Abstract
We reviewed the outcome of 671 patients 65 years of age or older with newly diagnosed acute myeloid leukemia (AML) treated at our institution between 2000 and 2010 with intensive chemotherapy (n = 557) or azacitidine- or decitabine-based therapy (n = 114). Both groups were balanced according to cytogenetics and performance status. The complete response rates with chemotherapy and epigenetic therapy were 42% and 28%, respectively (P = .001), and the 8-week mortality 18% and 11%, respectively (P = .075). Two-year relapse-free survival rates (28% vs 39%, P = .843) and median survival (6.7 vs 6.5 months, P = .413) were similar in the 2 groups. Multivariate analysis identified older age, adverse cytogenetics, poor performance status, elevated creatinine, peripheral blood and BM blasts, and hemoglobin, but not type of AML therapy, as independent prognostic factors for survival. No outcome differences were observed according to cytogenetics, FLT3 mutational status, age, or performance status by therapy type. Decitabine was associated with improved median overall survival compared with azacitidine (5.5 vs 8.8 months, respectively, P = .03). Survival after failure of intensive chemotherapy, azacitidine, or decitabine was more favorable in patients who had previously received decitabine (1.1 vs 0.9 vs 3.1 months, respectively, P = .109). The results of the present study show that epigenetic therapy is associated with similar survival rates as intensive chemotherapy in older patients with newly diagnosed AML. The studies reviewed are registered at www.clinicaltrials.gov as 2009-0172 (NCT00926731) and 2009-0217 (NCT00952588).
Introduction
The prognosis for several subsets of patients with acute myeloid leukemia (AML) is poor.1-3 Age, performance status, and karyotype remain powerful prognostic factors for survival in AML.1-8 Older patients with AML have a very poor prognosis with intensive chemotherapy, despite 40%-60% achieving a complete remission (CR). Older age is arbitrarily defined in different studies using different age cutoffs ranging from 60-70 years or more.1,9,10 The poor prognosis of older patients with AML is attributed to different factors, including comorbid conditions, a higher incidence of secondary AML or evolution from myelodysplastic syndrome (MDS), poor performance status, poor tolerance to chemotherapy, and a higher incidence of adverse karyotypes.9,10 These factors are associated with higher rates of early (4- to 8-week) mortality with intensive chemotherapy and with higher rates of resistance and relapse, resulting in poor long-term survival. The CR rates among older patients with AML treated with a standard combination of cytarabine and an anthracycline (eg, the 7 + 3 regimen) are 35%-60%, but the induction chemotherapy-related mortality can be high depending on several factors (4- to 8-week mortality rates of 20%-50%). The median survival of older patients with AML ranges from 4-7 months in different studies.5,8,11,12
Epigenetic therapy in cancer is a relatively recent concept that has produced positive results in some hematologic malignancies. Epigenetic therapy with azacitidine and decitabine is now standard of care in patients with MDS requiring therapy. Randomized studies and historical comparisons have shown that epigenetic therapy may result in significantly longer survival rates compared with intensive chemotherapy despite their association with lower CR rates. This suggested that epigenetic therapy in MDS may prolong survival through mechanisms independent of the achievement of CR.13,14 Epigenetic therapy with decitabine and azacitidine has also been investigated in older patients with AML who are judged not to be fit to receive intensive chemotherapy (based on an estimation of a high early mortality rate with the latter treatment). These studies have shown that epigenetic therapy results in lower CR and overall response rates (ORRs) than intensive chemotherapy (CR rates of 10%-30%; ORRs of 30%-50%), but were associated with reasonable median survival times. In a subset analysis of 113 patients who had a label of MDS but a BM blast percentage of 21%-30% (currently best classified as AML according to the new classification) treated in the randomized MDS trial comparing azacitidine with best standard of care, azacitidine therapy was associated with a median survival of 24.5 months versus 16 months with conventional care.15 These results suggested that epigenetic therapy might be a reasonable treatment approach in older patients with AML. However, this study may have excluded patients with worse prognosis AML (ie, secondary AML and proliferative AML) given that the control arm resulted in a median survival of 16 months, when historically the expected median survival of older patients with AML is less than 12 months. Therefore, an important question is whether epigenetic therapy would produce similar results compared with intensive chemotherapy when administered to an unselected cohort of older patients with AML. We present herein an analysis aimed at answering such question. Although many AML experts consider intensive chemotherapy to be well-tolerated and beneficial for patients younger than 65 years of age, fewer would consider intensive chemotherapy a safe and effective strategy in AML patients who are 65 years or older. Therefore, we restricted our analysis to patients with AML who were 65 years of age or older at the time of diagnosis. The studies reviewed are registered at www.clinicaltrials.gov as 2009-0172 (NCT00926731) and 2009-0217 (NCT00952588).
