of GFP+/Syndecan1+ cells were often also found in the BM and to a lesser degree, in the spleen (Figure 2C), showing that transformed plasma cells also disseminate to these organs in the CVA40 model. Importantly, these transfer studies show that the effect of Blimp1 gene dose on prevalence is tumor cell intrinsic.
Tumor prevalence in mice reconstituted with fetal liver cells bearing the v-abl transgene and the indicated Blimp1 genotypes
| Genotype . | N . | Tumor latency* (median), d . | Number with tumors† (%) . |
|---|---|---|---|
| +/+ | 15 | 177.0 ± 45.5 (181) | 10/15 (66.7) |
| +/gfp | 22 | 263.2 ± 72.7 (266) | 14/22 (63.6) |
| gfp/gfp | 18 | 275.0 ± 64.6 (296) | 5/18 (27.8) |
| Genotype . | N . | Tumor latency* (median), d . | Number with tumors† (%) . |
|---|---|---|---|
| +/+ | 15 | 177.0 ± 45.5 (181) | 10/15 (66.7) |
| +/gfp | 22 | 263.2 ± 72.7 (266) | 14/22 (63.6) |
| gfp/gfp | 18 | 275.0 ± 64.6 (296) | 5/18 (27.8) |
Mean ± SD.
Of donor cell origin.
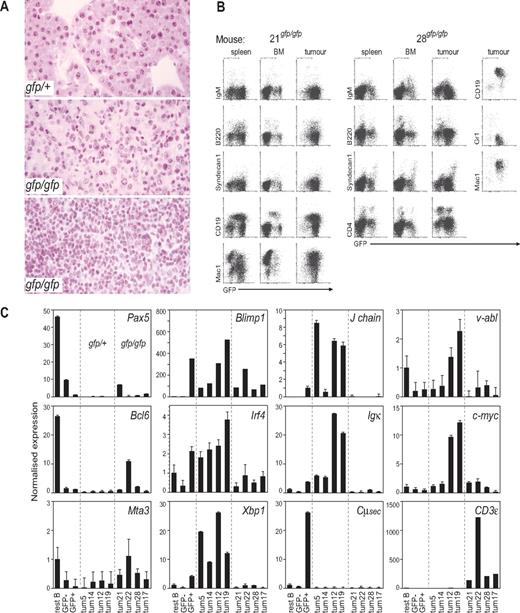
A small number of animals reconstituted with Blimp1gfp/gfp cells (5 of 18) developed tumors with a long median latency of 296 days, but none of these was a plasma cell tumor. This was evident by the lack of elevated Ig in the serum of the mice, as assessed by paraprotein gels (Figure 2B) or ELISA (Table 4), and by the lack of Syndecan1 expression on tumor cells (Figure 3B). The 5 donor-derived tumors in Blimp1gfp/gfp reconstituted mice were of 2 types. Four had the gross morphologic (enlarged, mottled spleen, and enlarged LN) and histologic appearance (Figure 3A middle) of reticulum cell sarcoma, leukemias typically of myeloid phenotype. The other mouse had an enlarged spleen, thymus, mesenteric and peripheral LNs, a pale liver, and the histologic appearance of a lymphoid leukemia (Figure 3A bottom). Measurements of expression of selected genes in GFP+ cells from the tumors distinguished the Blimp1gfp/+ PCTs (Blimp1+, Irf4+, Xbp1+, Igk+, J chain+, Pax5−, Bcl6−, Mta3−) from the nonplasma cell Blimp1gfp/gfp tumors, which had the opposite pattern. The latter group also showed somewhat lower v-abl and c-myc expression (Figure 3C). Flow cytometric analyses of tissues from these 5 mice confirmed an abundance of GFP+ cells in tumors and lymphoid tissues, with the reticulum cell sarcoma mice containing GFP+ cells that were Syndecan1−, B220lo/−, IgM−, and variably CD19+, Mac1+, or CD4+ (Figure 3B; data not sown). Tumor cells from mouse 28, which had a lymphoid leukemia, were GFP+, Syndecan1−, CD19+, Mac1+, B220−, and IgM−. It is important to note that some cells in these tumors or organs would be malignant, but others may simply be normal tissue or inflammatory cells, or tumor-reactive lymphoid cells.
