Abstract
Activation of p53 by murine double minute (MDM2) antagonist nutlin-3a or inhibition of X-linked inhibitor of apoptosis (XIAP) induces apoptosis in acute myeloid leukemia (AML) cells. We demonstrate that concomitant inhibition of MDM2 by nutlin-3a and of XIAP by small molecule antagonists synergistically induced apoptosis in p53 wild-type OCI-AML3 and Molm13 cells. Knockdown of p53 by shRNA blunted the synergy, and down-regulation of XIAP by antisense oligonucleotide (ASO) enhanced nutlin-3a–induced apoptosis, suggesting that the synergy was mediated by p53 activation and XIAP inhibition. This is supported by data showing that inhibition of both MDM2 and XIAP by their respective ASOs induced significantly more cell death than either ASO alone. Importantly, p53 activation and XIAP inhibition enhanced apoptosis in blasts from patients with primary AML, even when the cells were protected by stromal cells. Mechanistic studies demonstrated that XIAP inhibition potentiates p53-induced apoptosis by decreasing p53-induced p21 and that p53 activation enhances XIAP inhibition-induced cell death by promoting mitochondrial release of second mitochondria-derived activator of caspases (SMAC) and by inducing the expression of caspase-6. Because both XIAP and p53 are presently being targeted in ongoing clinical trials in leukemia, the combination strategy holds promise for expedited translation into the clinic.
Introduction
Chemotherapies are the primary treatment modality for acute myeloid leukemia (AML), but their effectiveness is limited largely by chemoresistance that almost invariably evolves in patients with this disease. We and others have demonstrated that apoptosis deregulation is the chief cause of this resistance. One important factor that contributes to chemoresistance is X-linked inhibitor of apoptosis (XIAP), a potent cellular inhibitor of apoptosis.1 XIAP is highly expressed in AML and other cancers, protecting malignant cells from apoptosis.2-6 Another key regulator of apoptosis is p53, a potent tumor suppressor. Although p53 mutations are rare in AML, p53 signaling is frequently inactivated by overexpression of murine double minute (MDM2), a negative regulator of p53.7-9 Both XIAP and MDM2 have been proven to be potential therapeutic targets in AML. Studies by our group have shown that inhibition of XIAP induced apoptosis, demonstrated antileukemia effects in vitro in both AML cell lines and samples from patients with primary AML, and showed chemosensitization in HL-60 cells.6,10 Furthermore, activation of p53 by inhibition of MDM2 induced death of AML cells in a p53-dependent manner.11-13 A phase 1/2 clinical trial of XIAP antisense oligonucleotide (ASO) in combination with standard chemotherapy (idarubicin [IDA] and cytosine arabinoside [ara-C]) in resistant/relapsed AML has shown promising results (10/11 induction therapy–resistant AML patients achieved complete response (CR) or complete remission without platelet recovery (CRp) with the XIAP ASO-IDA/ara-C combination14,15 ). A phase 1 clinical trial of MDM2 inhibition in leukemia is ongoing at The University of Texas M. D. Anderson Cancer Center.
However, the effectiveness of monotherapies in most cases is very limited because of the pleiotropic nature of cancers and the compensatory cellular mechanisms involved. Not only are XIAP and MDM2 overexpressed in many malignant cells, the functions of XIAP and p53 are mediated and the interplay of their activities is orchestrated by a network of numerous components. XIAP acts by binding to and inhibiting the activation and activity of caspases and is negatively regulated by multiple proteins, including serine protease HtrA2/omi, second mitochondria-derived activator of caspases (SMAC), and XIAP-associated factor 1.16-19 Clearly, the effectiveness of XIAP inhibition depends not only on the levels of XIAP, but also on the levels of caspases and cellular XIAP inhibitors. Increasing caspase levels and negative regulators of XIAP should tip the balance toward apoptosis and facilitate XIAP inhibition-induced cell death. Development of SMAC mimetics as a strategy to neutralize XIAP and induce cell death is under active investigation.20,21 ABT-10 is one such compound.22
p53 is a potent apoptosis inducer. Over the past few years, however, mounting evidence has demonstrated that p53 also transcriptionally activates a multitude of genes whose products counteract apoptosis (for review see Janicke et al23 ). The most studied, p21Waf1/Cip124,25 has been shown not only to block cell cycle progression, but also to inhibit apoptosis (for review see Liu et al26 ), in part by blocking the activation of procaspase-3.27 Therefore, p53-induced apoptosis can be blunted by p21, and removal of p21 can enhance p53-induced cell death.28
Interestingly, it was recently reported that p53 transcriptionally activates effector caspases-6 and -7.29 Furthermore, because p53 induces apoptosis largely by modulating B-cell leukemia 2 (Bcl-2) family proteins and thus permeabilizing mitochondrial outer membrane, it is possible that it promotes release of SMAC, the negative regulator of XIAP. Moreover, XIAP overexpression was shown to induce p21 and block cell proliferation in endothelial cells.3 Importantly, activated caspase-3 was found to cleave p21, converting growth arrest to apoptosis.30 Therefore, simultaneous inhibition of XIAP and activation of p53 could enhance each other's apoptogenic activities by maximizing proapoptotic and minimizing antiapoptotic effects associated with the 2 proteins. Studies in prostate cancer cells and neurons have suggested that accumulation of p53 and reduction of XIAP potentiate apoptosis.31-33 We therefore hypothesized that simultaneous inhibition of XIAP and activation of p53 could constitute a novel strategy for maximizing apoptotic signaling and improve treatment of AML. We here demonstrate that the simultaneous activation of p53 and inhibition of XIAP enhance the activation of apoptosis signaling pathways in AML and that decrease of p53-induced p21 via caspase cleavage mediated by inhibiting XIAP and the cytosolic release of SMAC and increase of effector caspase-6 protein level via p53 activation mediated by inhibiting MDM2 are all contributing to these synergistic effects.
Methods
Cells and cell cultures
OCI-AML3, OCI-AML3vec, OCI-AML3p53shRNA, and Molm13 cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 2mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. OCI-AML3 cells were kindly provided by Dr M. Minden (Ontario Cancer Institute). OCI-AML3 cells in which p53 was knocked down by short hairpin RNA (OCI-AML3p53shRNA) and vector control OCI-AML3vec cells were generated by retrovirus transfection, as reported previously.34 Molm13 cells were obtained from Fujisaki Cell Center, Hayashibara Biochemical Labs Inc. MS-5 stromal cells, an established murine mesenchymal stromal cell (MSC) line known to support primitive human progenitors and to mimic the bone marrow microenvironment35-37 was kindly provided by Dr K. Itoh (Department of Biology, Faculty of Science, Niigata University). Fresh samples from patients with primary AML with high blast counts (> 55%) were acquired after obtaining informed consent according to guidelines of the institution and the Declaration of Helsinki. Mononuclear cells were purified by Ficoll-Hypaque (Sigma Chemical Co) density-gradient centrifugation and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum. The clinical characteristics of the AML patients from whom samples were collected are summarized in Table 1. All studies were performed according to a protocol approved by the institutional review board of University of Texas M. D. Anderson Cancer Center.
Characteristics of AML patients whose samples were used in this study
| Patient no. . | Blasts, % . | Source . | Cytogenetics . | Treatments and responses at sampling . |
|---|---|---|---|---|
| 1 | 92 | Apheresis | Diploid female karyotype 46,XX[20] | New diagnosis |
| 2 | 92 | PB | 45,XY,t(1;12)(p34.3;q24.1),-7[20] | CR on IDA+ara-C then relapsed; relapsed on high-dose ara-C consolidation, transplant, and reinduction IDA+ara-C; and resistant to DAC+gemtuzumab ozogamicin |
| 3 | 85 | PB | Diploid female karyotype 46,XX[30] | CR on IDA+high-dose ara-C then relapsed; CR on infusion then relapsed;resistant to 5-AZA+ara-C and CP-4055 |
| 4 | 98 | PB | Diploid female karyotype 46,XX[20] | CR on IDA+ara-C then relapsed; consolidated with high dose ara-C;resistant to mitoxantrone+VP-16 and CP-4055 |
| 5 | 71 | PB | 46,XX,t(8;21)(q22;q22)[2],46,sl,-X, t(8;21)(q22;q22)[4],47,sl,X,+18,+der(21)t(8;21)(q22;q22)[4],sl,del(15)(q11.2q15),+18,add(20)(q11.2), +der(21)t(8;21)(q22;q22)[cp10] etc | CRp on bid FA+gemtuzumab ozogamicin then relapsed; resistant to oxyliplatin+FA |
| 6 | 56 | PB | Diploid male karyotype 46,XY[20] | Initial blast reduction, then increased on bortezomib+melphalan; resistant to 5-AZA+AC; stable disease and a gradual reduction of marrow blast on SAHA+DAC; resistant to Arry-520 |
| Patient no. . | Blasts, % . | Source . | Cytogenetics . | Treatments and responses at sampling . |
|---|---|---|---|---|
| 1 | 92 | Apheresis | Diploid female karyotype 46,XX[20] | New diagnosis |
| 2 | 92 | PB | 45,XY,t(1;12)(p34.3;q24.1),-7[20] | CR on IDA+ara-C then relapsed; relapsed on high-dose ara-C consolidation, transplant, and reinduction IDA+ara-C; and resistant to DAC+gemtuzumab ozogamicin |
| 3 | 85 | PB | Diploid female karyotype 46,XX[30] | CR on IDA+high-dose ara-C then relapsed; CR on infusion then relapsed;resistant to 5-AZA+ara-C and CP-4055 |
| 4 | 98 | PB | Diploid female karyotype 46,XX[20] | CR on IDA+ara-C then relapsed; consolidated with high dose ara-C;resistant to mitoxantrone+VP-16 and CP-4055 |
| 5 | 71 | PB | 46,XX,t(8;21)(q22;q22)[2],46,sl,-X, t(8;21)(q22;q22)[4],47,sl,X,+18,+der(21)t(8;21)(q22;q22)[4],sl,del(15)(q11.2q15),+18,add(20)(q11.2), +der(21)t(8;21)(q22;q22)[cp10] etc | CRp on bid FA+gemtuzumab ozogamicin then relapsed; resistant to oxyliplatin+FA |
| 6 | 56 | PB | Diploid male karyotype 46,XY[20] | Initial blast reduction, then increased on bortezomib+melphalan; resistant to 5-AZA+AC; stable disease and a gradual reduction of marrow blast on SAHA+DAC; resistant to Arry-520 |
AML indicates acute myeloid leukemia; PB, peripheral blood; CR, complete response; bid, twice daily; DAC, dacitabine; VP-16, etoposide; FA, fludarabine; 5-AZA+AC, 5-azacitidine + ara-c; and SAHA, suberoylanilide hydroyxamic acid.
Treatment of cells
Exponentially growing AML cells (0.4 × 106/mL) from cell lines or mononuclear cells from AML patients (0.5 × 106/mL) were treated with various concentrations of the MDM2 inhibitor nutlin-3a38 (Roche Pharmaceuticals), XIAP inhibitor 1396-1139 (Torrey Pines Institute for Molecular Studies), SMAC mimetic ABT-10,22 nutlin-3a+1396-11, or nutlin-3a+ABT-10 for 24 or 48 hours. Ninety-six–well plates (200 μL/well) were used for experiments requiring only cell viability and cell cycle determinations and 12-well plates (2-5 mL/well), for experiments also requiring Western blot and reverse-transcription–polymerase chain reaction (RT-PCR) analysis. An appropriate amount of dimethyl sulfoxide (≤ 0.1%) was used as control. In vitro–treated patient samples were cultured in suspension or cocultured in the same medium with MS-5 stromal cells. To block caspase activation, 20μM IDN-1965, a pan-caspase inhibitor5 (kindly provided by IDUN Pharmaceuticals), was added to cells 1 hour before the drug treatments.
To inhibit the levels of XIAP and MDM2 by ASO, exponentially growing OCI-AML3 cells (3 × 106) were electroporated as described previously6 with 9 μg of XIAP ASO, 8 μg of MDM2 ASO, or both, or their respective control oligonucleotides (all from ISIS Pharmaceuticals) using Nucleofector solution T and program X-001 according to the manufacturer's instructions (Amaxa Biosystems). For the combination of XIAP ASO and nutlin-3a, OCI-AML3 cells were electroporated with XIAP ASO (6 μg) for 24 hours and then treated with nutlin-3a for an additional 24 hours.
Cell viability
Cell viability was determined by trypan blue exclusion using a Vi-Cell XR Cell Counter (Beckman Coulter). Apoptosis was estimated by flow cytometry measurements of phosphatidylserine externalization40 with the Annexin-V-FLUOS staining kit (Roche Diagnostics) or annexin V–cyanin 5 (BD Biosciences) using a FACSCalibur or FACSArray Bioanalyzer (BD Biosciences). Membrane integrity was assessed simultaneously by propidium iodide (PI) or 7-amino-actinomycin D (7-AAD) exclusion in the annexin V–stained cells. For primary AML samples, apoptosis was assessed in CD34+ cells using a CD34-phycoerythrin antibody (8G12; BD Biosciences). Specific apoptosis (%) is defined as (% apoptosis in treated cells − % apoptosis in control cells)/% viable cells in control.
Cell cycle distribution
Cells were fixed with 70% ice-cold ethanol and stained with propidium iodide solution (12.5 μg/mL; containing 180 U/mL RNase, 0.1% Triton X-100, and 30 mg/mL polyethylene glycol in 4 mM citrate buffer, pH 7.8; Sigma Chemical Co). The DNA content was determined by FACSArray Bioanalyzer (BD Biosciences). The cell cycle distribution was analyzed by ModFit LT software (Verity Software House).
Real-time RT-PCR
RNA was extracted using Trizol solution (Invitrogen) and cDNA was generated with random hexamers and avian myeloblastosis virus reverse transcriptase (Roche Applied Science) at 42°C for 1 hour. Real-time PCR was performed in ABI 7900HT Fast RT-PCR system using Taqman Gene Expression Assays (Applied Biosystems). Reaction mixtures contained 1 μL of cDNA, 2 μL of probe/forward and reverse primers, and 2 × Taqman Fast Universal PCR Master Mix (Applied Biosystems) in a total volume of 20 μL. Probe/primers for caspase-6 (Hs00154250_ml) was purchased from Applied Biosystems. The reaction was initiated at 95°C for 10 minutes, followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. ABL1 RNA was used as an internal control. The abundance of each transcript relative to that of ABL1 was calculated using the 2−ΔCT method, where ΔCt is the mean Ct of the transcript of interest minus the mean Ct of the transcript for ABL1.
Western blot analysis
Western blot analysis to determine the levels of various proteins was performed as described elsewhere.6 Briefly, treated cells were washed with phosphate-buffered saline solution and subjected to lysis in 2 × protein lysis buffer (0.25M tris(hydroxymethyl)aminomethane-HCl, 2% sodium dodecyl sulfate, 4% β-mercaptoethanol, 10% glycerol, and 0.02% bromophenol blue). To analyze cytosolic releases of SMAC and cytochrome c from mitochondria, cells were suspended in an ice-cold buffer (25mM tris(hydroxymethyl)aminomethane and 5mM MgCl2, pH 7.4) and subjected to centrifugation for 5 minutes at 16 000g to isolate cytosolic lysates. The cytosolic fractions were mixed with 2 × protein lysis buffer. An equal amount of each cell lysate was loaded onto a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel (BioRad). After incubation with a second antibody, the membranes were reacted with enhanced chemiluminescence solution (Amersham Pharmacia Biotech). Signals were detected by a PhosphorImager (Storm 860 Version 4.0; Molecular Dynamics) and quantified by ImageJ (National Institutes of Health). β-Actin was included as a loading control.
Statistical analyses
All experiments were conducted at least 3 times, and results are expressed as mean (± SE), unless otherwise stated. The combination index (CI) was determined by the Chou-Talalay method41 and Calcusyn software and was expressed as the average of the CI values obtained at the 50%, 75%, and 90% effective doses. A CI of less than 1 was considered synergistic; CI of 1, additive; and CI of more than 1, antagonistic. P value (Student t test) less than .05 is defined as statistically significant.
Results
Concomitant inhibition of MDM2 and XIAP synergistically promotes apoptosis in p53 wild-type AML cells
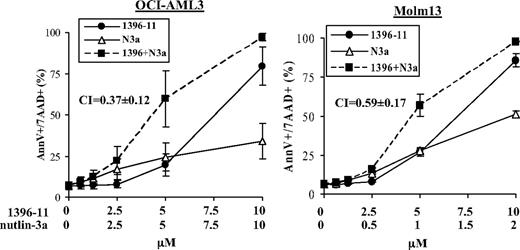
To assess the efficacy of simultaneous activation of p53 signaling and the caspase cascade on apoptosis in AML cells, we treated OCI-AML3 and Molm13 cells, 2 AML cell lines harboring wild-type (wt) p53, with nutlin-3a, an inhibitor of p53 antagonist MDM2; 1396-11, an antagonist of caspase inhibitor XIAP; or both. As demonstrated in Figure 1, the combination synergistically promoted apoptosis in both cells, with CIs of 0.37 (± 0.12) for OCI-AML3 cells and 0.59 (± 0.17) for Molm13 cells at 24 hours. To support this observation, OCI-AML3 and Molm13 cells were also treated with ABT-10, another XIAP antagonist, in combination with nutlin-3a. The results showed that this combination also synergistically promoted apoptosis in both cells, with CIs of 0.18 (± 0.12) for OCI-AML3 cells and 0.58 (± 0.11) for Molm13 cells at 24 hours (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Activation of p53 and inhibition of XIAP synergistically promote apoptosis in p53 wt acute myeloid leukemia (AML) cells. OCI-AML3 and Molm13 cells were treated with nutlin-3a, 1396-11, or both. Cell death was determined at 24 hours. N3a indicates nutlin-3a; 1396, 1396-11.
Activation of p53 and inhibition of XIAP synergistically promote apoptosis in p53 wt acute myeloid leukemia (AML) cells. OCI-AML3 and Molm13 cells were treated with nutlin-3a, 1396-11, or both. Cell death was determined at 24 hours. N3a indicates nutlin-3a; 1396, 1396-11.
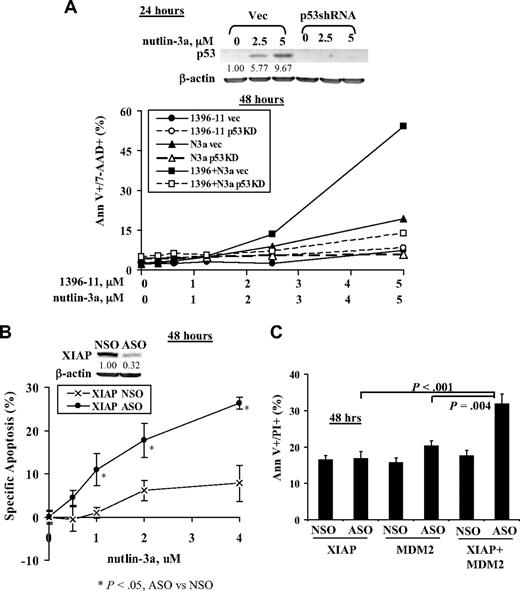
To demonstrate that the synergy is mediated via p53 activation, we treated p53 knockdown OCI-AML3 cells (OCI-AML3p53shRNA) and control cells (OCI-AML3vec) with nutlin-3a, 1396-11, or both. As shown in Figure 2A, both types of cells responded similarly to 1396-11; OCI-AML3vec cells were more sensitive, whereas OCI-AML3p53shRNA cells were resistant to nutlin-3a; the synergy of 1396-11 + nutlin-3a was significantly blunted in p53 knockdown OCI-AML3p53shRNA cells. To confirm that inhibition of XIAP contributes to the synergy, we first inhibited XIAP in OCI-AML3 cells by ASO for 24 hours and then treated with nutlin-3a for additional 24 hours: selective inhibition of XIAP by ASO sensitized OCI-AML-3 cells to nutlin-3a (Figure 2B). To further support the notion that synergy is mediated via p53 activation and XIAP inhibition, we transfected OCI-AML3 cells with MDM2 and XIAP ASOs. Results show that inhibition of both MDM2 and XIAP by their respective ASOs induced significantly more cell death than either ASO alone (Figure 2C).
The synergy of nutlin-3a and 1396-11 on apoptosis is mediated via p53 activation and XIAP inhibition. (A) Knockdown of p53 by shRNA blunts the synergy of nutlin-3a and 1396-11. OCI-AML3vec and OCI-AML3p53shRNA cells were treated with nutlin-3a, 1396-11, or both for 48 hours. p53 levels were determined at 24 hours by Western blot. N3a indicates nutlin-3a; Vec, OCI-AML3vec cells (solid lines); and p53KD, OCI-AML3p53shRNA cells (dashed lines). (B) Inhibition of XIAP by ASO sensitizes OCI-AML3 cells to nutlin-3a. OCI-AML3 cells were treated with XIAP ASO and control oligonucleotide (NSO; both 6 μg) by electroporation for 24 hours and then treated with nutlin-3a for an additional 24 hours. Inhibition of XIAP was confirmed by Western blot at 24 hours. (C) Inhibition of XIAP and MDM2 by their respective ASOs enhances apoptosis induction. OCI-AML3 cells were transfected with XIAP ASO (9 μg), MDM2 ASO (8 μg), or both, for 48 hours by electroporation. Apoptosis was assessed by annexin V (Ann V) staining in the presence of a vital dye.
The synergy of nutlin-3a and 1396-11 on apoptosis is mediated via p53 activation and XIAP inhibition. (A) Knockdown of p53 by shRNA blunts the synergy of nutlin-3a and 1396-11. OCI-AML3vec and OCI-AML3p53shRNA cells were treated with nutlin-3a, 1396-11, or both for 48 hours. p53 levels were determined at 24 hours by Western blot. N3a indicates nutlin-3a; Vec, OCI-AML3vec cells (solid lines); and p53KD, OCI-AML3p53shRNA cells (dashed lines). (B) Inhibition of XIAP by ASO sensitizes OCI-AML3 cells to nutlin-3a. OCI-AML3 cells were treated with XIAP ASO and control oligonucleotide (NSO; both 6 μg) by electroporation for 24 hours and then treated with nutlin-3a for an additional 24 hours. Inhibition of XIAP was confirmed by Western blot at 24 hours. (C) Inhibition of XIAP and MDM2 by their respective ASOs enhances apoptosis induction. OCI-AML3 cells were transfected with XIAP ASO (9 μg), MDM2 ASO (8 μg), or both, for 48 hours by electroporation. Apoptosis was assessed by annexin V (Ann V) staining in the presence of a vital dye.
XIAP inhibition potentiates p53-induced apoptosis by decreasing p53-induced p21
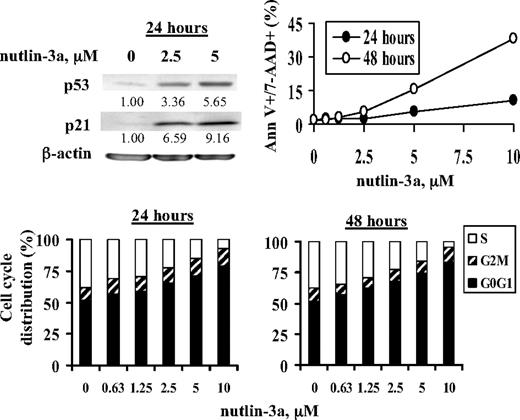
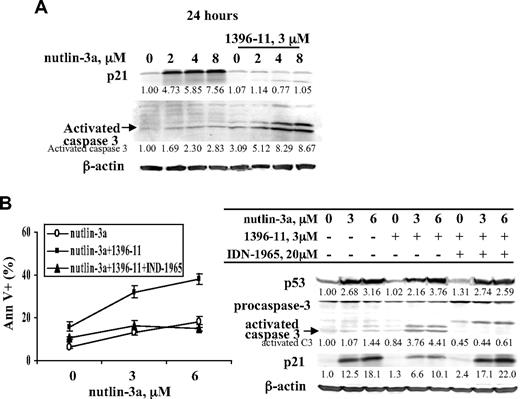
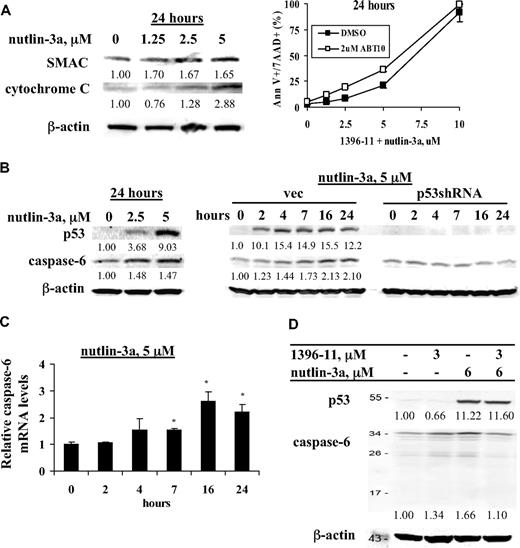
p21 is a transcriptional target of p53. As expected, increase in p53 levels by nutlin-3a greatly increased p21 protein, accompanied by G1 cell cycle block. Under this condition, only a limited number of annexin V+ cells was observed after treatment with 10μM nutlin-3a at 24 and 48 hours (Figure 3). Furthermore, nutlin-3a alone only minimally activated caspase-3 at 24 hours (Figure 4A). When nutlin-3a and 1396-11 were combined, in addition to the synergy determined by increased annexin V positivity as shown in Figure 1, significantly more caspase-3 was activated and p21 levels were greatly diminished (Figure 4A). To demonstrate that the decrease in p21 and the synergy in apoptosis are mediated by caspase activation via XIAP inhibition, these experiments were then performed in the presence of pan-caspase inhibitor IDN-1965. As shown in Figure 4B, caspase inhibition blocked both p21 decrease and apoptotic cell death.
Nutlin-3a activates p53 and its target p21 and induces G1 cell cycle block and low levels of apoptotic cells. OCI-AML3 cells were treated with nutlin-3a. p53 and p21 levels were determined by Western blot at 24 hours, and cell death and cell cycle distribution were assessed by annexin V/7-AAD and PI staining, respectively, at 24 and 48 hours.
Nutlin-3a activates p53 and its target p21 and induces G1 cell cycle block and low levels of apoptotic cells. OCI-AML3 cells were treated with nutlin-3a. p53 and p21 levels were determined by Western blot at 24 hours, and cell death and cell cycle distribution were assessed by annexin V/7-AAD and PI staining, respectively, at 24 and 48 hours.
XIAP inhibition–mediated caspase activation potentiates p53-induced apoptosis by decreasing p53-induced p21. OCI-AML3 cells were treated with various concentrations of nutlin-3a, 1396-11 (3μM), or both, with or without pan-caspase inhibitor IDN-1965 (20μM). p21 and p53 induction and caspase activation were determined by Western blot (A-B) and cell death by annexin V staining (B).
XIAP inhibition–mediated caspase activation potentiates p53-induced apoptosis by decreasing p53-induced p21. OCI-AML3 cells were treated with various concentrations of nutlin-3a, 1396-11 (3μM), or both, with or without pan-caspase inhibitor IDN-1965 (20μM). p21 and p53 induction and caspase activation were determined by Western blot (A-B) and cell death by annexin V staining (B).
Activation of p53 enhances XIAP inhibition-induced cell death by promoting mitochondrial release of SMAC and by inducing expression of caspase-6
p53 determines cell fate primarily by modulating the levels and activity of Bcl-2 family proteins, which control mitochondrial integrity. To investigate the role of p53 in the cytosolic release of SMAC, a XIAP antagonist from mitochondria, OCI-AML3 cells were treated with nutlin-3a and cytosolic SMAC protein levels were measured at 24 hours. As shown in Figure 5A, nutlin-3a induced release of SMAC from the mitochondria into the cytosol, along with cytochrome c. To support the notion that SMAC released from mitochondria plays a role in 1396-11 + nutlin-3a–induced cell death, we treated OCI-AML3 cells with 1396-11 + nutlin-3a in the presence and absence of SMAC mimetic ABT-10. As shown in Figure 5A, at 2 μM, ABT-10 itself had no effect on cell viability, but it further enhanced 1396-11 + nutlin-3a–induced cell death in OCI-AML3 cells.
Activation of p53 enhances XIAP inhibition-induced cell death by promoting mitochondrial release of SMAC and by inducing expression of caspase-6. (A) OCI-AML3 cells were treated for 24 hours with various concentrations of nutlin-3a and levels of cytosolic and mitochondrial SMAC were determined by Western blot. Cells were also treated with various concentrations of 1396-11 + nutlin-3a with or without 2μM ABT-10 and cell death was assessed. (B) OCI-AML3 cells were treated with nutlin-3a for 24 hours and OCI-AML3vec and OCI-AML3p53shRNA cells were treated with 5μM nutlin-3a for various times. p53 and caspase-6 protein levels were determined by Western blot. (C) OCI-AML3 cells were treated with 5μM nutlin-3a for different time periods, as indicated. Caspase-6 mRNA levels were determined by real-time RT-PCR. (D) OCI-AML3 cells were treated with 1396-11, nutlin-3a, or both for 24 hours. p53 and caspase-6 protein levels were determined by Western blot.
Activation of p53 enhances XIAP inhibition-induced cell death by promoting mitochondrial release of SMAC and by inducing expression of caspase-6. (A) OCI-AML3 cells were treated for 24 hours with various concentrations of nutlin-3a and levels of cytosolic and mitochondrial SMAC were determined by Western blot. Cells were also treated with various concentrations of 1396-11 + nutlin-3a with or without 2μM ABT-10 and cell death was assessed. (B) OCI-AML3 cells were treated with nutlin-3a for 24 hours and OCI-AML3vec and OCI-AML3p53shRNA cells were treated with 5μM nutlin-3a for various times. p53 and caspase-6 protein levels were determined by Western blot. (C) OCI-AML3 cells were treated with 5μM nutlin-3a for different time periods, as indicated. Caspase-6 mRNA levels were determined by real-time RT-PCR. (D) OCI-AML3 cells were treated with 1396-11, nutlin-3a, or both for 24 hours. p53 and caspase-6 protein levels were determined by Western blot.
Next we examined the levels of caspase-6 protein in response to nutlin-3a and found an increase of caspase-6 protein. This finding was further proven by the time-dependent induction of caspase-6 protein in OCI-AML3 vector control cells but not in p53 knockdown OCI-AML3p53shRNA cells (Figure 5B). To demonstrate that the increase in caspase-6 expression by nutlin-3a is related to enhanced gene expression, we treated OCI-AML3 cells with nutlin-3a and found by real-time RT-PCR that nutlin-3a indeed increased the level of caspase-6 mRNA in a time-dependent manner (Figure 5C). To further establish a role for caspase-6 in the observed synergism, we investigated whether caspase-6 was cleaved in response to the combined treatment. We found that the nutlin-3a–induced increase in caspase-6 was diminished when 1396-11 and nutlin-3a were combined, implying its degradation (Figure 5D). We did not detect the cleaved band of caspase-6, probably due to limitations in Western blot sensitivity. We observed no consistent increase of caspase-7 protein levels in nutlin-3a–treated OCI-AML3 cells (results not shown).
p53 activation and XIAP inhibition strongly enhanced apoptosis in blasts from primary AML patients
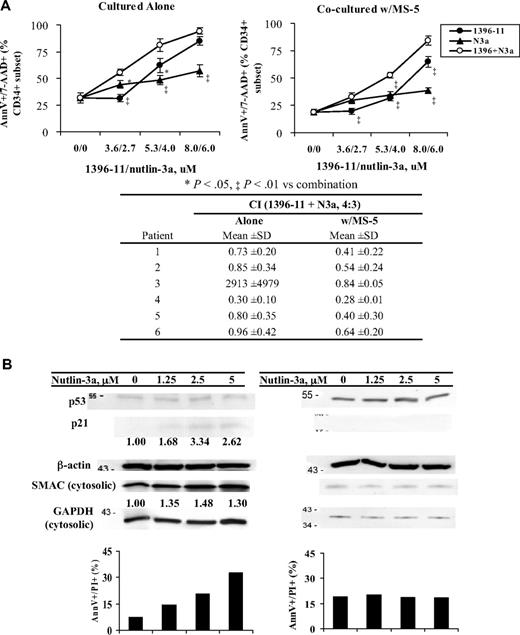
To test the potential effectiveness of the combination strategy in AML patients, blasts from 6 AML patients were treated with 1396-11, nutlin-3a, or both. Clinical characteristics of the patients are shown in Table 1. Cells from all 6 patients showed expression of XIAP and low or undetectable basal levels of p53 (not shown). Figure 6 shows that cells from these patients responded variably to 1396-11 or nutlin-3a alone, but when the 2 agents were combined, synergy was observed in all but 1 sample (patient 3 in Figure 6A and Table 1). Similar results were obtained when blasts were treated with ABT-10, nutlin-3a, or both (supplemental Figure 2). We and others have established that stromal cells protect leukemic cells from spontaneous apoptosis and from apoptosis induced by various therapeutic agents.35-37,42-44 We show here that the combination of nutlin-3a and 1396-11 or nutlin-3a and ABT-10 also synergistically promoted apoptosis in all 6 samples cocultured with MS-5 (Figure 6A and supplemental Figure 2).
Simultaneous inhibition of XIAP and MDM2 enhances cell death in samples from AML patients and cell death is enhanced by the combination even when they are protected by MSCs. (A) Mononucleocytes from blasts of AML patients were treated with 1396-11, nutlin-3a, or both for 24 hours, in suspension or cocultured with MS-5. Cell death was determined in the CD34+ population by annexin V/7-AAD staining. The CI of the combination for each patient is shown under the graph. 1396 indicates 1396-11; N3a, nutlin-3a. (B) Mononuclear cells from blasts of AML patients were treated with nutlin-3a for 24 hours. Cell death was determined by annexin V/PI staining and proteins by Western blot.
Simultaneous inhibition of XIAP and MDM2 enhances cell death in samples from AML patients and cell death is enhanced by the combination even when they are protected by MSCs. (A) Mononucleocytes from blasts of AML patients were treated with 1396-11, nutlin-3a, or both for 24 hours, in suspension or cocultured with MS-5. Cell death was determined in the CD34+ population by annexin V/7-AAD staining. The CI of the combination for each patient is shown under the graph. 1396 indicates 1396-11; N3a, nutlin-3a. (B) Mononuclear cells from blasts of AML patients were treated with nutlin-3a for 24 hours. Cell death was determined by annexin V/PI staining and proteins by Western blot.
To demonstrate that our finding of increased lethality in cell lines also applies to primary blasts, we treated AML samples with nutlin-3a. As shown in Figure 6B, the sample on the left (bone marrow, 84% blasts) was sensitive to nutlin-3a–induced apoptosis. Although induction of p53 was undetectable, p21 was induced suggesting that these cells had wt-p53. Nutlin-3a treatment indeed increased cytosolic SMAC levels. No increase in caspase-6 was detected (result not shown). The sample on the right (bone marrow, 70% blasts) was resistant to nutlin-3a–induced cell death. Neither induction of p21 nor increase of cytosolic SMAC (Figure 6B) or caspase-6 was found (results not shown), suggesting that p53 was mutated in cells from this patient. Unfortunately, sequence data could not be obtained from these samples.
Discussion
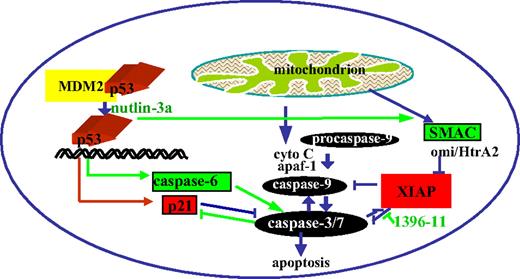
Combination chemotherapies for cancers and leukemias have long been designed to overcome problems of drug-related toxicity and resistance. We have demonstrated, using novel small molecule inhibitors and targeted ASO approaches, that concomitant inhibition of the p53 antagonist MDM2 and the caspase inhibitor XIAP synergistically promotes cell death in AML cell lines with wt p53 and in samples from patients with AML, even when they are protected by stromal cells. The synergy is mediated in part by the following mechanisms (as shown in Figure 7): caspase activation by XIAP inhibition eliminates antiapoptotic p21, which is induced by p53 activation, and p53 activation by MDM2 inhibition induces increases in caspase-6 protein levels and promotes the release of SMAC from the mitochondria into the cytosol, both of which promote caspase-dependent apoptosis.
p53 activation and XIAP inhibition synergistically promote cell death.
p21 has been shown not only to promote cell cycle block but also to inhibit apoptotic cell death. This is evident in our finding that nutlin-3a strongly induces p21 level and G1 cell cycle block but minimally activates caspase-3 and cell death in OCI-AML3 cells. The accumulated p21, along with XIAP, likely binds and inhibits caspase activation and prevents ultimate cell death even though the upstream mitochondrial pathway is activated by p53. In the presence of 1396-11, XIAP is antagonized, leading to caspase activation and p21 cleavage. Attenuation of apoptosis by p53-induced p21 in AML cells is further supported by data showing that wt p53 codon72-Arg induces apoptosis more efficiently than wt codon72-Pro, in part because it does not increase transcription of p21.45 Therefore, eliminating p53-induced p21 by activated caspases via XIAP inhibition, along with induction of caspase-6 and cytosolic release of SMAC by p53, clearly maximizes p53 effect and the caspase cascade and augments the shift of leukemic cells from survival toward death.
A recent study reported that cisplatin-induced p53 transcriptionally activates caspase-6 and caspase-7.29 We demonstrated that nutlin-3a–induced p53 increases caspase-6 mRNA and protein levels in a p53-dependent manner. However, no consistent increase in caspase-7 protein levels was observed. This could be due to either the fact that, when activated by different stimuli, p53 may have different transcriptional specificity or that, upon antagonism by 1396-11, caspase-7 is released from XIAP, resulting in its cleavage and activation and thus obscuring its increase. XIAP is known to inhibit caspase-7, but not caspase-6.
It is important to point out that p21, caspase-6, and SMAC are unlikely to be the only factors in the synergistic cell killing efforts observed in this study. p53 activates the transcription and activity of multiple components participating in the regulation of DNA repair, apoptosis, cell cycle, and senescence. XIAP inhibits apoptosis not only by directly inhibiting caspases but also by affecting cell signalings, such as nuclear factor κB, which is hyperactive in many human cancers. Cross-talk between p53 and nuclear factor κB regulatory networks has been identified,46,47 and simultaneous targeting of these 2 pathways as cancer therapies has been proposed.48
The importance of the microenvironment in the maintenance and differentiation of hematopoietic progenitors has been recognized only recently.49-53 It has become evident that MSCs interact with leukemic cells in vivo and provide a protective microenvironment that enables leukemic cells to proliferate and survive. This is supported by the fact that many drugs that potently kill leukemic cells in vitro are not effective in vivo and that leukemic cells resistant to apoptosis in vivo show high levels of spontaneous apoptosis in vitro. To partially mimic in vivo conditions, in vitro studies have been carried out coculturing leukemic cells with MSCs.54 We here show that nutlin-3a or 1396-11 alone induces less cell death when cells were cocultured with MS-5 cells and that the combined inhibition of MDM2 and XIAP synergistically promotes cell death in blasts from 5 of 6 patients with primary AML. Most importantly, the synergy was not abrogated by MSC coculture, suggesting that the combined targeting is also effective in overcoming the protective effects of the bone marrow microenvironment.
Combined treatment with 1396-11 + nutlin-3a strongly inhibited the clonogenic activity of both normal and AML bone marrow cells (results not shown). Although it is preferable for therapeutic agents to selectively eliminate or reduce the colony formation of leukemic blasts, this is not a critical determinant of the potential clinical applicability of a drug. In fact, ara-C, the most commonly used chemotherapeutic agent for the treatment of AML, shows little or no selectivity for malignant cells over normal cells in colony-forming assays.55 The safety and potential hematologic toxicity of the combination of p53 activation and XIAP inhibition as AML therapy will have to be carefully evaluated in preclinical in vivo and in phase 1 clinical trials.
Effective activation of apoptotic pathways will not only overcome chemoresistance, but also minimize chemotoxicity, 2 major obstacles in current therapy against AML. By simultaneous inhibition of XIAP and activation of p53, with associated maximal activation of apoptotic signaling, we hope to develop a novel therapeutic strategy that can overcome chemoresistance, the major obstacle for achievement of durable remissions in patients with AML. Both XIAP and p53 are critical targets in AML. A phase 1/2 trial of XIAP ASO in combination with chemotherapy in patients with relapsed/refractory AML has shown promising results in a subset of patients,14 and a phase 1 trial with nutlin-3a in AML is ongoing. Therefore, the combination strategy tested in this study holds promise for rapid translation into the clinic.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Wenjing Chen for collecting patient information and Bradley S. Tadlock and Kathryn Hale for helping with the paper preparation.
This work was supported in part by grants from the National Institutes of Health (P01 CA49639, P01 CA55164, and CA16672 to M.A.; and CA113318 to J.C.R.).
National Institutes of Health
Authorship
Contribution: B.Z.C. designed and performed the experiments and wrote the paper; D.H.M. performed the experiments and analyzed data; W.D.S. performed experiments; E.K., C.P., L.T.V., and J.C.R. provided materials and scientific input; and M.A. provided infrastructure and support for the work and edited the paper.
Conflict-of-interest disclosure: E.K. works for Isis. L.T.V. works for Roche. J.C.R. is a consultant for and shareholder of Apoptos Inc. The remaining authors declare no competing financial interests.
Correspondence: Michael Andreeff, Section of Molecular Hematology and Therapy, Department of Stem Cell Transplantation and Cellular Therapy, Unit 448, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: mandreef@mdanderson.org.