Methods
Patient cohorts
A total of 671 patients 65 years of age or older who were treated for newly diagnosed AML at our institution from 2000-2010 were reviewed. All patients received therapy in clinical trials conducted at our institution after signing an institutional review board–approved consent form to participate in such studies. The study was conducted in compliance with institutional guidelines and with the Declaration of Helsinki. The 2 groups of patients were those who had received intensive chemotherapy and those who received epigenetic therapy. The baseline characteristics of the study groups are shown in Table 1. Significant differences in the baseline characteristics of patients treated with intensive chemotherapy versus those treated with epigenetic therapy were: median age (71 vs 74 years; P = .033), median hemoglobin level (8.3 vs 8.7 g/dL; P = .015), median BM blasts (43% vs 36%; P = .002), and peripheral blood blasts (15% vs 7%; P = .004). However, no differences were observed regarding cytogenetic grouping (P = .738), including the proportion of patients with poor karyotypes (34% vs 35%, P = .936) or regarding performance status 0-2 (527 [95%] vs 108 [95%], P = .96).
Baseline characteristics of elderly patients with AML treated with chemotherapy or epigenetic therapy
| Characteristic . | Chemotherapy . | Azacitidine . | Decitabine . | 3-way P value . | Epigenetic . | Chemotherapy vs epigenetic P value . |
|---|---|---|---|---|---|---|
| Patients, n (%) | 557 (83) | 47 (7) | 67 (10) | 114 (17) | ||
| Male sex, n (%) | 358 (64) | 34 (72) | 44 (66) | .53 | 78 (68) | .4 |
| Median age, y (range) | 71 (65-89) | 74 (65-84) | 73 (65-86) | .1 | 74 (65-86) | .033 |
| ECOG performance status 0-2, n (%) | 527 (95) | 41 (87) | 67 (100) | .012‡ | 108 (95) | .9 |
| Median WBC count, × 109/L (range) | 5.8 (0.4-433) | 8.1 (0.7-79.1) | 2.8 (1.0-56.4) | .01 | 4.6 (0.7-79.1) | .12 |
| Median hemoglobin, g/dL (range) | 8.3 (3.8-14.2) | 8.4 (4.5-11.9) | 8.8 (4.0-14.6) | .01 | 8.7 (4-14.6) | .01 |
| Platelets, × 109/L | 51 (3-787) | 49 (2-437) | 58 (3-470) | .37 | 55 (2-470) | .23 |
| Median BM, % (range) | 43 (0-98) | 42 (1-85) | 32 (6-87) | .001 | 36 (1-87) | .002 |
| Median PB blasts, % (range) | 15 (0-99) | 5 (0-89) | 7 (0-69) | .001 | 7 (0-89) | .003 |
| Cytogenetics, n (%) | ||||||
| −5 or −7 alone | 15 (3) | 2 (4) | 0 (0) | .85* | 2 (2) | .74 |
| −5 or −7 with other abnormalities | 127 (23) | 10 (21) | 19 (28) | 29 (25) | ||
| Complex (≥ 3 abnormalities) | 42 (8) | 3 (6) | 4 (6) | 7 (6) | ||
| Noncomplex (≤ 2 abnormalities) | 98 (18) | 11 (23) | 13 (19) | 24 (21) | ||
| 11q23 | 14 (3) | 1 (2) | 0 (0) | 1 (1) | ||
| Diploid | 238 (43) | 19 (40) | 27 (40) | 46 (40) | ||
| Not done/insufficient metaphases* | 23 (4) | 1 (2) | 4 (6) | 5 (4) | ||
| FLT3-ITD, n (%) | 47 (8) | 4 (9) | 7 (10) | .85 | 11 (10) | .67 |
| NPM1 mutation, n (%)† | 23 (16) | 3 (13) | 0 (0) | .32 | 3 (9) | .26 |
| Characteristic . | Chemotherapy . | Azacitidine . | Decitabine . | 3-way P value . | Epigenetic . | Chemotherapy vs epigenetic P value . |
|---|---|---|---|---|---|---|
| Patients, n (%) | 557 (83) | 47 (7) | 67 (10) | 114 (17) | ||
| Male sex, n (%) | 358 (64) | 34 (72) | 44 (66) | .53 | 78 (68) | .4 |
| Median age, y (range) | 71 (65-89) | 74 (65-84) | 73 (65-86) | .1 | 74 (65-86) | .033 |
| ECOG performance status 0-2, n (%) | 527 (95) | 41 (87) | 67 (100) | .012‡ | 108 (95) | .9 |
| Median WBC count, × 109/L (range) | 5.8 (0.4-433) | 8.1 (0.7-79.1) | 2.8 (1.0-56.4) | .01 | 4.6 (0.7-79.1) | .12 |
| Median hemoglobin, g/dL (range) | 8.3 (3.8-14.2) | 8.4 (4.5-11.9) | 8.8 (4.0-14.6) | .01 | 8.7 (4-14.6) | .01 |
| Platelets, × 109/L | 51 (3-787) | 49 (2-437) | 58 (3-470) | .37 | 55 (2-470) | .23 |
| Median BM, % (range) | 43 (0-98) | 42 (1-85) | 32 (6-87) | .001 | 36 (1-87) | .002 |
| Median PB blasts, % (range) | 15 (0-99) | 5 (0-89) | 7 (0-69) | .001 | 7 (0-89) | .003 |
| Cytogenetics, n (%) | ||||||
| −5 or −7 alone | 15 (3) | 2 (4) | 0 (0) | .85* | 2 (2) | .74 |
| −5 or −7 with other abnormalities | 127 (23) | 10 (21) | 19 (28) | 29 (25) | ||
| Complex (≥ 3 abnormalities) | 42 (8) | 3 (6) | 4 (6) | 7 (6) | ||
| Noncomplex (≤ 2 abnormalities) | 98 (18) | 11 (23) | 13 (19) | 24 (21) | ||
| 11q23 | 14 (3) | 1 (2) | 0 (0) | 1 (1) | ||
| Diploid | 238 (43) | 19 (40) | 27 (40) | 46 (40) | ||
| Not done/insufficient metaphases* | 23 (4) | 1 (2) | 4 (6) | 5 (4) | ||
| FLT3-ITD, n (%) | 47 (8) | 4 (9) | 7 (10) | .85 | 11 (10) | .67 |
| NPM1 mutation, n (%)† | 23 (16) | 3 (13) | 0 (0) | .32 | 3 (9) | .26 |
PB indicates peripheral blood.