Frequency of paraprotein or hypergammaglobulinemia in serum of tumor-bearing animals, according to Blimp1 genotype
| Genotype . | N . | Paraprotein or hyper-Ig (%) . | Isotype, n (%) . |
|---|---|---|---|
| +/+ | 10 | 9 (90) | 6 IgA+ (67) |
| 1 IgG1+ | |||
| 2 IgM+ | |||
| +/gfp | 14 | 13 (93) | 9 IgA+ (64) |
| 2 IgG1+/A+ | |||
| 1 Igk/IgH? | |||
| gfp/gfp | 5 | 0 | 0 |
| Genotype . | N . | Paraprotein or hyper-Ig (%) . | Isotype, n (%) . |
|---|---|---|---|
| +/+ | 10 | 9 (90) | 6 IgA+ (67) |
| 1 IgG1+ | |||
| 2 IgM+ | |||
| +/gfp | 14 | 13 (93) | 9 IgA+ (64) |
| 2 IgG1+/A+ | |||
| 1 Igk/IgH? | |||
| gfp/gfp | 5 | 0 | 0 |
indicates isotype not determined.
Characterization of rare Blimp1gfp/gfp tumors. (A) Representative histologic appearance of tumors by Blimp1 genotype. (Top) PCT in a Blimp1gfp/+ mouse, characteristic of tumors seen in all Blimp1+/+ and Blimp1gfp/+ mice. (Middle) Reticulum cell sarcoma, as seen in 4 Blimp1gfp/gfp mice. (Bottom) Lymphoid leukemia in Blimp1gfp/gfp mouse no. 28. Images were viewed using a Zeiss Axiophot microscope (Oberkochen, Germany) with a Plan-apo 20×/0.60 objective and hematoxylin and eosin stain and captured with a Zeiss Axiophot camera. (B) Flow cytometric analysis of BM, spleens, and enlarged LN (“tumors”) of 2 representative Blimp1gfp/gfp mice that developed tumors. The tissues of mouse no. 21 had a pattern characteristic of mice that bore reticulum cell sarcomas (nos. 17, 21, and 22). Mouse no. 28 had a lymphoid leukemia. The panel on the right for mouse no. 28 is gated on GFP+ cells in the tumor. (C) Quantitative reverse transcription PCR measuring expression levels of genes in normal resting B cells, GFP− B-cell blasts, GFP+ plasmablasts generated in vitro (27 ) and GFP+ cells sorted from 4 Blimp1gfp/+ and all 5 Blimp1gfp/gfp tumors. Note that the Blimp1 PCR amplifies exons that are not affected by the Blimp1/GFP modification, so both Blimp1+ and Blimp1gfp alleles are detected.
Characterization of rare Blimp1gfp/gfp tumors. (A) Representative histologic appearance of tumors by Blimp1 genotype. (Top) PCT in a Blimp1gfp/+ mouse, characteristic of tumors seen in all Blimp1+/+ and Blimp1gfp/+ mice. (Middle) Reticulum cell sarcoma, as seen in 4 Blimp1gfp/gfp mice. (Bottom) Lymphoid leukemia in Blimp1gfp/gfp mouse no. 28. Images were viewed using a Zeiss Axiophot microscope (Oberkochen, Germany) with a Plan-apo 20×/0.60 objective and hematoxylin and eosin stain and captured with a Zeiss Axiophot camera. (B) Flow cytometric analysis of BM, spleens, and enlarged LN (“tumors”) of 2 representative Blimp1gfp/gfp mice that developed tumors. The tissues of mouse no. 21 had a pattern characteristic of mice that bore reticulum cell sarcomas (nos. 17, 21, and 22). Mouse no. 28 had a lymphoid leukemia. The panel on the right for mouse no. 28 is gated on GFP+ cells in the tumor. (C) Quantitative reverse transcription PCR measuring expression levels of genes in normal resting B cells, GFP− B-cell blasts, GFP+ plasmablasts generated in vitro (27 ) and GFP+ cells sorted from 4 Blimp1gfp/+ and all 5 Blimp1gfp/gfp tumors. Note that the Blimp1 PCR amplifies exons that are not affected by the Blimp1/GFP modification, so both Blimp1+ and Blimp1gfp alleles are detected.
In human MM, an alternative PRDM1 transcript has been described, initiated in the intron upstream of exon 4.33,34 The truncated protein product of this transcript, the “β isoform,” has reduced repressor function in vitro and has been postulated to contribute to the malignant phenotype.33 The β isoform transcript has not been described in the mouse, but the sequence is conserved. We designed primers to detect both the normal Blimp1 α isoform and the β isoform, to assess whether the latter was specifically up-regulated in the tumors described here. As shown in Figure S1, the β isoform transcript was expressed consistently at a very low level (∼ 30-fold less than the α isoform) in the samples tested, and it was found sporadically in both normal and malignant tissues. The Blimp1gfp/+ reporter allele, which also encodes a truncated protein,5 was expressed at roughly the same level in tumors as in normal plasma cells and plasmablasts, consistent with the level of GFP expression measured by flow cytometry (Figure S2). Thus, although it is a formal possibility, it appears unlikely that elevated expression of alternative Blimp1 transcripts are responsible for the effect on plasmacytoma development that we describe here for Blimp1-sufficient mice but may have an effect on tumor formation in Blimp1-deficient animals. Overall, these findings indicate that Blimp1 is required for the generation of plasma cell tumors in this model. We did not find evidence for an acceleration of nonplasma cell tumors in the absence of Blimp1, but, in its absence, other lymphomyeloid malignancies did occur. These expressed some T and myeloid lineage genes (CD3, Mac1; Figure 3; data not shown), and they arose at a low frequency and after a long latency.
Discussion
Given the essential role of Blimp1 in plasma cell differentiation and longevity and its universal expression in PCT and MM, it is important to clarify the role PDM1/BLIMP1 plays during plasma cell transformation. This project aimed to determine the involvement of Blimp1 in susceptibility to plasma cell transformation in a mouse model. We have assessed whether loss of one or both alleles of the Blimp1 gene affects tumor prevalence, latency, or phenotype.
In the work described here, all tumors in Blimp1-sufficient mice were plasma cell tumors. Decreasing the dose of Blimp1 from 2 to 1 functional gene copy in the v-abl mice reduced disease significantly, whereas the total loss of functional Blimp1 prevented PCT development. Thus, Blimp1 does not have tumor suppressor activity in plasma cells. Indeed, Blimp1 may contribute directly to the transformed phenotype in PCT, because we found no numerical difference in ASC numbers in nontransgenic mice bearing 1 or 2 functional Blimp1 alleles, although PCT prevalence was significantly affected by Blimp1 dose. The mechanism through which Blimp1 levels mediate plasma cell transformation will require investigation. Indeed, through its known target genes, Blimp1 has the capacity to regulate both cell division (via c-myc)8 and cell survival (via Bim and Mcl1).16
Two recent studies suggested that Blimp1 may act as a tumor suppressor in a small subset of human DLBCL, because these had suffered mutations silencing both Blimp1/PRDM1 alleles.17,18 The investigators proposed that the Blimp1-deficient tumors formed because the activated B cells carrying the transforming gene mutations could no longer differentiate into antibody-secreting cells in the absence of Blimp1. In the present study, a small number of tumors did arise in mice reconstituted with Blimp1-deficient cells that were unlike those occurring in mice reconstituted with Blimp1+/+ or Blimp1gfp/+ cells. They arose late, and so it is difficult to know whether they may have also occurred in the other cohorts but were overshadowed by the more rapid and frequent PCTs. These 5 Blimp1-deficient tumors had histologic characteristics, gene expression, and surface phenotypes, indicating they involved less-mature B lineage cells (in one case), or an unusual lymphomyeloid cell (4 cases). Similar tumors are typical of older mice35 and have also been observed in non–v-abl-transgenic Blimp1gfp/gfp mice (A.K., unpublished observations, 2008). They may reflect a cell type or stage in which Blimp1 plays a different role, suppressing transformation.