Not included in the calculation of the P value.
NPM1 mutational status was available in 181 patients.
By the Fisher exact test.
Treatment regimens
Of the 557 patients receiving intensive chemotherapy, 177 (32%) received idarubicin and high-dose cytarabine (cytarabine 1.5 g/m2 by continuous infusion over 24 hours daily for 3 days; idarubicin 12 mg/m2 by IV short infusion daily for 3 days).16 In addition, 83 (17%) patients received other high-dose cytarabine-based induction chemotherapy regimens (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).17-20 Among the 114 patients receiving epigenetic therapy, the agents most frequently used were azacitidine (n = 47) and decitabine (n = 67), either alone or in combination with histone deacetylase inhibitors.20-25 Patients were assigned to each study as they became available. Elderly patients with AML were preferentially offered epigenetic-based therapy since November 2003.
Statistical considerations
Differences in the study groups regarding a variety of characteristics were evaluated using the χ2 test or the Fisher exact test for discrete variables, and the Mann-Whitney U test for continuous variables. The survival of patients was analyzed using the Kaplan-Meier method and differences were compared using the log-rank test. To determine the relative impact of each variable on survival, a Cox proportional hazard model was constructed entering covariates with P ≤ .10 in the univariate analysis. Because the focus of our study was to determine whether the treatment regimen received (intense chemotherapy vs epigenetic therapy) was associated with survival, we included this variable a priori in the model. Because performance status is well established to affect survival, it was also entered a priori in the multivariate model. P < .05 was considered statistically significant. IBM PASW Statistics Version 19 software for Windows (SPSS) was used for statistical analysis.
Results
Outcomes by therapeutic modality
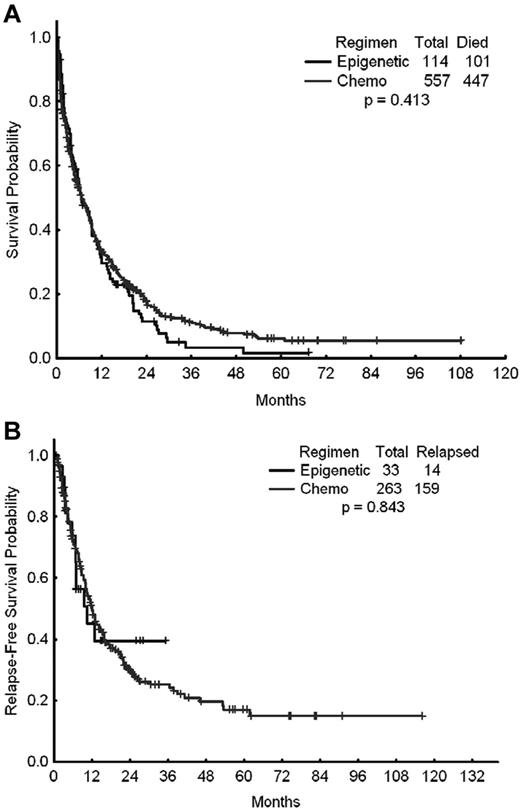
A total of 671 patients were included in this analysis, of whom 557 (83%) received intensive chemotherapy and 114 (17%) received epigenetic therapy with a hypomethylating agent (azacitidine or decitabine) either alone or in combination with a histone deacetylase inhibitor as their initial induction therapy for AML. The outcomes of these 2 cohorts of patients are shown in Table 2. The CR rates for patients treated with chemotherapy and epigenetic therapy were 42% and 28%, respectively (P = .001). The ORR (ORR = CR + CR with incomplete platelet recovery) were 47% and 29%, respectively (P < .001). The early mortality rates (mortality within the first 8 weeks of therapy) were 18% with intensive chemotherapy and 11% with hypomethylating agents (P = .075). Despite the higher CR and ORRs achieved with intensive chemotherapy, the latter did not translate into improved long-term benefit compared with hypomethylating therapy. The 2-year relapse-free survival rates with intensive chemotherapy and epigenetic therapy were 30% and 40%, respectively (P = .843). The median survival times were 6.7 and 6.5 months (P = .413), respectively (Figure 1A-B).
Outcomes after standard chemotherapy or epigenetic therapy
| . | Chemotherapy . | Epigenetic therapy . | P . |
|---|---|---|---|
| Total patients, n (%) | 557 (83%) | 114 (17%) | |
| Outcome, n (%) | |||
| ORR | 263 (47%) | 33 (29%) | < .001 |
| CR | 235 (42%) | 32 (28%) | .005 |
| CRp | 28 (5%) | 1 (1%) | NR |
| No response | 189 (34%) | 63 (55%) | NR |
| ED < 8 wk | 102 (18%) | 13 (11%) | .075 |
| Median OS, mo | 6.7 | 6.5 | .413 |
| 1-y | 34% | 31% | |
| 2-y | 18% | 11% | |
| Median RFS, mo | 12.0 | 10.5 | .843 |
| 1-y | 50% | 45% | |
| 2-y | 30% | 40% | |
| . | Chemotherapy . | Epigenetic therapy . | P . |
|---|---|---|---|
| Total patients, n (%) | 557 (83%) | 114 (17%) | |
| Outcome, n (%) | |||
| ORR | 263 (47%) | 33 (29%) | < .001 |
| CR | 235 (42%) | 32 (28%) | .005 |
| CRp | 28 (5%) | 1 (1%) | NR |
| No response | 189 (34%) | 63 (55%) | NR |
| ED < 8 wk | 102 (18%) | 13 (11%) | .075 |
| Median OS, mo | 6.7 | 6.5 | .413 |
| 1-y | 34% | 31% | |
| 2-y | 18% | 11% | |
| Median RFS, mo | 12.0 | 10.5 | .843 |
| 1-y | 50% | 45% | |
| 2-y | 30% | 40% | |
CRp indicates CR with incomplete platelet recovery; ED, early death; RFS, relapse-free survival; and NR, not reported.
Survival times of patients treated with standard intensive AML chemotherapy. Shown are the OS (A) and relapse-free survival (B) of patients treated with standard intensive AML chemotherapy (Chemo) or with epigenetic therapy (Epigenetic).
Survival times of patients treated with standard intensive AML chemotherapy. Shown are the OS (A) and relapse-free survival (B) of patients treated with standard intensive AML chemotherapy (Chemo) or with epigenetic therapy (Epigenetic).
Impact of cytogenetics and FLT3/NPM1 mutational status
Because one of the major determinants of long-term prognosis in AML is the presence of specific cytogenetic alterations, we evaluated the effect of intensive chemotherapy versus epigenetic therapy in older patients with AML by the presence or absence of karyotypic abnormalities (Table 3). Among the 225 patients (187 treated with chemotherapy and 38 with epigenetic therapy) with poor-risk cytogenetics (eg, patients carrying −5 and/or −7, both as single abnormalities or in combination with other cytogenetic abnormalities, and those with complex karyotype), the CR rate was 28% with intensive chemotherapy and 26% with epigenetic therapy (P = .849); the median overall survival (OS) was 3.2 months with intensive chemotherapy and 4.2 months with epigenetic therapy (P = .315). Among patients with intermediate-risk cytogenetics (n = 421, 350 treated with chemotherapy and 71 treated with epigenetic therapy), the CR rate was 50% with intensive chemotherapy and 31% with epigenetic therapy (P < .001). Again, this higher response rate among patients receiving intensive chemotherapy did not translate into a survival advantage, because the median OS was 9.5 months for patients treated with intensive chemotherapy and 8.8 months for those receiving epigenetic therapy (P = .134). Among patients carrying the FLT3-ITD mutation, which has been extensively associated with poor prognosis in AML, the CR rates were 51% with intensive chemotherapy and 45% with hypomethylating agents (P = .741); the corresponding median survival times were 5.8 and 9.5 months, respectively (P = .406). Conversely, of patients carrying NPM1-mutated alleles who were treated with intensive chemotherapy or hypomethylating agents, 70% and 0% achieved CR, respectively (P = .02), and the median OS was 14.1 and 3.7 months (P = .16), respectively. Therefore, although the number of patients with NPM1-mutated alleles was very small, the use of standard chemotherapy appears to render superior results compared with hypomethylating therapy.
Outcomes after standard chemotherapy or epigenetic therapy in patients with poor- and intermediate-risk cytogenetics
| . | Chemotherapy . | Epigenetic . | P . |
|---|---|---|---|
| Poor-risk CG patients, n (%) | 187 (83%) | 38 (17%) | |
| Outcome | |||
| ORR | 61 (33%) | 10 (26%) | .447 |
| CR | 52 (28%) | 10 (26%) | .849 |
| CRp | 9 (5%) | 0 (0%) | NR |
| No response | 78 (42%) | 19 (0%) | NR |
| ED < 8 wk | 46 (25%) | 6 (16%) | .242 |
| Median OS, mo | 3.2 | 4.2 | .315 |
| 1-y | 17% | 26% | |
| 2-y | 7% | 11% | |
| Median RFS, mo | 9.3 | 10.5 | .104 |
| 1-y | 42% | 46% | |
| 2-y | 8% | 46% | |
| Intermediate-risk CG patients, n (%) | 350 (81%) | 71 (19%) | |
| Outcome | |||
| ORR | 194 (55%) | 23 (32%) | .0006 |
| CR | 176 (50%) | 22 (31%) | < .001 |
| CRp | 18 (5%) | 1 (1%) | NR |
| No response | 105 (30%) | 41 (58%) | NR |
| ED < 8 wk | 50 (14%) | 6 (8%) | .187 |
| Median OS, mo | 9.5 | 8.8 | .134 |
| 1-y | 43% | 34% | |
| 2-y | 24% | 12% | |
| Median RFS, mo | 13 | 9.5 | .673 |
| 1-y | 53% | 46% | |
| 2-y | 35% | 37% | |
| . | Chemotherapy . | Epigenetic . | P . |
|---|---|---|---|
| Poor-risk CG patients, n (%) | 187 (83%) | 38 (17%) | |
| Outcome | |||
| ORR | 61 (33%) | 10 (26%) | .447 |
| CR | 52 (28%) | 10 (26%) | .849 |
| CRp | 9 (5%) | 0 (0%) | NR |
| No response | 78 (42%) | 19 (0%) | NR |
| ED < 8 wk | 46 (25%) | 6 (16%) | .242 |
| Median OS, mo | 3.2 | 4.2 | .315 |
| 1-y | 17% | 26% | |
| 2-y | 7% | 11% | |
| Median RFS, mo | 9.3 | 10.5 | .104 |
| 1-y | 42% | 46% | |
| 2-y | 8% | 46% | |
| Intermediate-risk CG patients, n (%) | 350 (81%) | 71 (19%) | |
| Outcome | |||
| ORR | 194 (55%) | 23 (32%) | .0006 |
| CR | 176 (50%) | 22 (31%) | < .001 |
| CRp | 18 (5%) | 1 (1%) | NR |
| No response | 105 (30%) | 41 (58%) | NR |
| ED < 8 wk | 50 (14%) | 6 (8%) | .187 |
| Median OS, mo | 9.5 | 8.8 | .134 |
| 1-y | 43% | 34% | |
| 2-y | 24% | 12% | |
| Median RFS, mo | 13 | 9.5 | .673 |
| 1-y | 53% | 46% | |
| 2-y | 35% | 37% | |
CRp indicates CR with incomplete platelet recovery; ED, early death; RFS, relapse-free survival; CG, cytogenetics; and NR, not reported.
Impact of age and performance status
Age and performance status are powerful prognostic factors in AML. We investigated potential differences in AML outcomes by therapeutic modality in patients 65 years of age or older and in those 70 years or older. The CR rates and median survival times in the former group treated with intensive chemotherapy or epigenetic therapy were 44% versus 28% (P = .002) and 6.7 versus 6.5 months (P = .413), respectively. The same analysis in patients over the age of 70 years showed also higher CR rates with intensive chemotherapy compared with epigenetic therapy (38% vs 28%, P = .097), but no survival advantage of one therapeutic approach over the other (median OS 6 vs 6 months, P = .285). Because the prognostic effect of age in AML is heavily modulated by the presence of comorbidities and the patient's overall performance status,1 we also investigated the impact of different treatment modalities by performance status. We divided patients into those with an Eastern Cooperative Oncology Group (ECOG) performance status of 0-1 (good) and those with an ECOG performance status 2-4 (worse). Patients with an ECOG performance status of 0-1 treated with intensive chemotherapy had higher CR rates than those receiving epigenetic therapy (45% vs 33%, respectively, P = .043). However, the median survival times were 8.8 versus 10.6 months (P = .937). Among patients with ECOG performance status of 2-4, those treated with intensive chemotherapy had a CR rate of 33% versus 17% with epigenetic therapy (P = .061) and the median survival times were 2.5 versus 3 months, respecively (P = .441). Renal insufficiency is a comorbidity that frequently affects the OS of patients with AML undergoing induction therapy. Similar median OS was observed among patients treated with chemotherapy versus those treated with epigenetic therapy when divided according to their baseline creatinine: 8.8 vs 9.5 months (P = .15) for patients with creatinine 1.5 mg/dL or lower and 2.8 vs 5.1 (P = .82) for those with a creatinine greater than 1.5 mg/dL, respectively.
Impact of specific therapeutic regimens
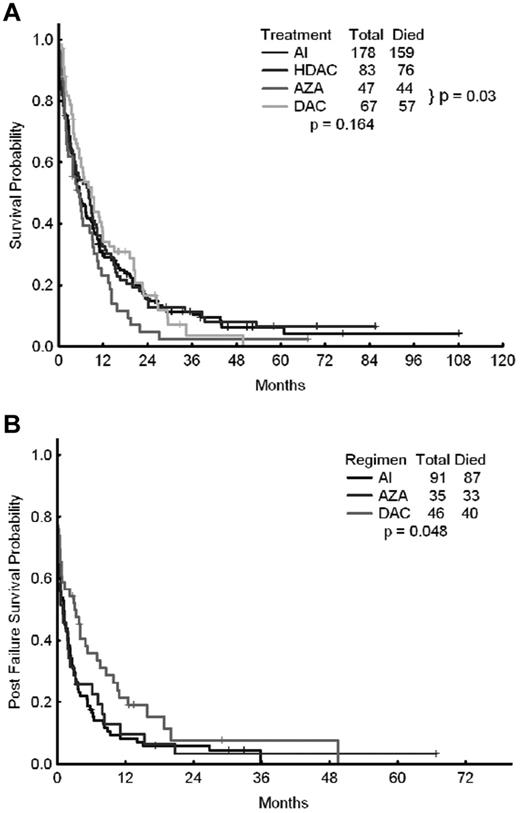
We also investigated potential differences in outcomes of patients receiving intensive chemotherapy with a combination of cytarabine and idarubicin (AI; n = 178) or other high-dose cytarabine-containing regimens (n = 83), as well as patients treated with azacitidine-based regimens (n = 47) or decitabine-based regimens (n = 67). The CR rates for each of the 4 approaches were 44%, 46%, 26%, and 30%, respectively (P = .027). The corresponding median survival times were 5.5, 8.1, 5.5, and 8.8 months, respectively (P = .16; Figure 2A and Table 4). The corresponding 8-week mortality rates were 21%, 20%, 15%, and 9%, respectively (P = .149). The corresponding median relapse-free survival times were 10, 8.8, 7.0, and 12.8 months (P = .89). When azacitidine- and decitabine-based therapies were compared directly, the median survival times were 5.5 months with azacitidine and 8.8 months with decitabine (P = .03).
OS of patients treated with epigenetic therapy compared with patients treated with AI or other high-dose ara-C–containing regimens. Shown are OS rates of patients treated with epigenetic therapy (azacitidine vs decitabine) compared with that of patients treated with AI or other high-dose ara-C (HDAC)–containing regimens (A) and OS after failure of AI, azacitidine, or decitabine (B).
OS of patients treated with epigenetic therapy compared with patients treated with AI or other high-dose ara-C–containing regimens. Shown are OS rates of patients treated with epigenetic therapy (azacitidine vs decitabine) compared with that of patients treated with AI or other high-dose ara-C (HDAC)–containing regimens (A) and OS after failure of AI, azacitidine, or decitabine (B).
Outcome according to specific therapeutic regimens
| . | AI . | HDAC . | Aza . | DAC . | P . |
|---|---|---|---|---|---|
| Total patients, n (%) | 178 (47%) | 83 (22%) | 47 (13%) | 67 (18%) | |
| Outcome | |||||
| ORR, n (%) | 87 (49%) | 44 (33%) | 12 (26%) | 21 (31%) | .001 |
| CR | 78 (44%) | 38 (46%) | 12 (26%) | 20 (30%) | .027 |
| CRp | 9 (5%) | 6 (7%) | 0 (0%) | 1 (1%) | NR |
| ED < 8 wk, n (%) | 37 (21%) | 17 (20%) | 7 (15%) | 6 (9%) | .149 |
| Median OS, mo | 5.5 | 8.1 | 5.5 | 8.8 | .164 |
| 1-y | 30% | 31% | 23% | 36% | |
| 2-y | 15% | 14% | 2% | 14% | |
| Median RFS, mo | 10.0 | 8.8 | 7.0 | 12.8 | .899 |
| 1-y | 41% | 36% | 30% | 45% | |
| 2-y | 20% | 28% | 30% | 45% | |
| . | AI . | HDAC . | Aza . | DAC . | P . |
|---|---|---|---|---|---|
| Total patients, n (%) | 178 (47%) | 83 (22%) | 47 (13%) | 67 (18%) | |
| Outcome | |||||
| ORR, n (%) | 87 (49%) | 44 (33%) | 12 (26%) | 21 (31%) | .001 |
| CR | 78 (44%) | 38 (46%) | 12 (26%) | 20 (30%) | .027 |
| CRp | 9 (5%) | 6 (7%) | 0 (0%) | 1 (1%) | NR |
| ED < 8 wk, n (%) | 37 (21%) | 17 (20%) | 7 (15%) | 6 (9%) | .149 |
| Median OS, mo | 5.5 | 8.1 | 5.5 | 8.8 | .164 |
| 1-y | 30% | 31% | 23% | 36% | |
| 2-y | 15% | 14% | 2% | 14% | |
| Median RFS, mo | 10.0 | 8.8 | 7.0 | 12.8 | .899 |
| 1-y | 41% | 36% | 30% | 45% | |
| 2-y | 20% | 28% | 30% | 45% | |
HDAC indicates high-dose ara-C; CRp, CR with incomplete platelet recovery; ED, early death; RFS, relapse-free survival; and NR, not reported.
Prognostic factors associated with survival
We also evaluated independent prognostic factors for survival. By multivariate analysis, older age (P < .001), adverse cytogenetics (P < .001), poor performance status (P < .001), elevated creatinine (P < .001), percentage of peripheral blood (P = .001) and BM blasts (P = .016), and hemoglobin (P = .023), but not chemotherapy versus epigenetic therapy (P = .492), were identified as independent adverse prognostic factors. The same factors, with the exception of BM blasts (P = .6) and hemoglobin (< 8g/dL; P = .055), were identified when the multivariate analysis was conducted using either categorical cutoffs (Table 5) or patients 70 years of age or older.
Prognostic factors for survival identified by multivariate analysis in older patients with AML
| . | Groups . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Age, y | ≥ 70 | 1.289 | 1.082-1.536 | .004 |
| 65-69 | 1 | |||
| ECOG performance status | > 2 | 2.095 | 1.428-3.073 | < .001 |
| 0-2 | 1 | |||
| Poor-risk cytogenetics | −7, −5, and/or complex | 1.866 | 1.566-2.224 | < .001 |
| Other | 1 | |||
| BM blasts | ≤ 47% | 1.006 | 0.875-1.260 | .60 |
| > 47% | 1 | |||
| Creatinine, mg/dL | ≥ 1.5 | 1.640 | 1.286-2.090 | < .001 |
| < 1.5 | 1 | |||
| Peripheral blood blasts | ≤ 13% | 0.651 | 0.544-0.779 | < .001 |
| > 13% | 1 | |||
| Hemoglobin, g/dL | ≤ 8 | 1.177 | 0.996-1.391 | .055 |
| > 8 | 1 | |||
| Type of therapy | Epigenetic | 1.116 | 0.895-1.391 | .330 |
| Chemotherapy | 1 |
| . | Groups . | HR . | 95% CI . | P . |
|---|---|---|---|---|
| Age, y | ≥ 70 | 1.289 | 1.082-1.536 | .004 |
| 65-69 | 1 | |||
| ECOG performance status | > 2 | 2.095 | 1.428-3.073 | < .001 |
| 0-2 | 1 | |||
| Poor-risk cytogenetics | −7, −5, and/or complex | 1.866 | 1.566-2.224 | < .001 |
| Other | 1 | |||
| BM blasts | ≤ 47% | 1.006 | 0.875-1.260 | .60 |
| > 47% | 1 | |||
| Creatinine, mg/dL | ≥ 1.5 | 1.640 | 1.286-2.090 | < .001 |
| < 1.5 | 1 | |||
| Peripheral blood blasts | ≤ 13% | 0.651 | 0.544-0.779 | < .001 |
| > 13% | 1 | |||
| Hemoglobin, g/dL | ≤ 8 | 1.177 | 0.996-1.391 | .055 |
| > 8 | 1 | |||
| Type of therapy | Epigenetic | 1.116 | 0.895-1.391 | .330 |
| Chemotherapy | 1 |
HR indicates hazard ratio; and CI, confidence interval.
Outcomes after failure of primary therapy
The outcome of patients who failed (but did not die) after initial AML therapy was available for 172 patients: 91 treated with intensive chemotherapy (AI regimen), 35 treated with azacitidine, and 46 treated with decitabine (Figure 2A and Table 6). The 8-week mortality with first salvage therapy was significantly lower among patients who had previously failed azacitidine (20%) or decitabine (13%) compared with intensive chemotherapy (41%, P = .001). The 1-year survival rate was 10% after failure with azacitidine, 21% with decitabine, and only 8% with intensive chemotherapy (ie, the AI regimen; P = .048). The median survival rates after first salvage therapy were 1.1 months after intensive chemotherapy, 0.9 months after azacitidine, and 3.1 months after decitabine (P = .048), suggesting a modest but significant survival advantage for patients receiving salvage therapy after decitabine failure (Figure 2B).
Outcomes of patients receiving salvage therapy after failure of AI, azacitidine, or decitabine
| . | After AI . | After azacitidine . | After decitabine . | P . |
|---|---|---|---|---|
| Total patients, n (%) | 91 (53%) | 35 (20%) | 46 (27%) | |
| Outcome, n (%) | ||||
| No response | 54 (59%) | 27 (77%) | 36 (78% | .001 |
| Early death (< 8 wk) | 37 (41%) | 7 (20%) | 6 (13%) | |
| Median postfailure survival, mo | 1.1 | 0.9 | 3.1 | .048 |
| 1-y | 8% | 10% | 21% | |
| 2-y | 6% | 3% | 8% | |
| . | After AI . | After azacitidine . | After decitabine . | P . |
|---|---|---|---|---|
| Total patients, n (%) | 91 (53%) | 35 (20%) | 46 (27%) | |
| Outcome, n (%) | ||||
| No response | 54 (59%) | 27 (77%) | 36 (78% | .001 |
| Early death (< 8 wk) | 37 (41%) | 7 (20%) | 6 (13%) | |
| Median postfailure survival, mo | 1.1 | 0.9 | 3.1 | .048 |
| 1-y | 8% | 10% | 21% | |
| 2-y | 6% | 3% | 8% | |
Discussion
Hypomethylating agents such as azacitidine and decitabine are now established standard of care for the treatment of patients with MDS. In one randomized trial and in a retrospective analysis from our institution, a comparison between hypomethylating therapy and standard of care treatment (including intensive chemotherapy) demonstrated the former to be associated with improved survival.15,20 Hypomethylating agents have also shown activity in AML. Several studies with decitabine and azacitidine in older patients with AML showed reasonable efficacy, with low CR rates of 10%-20% and ORRs of 30%-50%. An important unanswered question is whether therapy with hypomethylating agents in older patients with AML may improve long-term outcomes compared with intensive chemotherapy. In the present study, we have shown that although hypomethylating therapy results in lower CR rates, the survival of patients treated with that approach does not appear to be inferior to that of patients treated with intensive chemotherapy. This suggests that survival in AML is not solely determined by the achievement of CR and that other forms of response produced by hypomethylating agents may result in leukemia control and reduction of mortality that result in similar long-term outcomes compared with those produced by intensive chemotherapy. Our study also suggests that certain subsets of patients with AML may benefit selectively from hypomethylating agents, in particular patients who fare poorly with intensive chemotherapy (eg, patients 70 years of age or older and those with adverse cytogenetics). In that context, and within the constraints of this retrospective analysis, decitabine appears to provide an outcome benefit over azacitidine.
Similar observations have been reported in 2 recent studies comparing intensive chemotherapy to hypomethylating agents in AML. Lestang et al compared the French experience with azacitidine (n = 36) and with intensive chemotherapy (n = 36). The ORR was 28% with azacitidine and 63% with intensive chemotherapy. However, the median survival times were 10.4 and 10.3 months, respectively (P = .3).26 Spanish investigators also reported on their results with azacitidine (n = 67) versus intensive chemotherapy (n = 47) in older patients with AML, reporting median survival times of 13.7 versus 11.2 months (P = .75).27 The cumulative experiences from these studies and our present findings in older patients with AML suggest a potential role for hypomethylating therapy among patients who cannot tolerate chemotherapy, in particular very old patients or those with adverse karyotypes. Stratification by karyotype (adverse vs diploid), the presence of FLT3-ITD (wild-type versus mutant), age (60-70 vs more than 70 years of age), and performance status (ECOG 0-1 vs 2-4), the presence of renal dysfunction (creatinine above or below 1.5 mg/dL), also demonstrated the noninferiority of hypomethylating therapy compared with intensive chemotherapy.
Recently, a large phase 3 study in older patients with AML unfit for intensive chemotherapy compared decitabine with accepted standards of care, including low-dose cytarabine, which was given to most patients. A total of 485 patients 65 years of age and older with newly diagnosed or secondary AML and poor- or intermediate-risk cytogenetics were randomized to receive decitabine at 20 mg/m2/d for 5 days per cycle or conventional care (supportive care only or low-dose cytarabine).28 The protocol-specified planned analysis (the primary end point) conducted after 82% of patients had died showed median survival times of 7.7 months with decitabine versus 5.0 months with conventional care (hazard ratio = 0.85, 95% confidence interval, 0.69-1.04, P = .10). A final analysis with an additional year of follow-up showed the same differences regarding median survival, but this time with a P value = .03. This randomized study again suggests the potential role of hypomethylating agents in older patients with AML who cannot tolerate intensive chemotherapy.
The equivalent but not improved survival with epigenetic strategies over that afforded by standard therapy underscores the critical need to further discover and develop novel effective treatments for elderly patients with AML. Several agents are currently under investigation for the treatment of older patients with AML, including clofarabine, sapacitabine, FLT3 inhibitors (eg, sorafenib and AC220), or tosedostat. Hypomethylating agents are also being tested in older patients with AML in combination with other investigational agents such as histone deacetylase inhibitors,29 lenalidomide,30 and sapacitabine,31 as well as in sequential strategies using non-cross-resistant agents (eg, clofarabine plus low-dose ara-C alternating with decitabine).32 Prospective randomized studies will be necessary to demonstrate the superiority of such approaches over conventional strategies.
In conclusion, our retrospective analysis in a large and unselected cohort of older patients with AML treated at our institution over the last decade showed similar long-term outcomes with epigenetic therapy compared with intensive chemotherapy. The persistent poor outcome of older patients with AML underscores the need to identify novel agents and/or combinations of existing ones with potential to improve the outcome of AML among older patients.
The online version of this article contains a data supplement.
Presented in abstract form at the 53rd Annual Meeting of the American Society of Hematology, San Diego, CA, December 11, 2011.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.Q.-C. and H.K. designed the research, treated the patients, interpreted the data, and wrote the manuscript; F.R., T.L.-D, S.F., G.B., G.G-M., and J.C. treated the patients and approved the final version of the manuscript; and M.B. and S.P. analyzed the data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Alfonso Quintás-Cardama or Dr Hagop Kantarjian, Department of Leukemia, Unit 428, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030-4095; e-mail: aquintas@mdanderson.org or hkantarj@mdanderson.org.