Several transgenic mouse models of PCT have been described. In the earliest versions, pristane was used to induce granulomas, inflammatory lesions composed of transformed plasma cells and supporting stroma, to accelerate disease in susceptible strains. In later models, enforced transgenic expression of oncogenes such as c-myc,36 the growth factor IL-6,37 or cell survival genes38 were shown to contribute to PCT development. In most of these models, PCTs developed in gut-associated lymphoid tissues and shared other pathologic features. Potter has reviewed much work in this area and has generated a model for PCT development in which inflammation, enhanced proliferation and survival all contribute.3 The gut microenvironment provides growth signals for B cells and inflammatory signals, particularly by IL-6–secreting stromal cells. B-cell growth is enhanced by translocated c-myc. (Indeed, a rearranged c-myc allele is unlikely to be regulated by Blimp1, because the Blimp1 responsive region8 of the c-myc promoter is typically replaced with sequences from the translocated chromosome.) In the CVA40 and related models, v-Abl, a constitutively active tyrosine kinase, may also deliver a strong growth or survival signal. All of these influences are tempered by the genetic background of the mouse. Although the plasmacytoma-prone transgenic mice together emphasize the contributions of inflammation and deregulated growth and survival signals to malignant transformation, they do not explain why the plasma cell is specifically affected. Both v-abl and aberrantly expressed c-myc can efficiently transform precursor B cells.20,39 Spontaneous c-myc translocations are thought to occur in mature B cells in the context of the germinal center under the influence of AID, the mediator of somatic hypermutation and class switch recombination.21-23 However, neither precursor nor mature B-cell tumors occur in the CVA40 mouse line if Blimp1 is functional; the mice develop exclusively PCTs (Rosenbaum19 ; this study). Blimp1 appears to be required for generation of a cell that is responsive to all of the signals that conspire to transform a plasma cell and may also contribute to the transformed cell's capacity to expand or survive in the microenvironment. The Blimp1-independent pre-plasmablast stage appears not to be susceptible to transformation in the CVA40 model, because none of Blimpgfp/gfp tumors had the phenotype reported for pre-plasmablasts: low Ig-secreting cells with elevated Xbp1 and J chain expression.12 In this context, it may be relevant that a cell with the phenotype of a memory B cell, rather than a CD138/Syndecan1+ plasma cell, has been proposed to be the stem cell in MM.40 Combining the Blimp1/GFP reporter with other plasmacytoma-prone mouse models should help to identify the plasmacytoma stem cell. Taken together, the studies reported here highlight the importance of Blimp1 not only in the persistence and phenotype of normal plasma cells but also in the generation of malignant plasma cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are thankful for the thoughtful and generous advice given to us in the early phases of this project by our colleague, Dr Alan Harris, now deceased. Dr Harris also provided the CVA40 mice and advice on their care and breeding. Charity Law provided valuable help with statistical analyses. We also thank Dr T. Thomas for advice on genotyping for sex.
This work was supported by the Australian National Health and Medical Research Council (NHMRC; IRIISS grant 361646), an NHMRC Program grant (356202), and a Victorian State Government OIS grant.
Authorship
Contribution: K.D. performed all genotyping, paraprotein analyses, and ELISA; D.E. performed the genomic Southern analysis and all PCRs; D.M. performed the histologic analyses; G.K.S. performed the statistical analyses; A. Karnowski, A. Kallies, and S.L.N. helped with mouse generation and analysis of tissues from sick animals, as well as assisting in the writing of the manuscript; and L.M.C. designed the experiments, performed most of the analyses on sick mice, performed the EliSpot assays, performed the fetal liver reconstitutions, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lynn M. Corcoran, Immunology Division, 1G Royal Parade, Parkville, Victoria, Australia 3050; e-mail: corcoran@wehi.edu.au.
References
Author notes
*K.D. and D.E. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal