Abstract
Activating mutations in the receptor tyrosine kinase FLT3 are frequently found in acute myelogenous leukemia patients and confer poor clinical prognosis. It is unclear how leukemic blasts escape cytokine control that regulates normal hematopoiesis. We have recently demonstrated that FLT3-internal tandem duplication (ITD), when localized to the biosynthetic compartment, aberrantly activates STAT5. Here, we show that one of the target genes induced by STAT5 is suppressor of cytokine signaling (SOCS)1—a surprising finding for a known tumor suppressor. Although SOCS1 expression in murine bone marrow severely impaired cytokine-induced colony growth, it failed to inhibit FLT3-ITD–supported colony growth, indicating resistance of FLT3-ITD to SOCS1. In addition, SOCS1 coexpression did not affect FLT3-ITD–mediated signaling or proliferation. Importantly, SOCS1 coexpression inhibited interferon-α and interferon-γ signaling and protected FLT3-ITD hematopoietic cells from interferon-mediated growth inhibitory effects. In a murine bone marrow transplantation model, the coexpression of SOCS1 and FLT3-ITD significantly shortened the latency of a myeloproliferative disease compared with FLT3-ITD alone (P < .01). Mechanistically, SOCS proteins shield FLT3-ITD from external cytokine control, thereby promoting leukemogenesis. The data demonstrate that SOCS1 acts as a conditional oncogene, providing novel molecular insights into cytokine resistance in oncogenic transformation. Restoring cytokine control may provide a new way of therapeutic intervention.
Introduction
Normal hematopoiesis is regulated by growth factors and cytokines to promote diverse biologic functions such as proliferation, differentiation, or growth arrest. Perturbations in cytokine signaling alter homeostasis of blood cells.1 Depending on the cellular context, cytokines stimulate their respective receptors that, in turn, activate specific Jak kinases (Jak1, -2, and -3 and Tyk2), leading to phosphorylation, dimerization, and nuclear translocation of 1 or combinations of 6 signal transducers and activators of transcription (STAT) transcription factors, thereby controlling the above-mentioned biologic processes (reviewed by O'Shea et al2 ). Cytokine-activated Jak/STAT signaling is tightly controlled by negative regulatory loops, such as protein phosphatases; protein inhibitors of activated STATs; and importantly, suppressor of cytokine signaling (SOCS) proteins.
FLT3 is a class III receptor tyrosine kinase expressed on the surface of early hematopoietic progenitor cells. It plays a key role in the development of myeloid and lymphoid lineages.3,4 Although FLT3 knockout mice are healthy, they display deficiencies in primitive B-lymphoid progenitors and a reduced ability of stem cells to reconstitute lymphoid and myeloid compartments.3 These data implicate FLT3 in the development of multipotent hematopoietic stem cells and lymphopoiesis. FLT3 is highly expressed on leukemia and lymphoma cell lines.5,6
Activating mutations of FLT3 with an in-frame internal tandem duplication (ITD) in the juxtamembrane domain are found in 20% to 25% of acute myelogenous leukemia (AML) patients and are associated with poor prognosis.7,8 Expression of FLT3-ITD in 32D or Ba/F3 cell lines constitutively activates the STAT5, MAPK-ERK, and PI3K-AKT pathways and confers IL-3–independent growth.9,10 We have previously compared the gene expression profile of FLT3-ligand (FL)–stimulated FLT3-wild type (FLT3-WT) with FLT3-ITD–expressing cells, and we found STAT5 target genes most differentially expressed.11 Among these, several SOCS family members were induced by FLT3-ITD.11
The SOCS family consists of SOCS1 to 7 and cytokine-inducible Src homology 2 protein (CIS), which inhibit Jak kinases by multiple mechanisms. The Src homology 2 domain of SOCS proteins binds activated cytokine receptors, whereas the SOCS-box contains E3-ligase activity and promotes ubiquitination of associated proteins, including Jak kinases.12,13 SOCS1 and SOCS3 have an N-terminal kinase inhibitory domain that binds to tyrosine 1007 of Jak2 and inhibits kinase activity by acting as a pseudosubstrate.14,15 Therefore, SOCS proteins are key components of the negative feedback machinery that terminate cytokine signals. SOCS1 has been shown to have tumor suppressive properties,16 and it is silenced by hypermethylation in hepatocellular carcinoma17 and multiple myeloma.18 Finally, inactivating mutations of SOCS1 are found in lymphoma, with consecutive increase in Jak2 activity and accumulation of phosphorylated STAT5 and STAT6.19,20
We have shown previously that STAT5 is aberrantly activated by FLT3-ITD but not by ligand-activated FLT3-WT.10,11 STAT5 is directly phosphorylated by FLT3-ITD, independently of Jak and Src kinases.21 Furthermore, we have discovered recently that FLT3-ITD is aberrantly activated at the endoplasmic reticulum and phosphorylates STAT5, whereas the plasma membrane–bound pool of FLT3-ITD activates the AKT and MAPK signaling pathways.22
Constitutive STAT5 phosphorylation leads to its nuclear translocation and induction of target genes such as SOCS1. Because SOCS1 has known growth inhibitory functions in hematopoiesis, we were intrigued by its induction by an oncogene such as FLT3-ITD. Here, we observed that overexpression of SOCS1 in primary bone marrow cells severely impaired their colony growth in the presence of growth-promoting cytokines (stem cell factor [SCF], IL-3, and IL-6). This inhibition was reversed by coexpression of FLT3-ITD, suggesting resistance of FLT3-ITD to SOCS1 in murine bone marrow cells. Importantly, when colony assays were performed in the presence of the growth inhibitory cytokine IFN-α and IFN-γ, coexpression of SOCS1 and FLT3-ITD protected bone marrow cells from the IFN-α– and IFN-γ–mediated growth inhibition. Finally, in a murine bone marrow transplant model, the coexpression of SOCS1 and FLT3-ITD led to the rapid development of a myeloproliferative disease (MPD) or AML with significantly faster onset of disease and shorter median survival compared with FLT3-ITD. These data suggest that in the context of FLT3-ITD, SOCS1 contributes to FLT3-ITD–mediated leukemogenesis by inhibiting differentiation and growth inhibitory cytokine signals, rather than by functioning as tumor suppressor.
Methods
Cytokines, growth factors, and antibodies
Recombinant murine IL-3, IL-6, and SCF were purchased from PeproTech. IFN-α and IFN-γ were purchased from R&D Systems and Sigma-Aldrich, respectively. The FLT3-kinase inhibitors sorafenib and PKC412 were kind gifts from Bayer and Novartis Pharmaceuticals, respectively. The anti–phospho-STAT5 antibody was purchased from Millipore. Anti–phospho-ERK1/2, anti–phospho-FLT3Y591, anti–phospho-STAT1Y701, and anti–phospho-AktS473 were purchased from Cell SignalingTechnology. Total anti-ERK1/2, total anti-STAT5a/b, anti–green fluorescent protein (GFP), anti-Pim1, and anti-Pim2 antibodies were obtained from Santa Cruz Biotechnology. The mouse monoclonal anti-actin antibody was purchased from Sigma-Aldrich. Total anti-STAT1 antibody and anti-SOCS1 were purchased from BD Biosciences Pharmingen and MBL, respectively. Lipofectamine and Plus reagents were purchased from Invitrogen. 5-fluorouracil was purchased from GRY-Pharma. RetroNectin was purchased from Takara Bio. All FACS antibodies were purchased from BD Biosciences Pharmingen. Mouse anti-myeloperoxidase (MPO) antibody for intracellular FACS was purchased from Abcam. Alexa Fluor-647–labeled donkey anti–mouse secondary antibody was purchased from Invitrogen.
Cell lines
The IL-3–dependent murine myeloid 32Dcl3 cell line (hereafter 32D) and the murine lymphoid cell line BaF/3 were cultured in RPMI 1640, supplemented with 10% WEHI (IL-3)–conditioned medium with 10% fetal calf serum (FCS) at 37°C with 5% CO2 as described previously.9,23 The SC1 and Plat-E cell lines (retroviral packaging cell line)24 were cultured in DMEM with 10% FCS.21 MV4-11 and MOLM-13 were cultured in RPMI 1640 with 10% FCS at 37°C with 5% CO2.
Patient samples
The use of patient samples was approved by the Ethics Committee of the Medical Faculty of the University of Muenster. Samples were collected from patients after written informed consent was obtained in accordance with the Declaration of Helsinki. Control CD34+ bone marrow samples were obtained from healthy donors.
RNA preparation and quantitative PCR
RNA isolation and quantitative real-time PCR was done as described previously.23 Primer and probe sequences are listed in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
[3H]thymidine incorporation assay
In total, 2 × 104 32D cells were starved from IL-3 and incubated in medium containing 0.5% FCS for 12 hours in 200 μL of medium in a 96-well plate. Subsequently, cells were placed in medium with 10% FCS, supplemented with the indicated concentrations of 100nM PKC412, IFN-α (1000 U/mL), IFN-γ (20 ng/mL), and IL-3 (2 ng/mL). The PKC412 was added 1 hour before addition of growth factors. Addition of radiolabeled [3H]thymidine and measurement of incorporation was done as described previously.25
Western blotting
32D cells were transduced with pMY (vector control), pMY FLT3-ITD, and pMY SOCS1-T2A-FLT3-ITD and sorted for GFP to generate stable cell lines. For each experiment, cells were starved overnight in RPMI 1640 containing 0.5% serum. Cell lysis and Western blot analysis were performed as described previously.21
Primary murine bone marrow
Balb/c mice were injected with 150 μg of 5-flurouracil per gram of body weight, and after 3 days the mice were killed and bone marrow was harvested. Cells were prestimulated overnight in Iscove modified Dulbecco medium supplemented with 20% FCS with mouse SCF (50 ng/mL), mouse IL-3 (10 ng/mL), mouse IL-6 (10 ng/mL), and 100μM β-mercaptoethanol.
Retroviral and lentiviral supernatants and transduction of murine bone marrow
All retroviral vectors (pMY empty, pMY mSOCS1, pMY FLT3-ITD, and pMY SOCS1-T2A-FLT3-ITD) were transfected into the Plat-E packaging cell line with Lipofectamine and Plus reagent (Invitrogen) according to manufacturer's instructions. Supernatants were collected after 36 hours for every 12 hours until 60 hours and then tested for titer on SC1 cells. Retroviral supernatants with equal titers were coated on RetroNectin-coated 6-well plates according to manufacturer's instructions (7 × 106 transducing units). Equal numbers of bone marrow cells (1 × 106 cells/well) were placed on the retrovirally coated wells overnight, and this procedure was repeated for 2 consecutive days. The cells were FACS-analyzed for GFP expression.
For SOCS1 knockdown experiments, 293T cells were transfected with short hairpin RNA (shRNA) pLKO lentiviral vectors (together with lentiviral packaging plasmids) purchased from Sigma-Aldrich. Vectors express either SOCS1-specific shRNA (1-5) or a nonspecific shRNA. Lentiviral supernatants were collected after 36 hours for every 12 hours until 60 hours. GFP-sorted FLT3-ITD–expressing bone marrow cells were then transduced with lentiviral supernatants, and positive clones were selected in puromycin (1 μg/mL) for 8 days.
Colony assay of murine bone marrow cells
Retrovirally transduced bone marrow was sorted for GFP on an FACSAria cell sorter (BD Biosciences). These GFP+ cells were washed 3 times with PBS, and 2000 cells were plated in 1 mL of methylcellulose per dish in triplicates. Methylcellulose was purchased from StemCell Technologies and was supplemented with either IFN-α (300 U/mL) or IFN-γ (100 ng/mL; Methocult 3231) or contained 50 ng/mL mouse SCF, 10 ng/mL mouse IL-3, and 10 ng/mL human IL-6 (Methocult 3534). Colonies were counted after 12 days, and an equal number of cells (2000 cells/mL) from the colony assay was serially replated.
Transplantation of murine bone marrow
GFP-positive cells (2 × 104) containing more than 150 000 propidium iodide–negative cells were injected into the tail veins of lethally irradiated (8 Gy) female Balb/c recipient mice. In total, the groups were pMY (empty vector, n = 7), pMY SOCS1 (n = 10), pMY FLT3-ITD (n = 18), and pMY SOCS1-T2A-FLT3-ITD (n = 22). Peripheral blood counts were monitored on days 10, 20, and 30 after transplantation on a Hemavet 950 (Drew Scientific) and by FACS analysis for GFP expression. All moribund mice were then killed, and peripheral blood, bone marrow, and spleen cells were analyzed for GFP, CD19, B220, Gr1, CD117, and MPO expression. At day 120, all remaining mice were killed and analyzed. Experimental protocols were approved by the local animal experimentation committee.
Histology and microscopy
Organs were formalin-fixed and paraffin-embedded, and sections were stained with H&E. Representative images were acquired via an AxioCam camera and Axiovision 4.0 software (Carl Zeiss).
Results
FLT3-ITD induces expression of SOCS family members
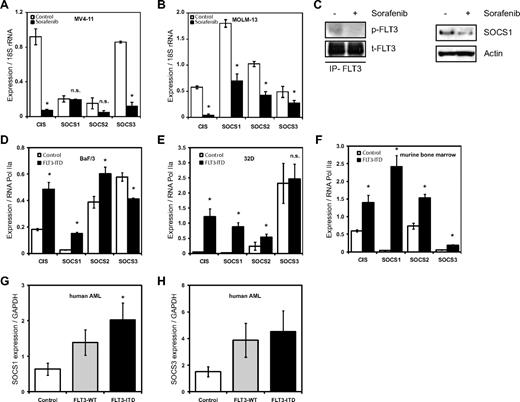
We have reported previously on the gene expression profile of FLT3-ITD, showing the induction of STAT5 target genes such as Pim2, SOCS2, and SOCS3.11 Here, we first analyzed the relative mRNA expression levels of CIS and SOCS1 to 3 in the FLT3-ITD+ AML cell lines MV4-11 and MOLM-13 after treatment with the FLT3 kinase inhibitor sorafenib. Expression of CIS and SOCS3 mRNA in the MV4-11 cell line decreased after sorafenib treatment (Figure 1A), whereas expression of CIS and SOCS1 to 3 was decreased in the MOLM-13 cell line (Figure 1B). This demonstrates that the expression of these SOCS family members requires FLT3-ITD kinase activity. Furthermore, SOCS1 protein levels decreased on FLT3-ITD inhibition with sorafenib (Figure 1C). Immunoprecipitates of FLT3 receptor showed reduced pFLT3, indicating FLT3 kinase activity is indeed inhibited by sorafenib (Figure 1C).
FLT3-ITD induces expression of SOCS family members. (A-B) FLT3 kinase–dependent CIS, SOCS1, SOCS2, and SOCS3 mRNA expression in FLT3-ITD+ AML cell lines. Real-time quantitative PCR analysis of CIS and SOCS1-3 mRNA expression levels in MV4-11 (A) and MOLM-13 (B) cell lines. MV4-11 and MOLM-13 cells were incubated overnight with or without the FLT3 kinase inhibitor sorafenib (20nM) as indicated. Normalized mRNA expression is plotted with SD as error bars. Representative data from 1 of the 2 independent experiments is shown. (C) SOCS1 protein expression depends on FLT3 kinase activity. MOLM-13 cells were incubated overnight, with or without FLT3 kinase inhibitor sorafenib (20nM), and SOCS1 protein levels were determined by Western blot. Actin serves as a loading control. From these lysates, FLT3 was immunoprecipitated and pFLT3 was immunoblotted. (D-F) FLT3-ITD expression led to increased CIS, SOCS1, SOCS2, and SOCS3 mRNA levels in BaF/3 and 32D cell lines and in primary murine bone marrow. Real-time quantitative PCR analysis of mRNA expression levels of CIS and SOCS1-3 expression in BaF/3 (D), 32D (E), and murine bone marrow (F). BaF/3 and 32D cell lines and murine bone marrow were transduced with either empty vector (control) or with FLT3-ITD retrovirus and sorted for GFP. RNA was isolated from cytokine and serum-starved BaF/3 and 32D cells and murine primary bone marrow. The normalized mRNA expression is plotted, with SD as error bars. Representative data from 1 of the 3 independent experiments is shown. (G-H) SOCS1 and SOCS3 mRNA is highly expressed in AML patient bone marrow. Bone marrow samples from a cohort of 77 AML patients (38 of FLT3-ITD and 39 FLT3-WT) and 7 healthy CD34+ donors were analyzed for mRNA expression of SOCS1 (G) and SOCS3 (H). The normalized mRNA expression is plotted, with SD as error bars (*P < .05).
FLT3-ITD induces expression of SOCS family members. (A-B) FLT3 kinase–dependent CIS, SOCS1, SOCS2, and SOCS3 mRNA expression in FLT3-ITD+ AML cell lines. Real-time quantitative PCR analysis of CIS and SOCS1-3 mRNA expression levels in MV4-11 (A) and MOLM-13 (B) cell lines. MV4-11 and MOLM-13 cells were incubated overnight with or without the FLT3 kinase inhibitor sorafenib (20nM) as indicated. Normalized mRNA expression is plotted with SD as error bars. Representative data from 1 of the 2 independent experiments is shown. (C) SOCS1 protein expression depends on FLT3 kinase activity. MOLM-13 cells were incubated overnight, with or without FLT3 kinase inhibitor sorafenib (20nM), and SOCS1 protein levels were determined by Western blot. Actin serves as a loading control. From these lysates, FLT3 was immunoprecipitated and pFLT3 was immunoblotted. (D-F) FLT3-ITD expression led to increased CIS, SOCS1, SOCS2, and SOCS3 mRNA levels in BaF/3 and 32D cell lines and in primary murine bone marrow. Real-time quantitative PCR analysis of mRNA expression levels of CIS and SOCS1-3 expression in BaF/3 (D), 32D (E), and murine bone marrow (F). BaF/3 and 32D cell lines and murine bone marrow were transduced with either empty vector (control) or with FLT3-ITD retrovirus and sorted for GFP. RNA was isolated from cytokine and serum-starved BaF/3 and 32D cells and murine primary bone marrow. The normalized mRNA expression is plotted, with SD as error bars. Representative data from 1 of the 3 independent experiments is shown. (G-H) SOCS1 and SOCS3 mRNA is highly expressed in AML patient bone marrow. Bone marrow samples from a cohort of 77 AML patients (38 of FLT3-ITD and 39 FLT3-WT) and 7 healthy CD34+ donors were analyzed for mRNA expression of SOCS1 (G) and SOCS3 (H). The normalized mRNA expression is plotted, with SD as error bars (*P < .05).
Next, we tested whether overexpression of FLT3-ITD leads to increased SOCS gene expression. As shown in Figure 1D, BaF/3 cells stably expressing FLT3-ITD have significantly higher expression of CIS (2.6-fold), SOCS1 (5.9-fold), and SOCS2 (1.5-fold) compared with controls. Similarly, 32D cells expressing FLT3-ITD have a significantly higher expression of CIS (28-fold) and SOCS1 (46-fold) compared with controls (Figure 1E). Furthermore, murine bone marrow cells overexpressing FLT3-ITD showed a very strong induction of SOCS1 (59-fold) and a moderate induction of CIS (2.3-fold), SOCS2 (2-fold), and SOCS3 (3.2-fold), compared with controls (Figure 1F).
Finally, we investigated expression of SOCS1 and SOCS3 mRNA in primary human AML samples and compared their expression in FLT3-ITD+ AML, FLT3-WT AML, and controls (CD34+ cells from healthy donors). SOCS1, but not SOCS3, expression was significantly higher in FLT3-ITD+ AML samples compared with FLT3-WT AML (Figure 1G-H).
Simultaneous expression of SOCS1 and FLT3-ITD
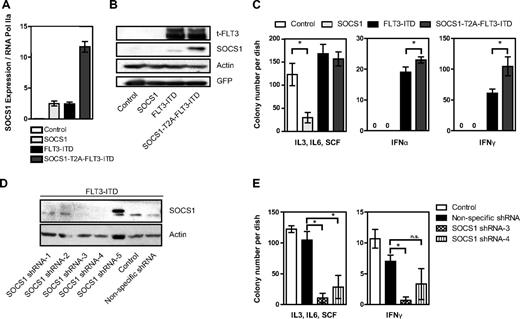
Knowing the tumor-suppressive and antiproliferative functions of SOCS1, we were intrigued by its high induction by the oncogene FLT3-ITD. To study the role of SOCS1 in FLT3-ITD–mediated transformation, we constructed a retroviral vector encoding both SOCS1 and FLT3-ITD in a single open reading frame separated by an in-frame self-cleaving T2A peptide sequence.26 The resulting vector pMY-SOCS1-T2A-FLT3-ITD-IRES-GFP (hereafter SOCS1-T2A-FLT3-ITD) ensures simultaneous expression of SOCS1 and FLT3-ITD from the same mRNA transcript while internal ribosome entry site (IRES)–driven GFP expression is used as a selectable marker. Western blot analysis revealed that SOCS1 and FLT3-ITD were expressed as separate proteins, and no SOCS1-T2A-FLT3-ITD fusion protein was detected (data not shown). It was not possible to generate 32D cell lines stably overexpressing SOCS1, because we and others have shown that the IL-3 signal is abrogated by SOCS1 expression in this IL-3–dependent cell line.21,27 As shown in Figure 2A, we stably overexpressed FLT3-ITD alone or in combination with SOCS1 in 32D cells. Importantly, equal FLT3 protein levels were observed in stable cell lines expressing either FLT3-ITD or SOCS1-T2A-FLT3-ITD. To circumvent the possibility of clonal selection, 2 separate bulk cultures were used (Figure 2A). Furthermore, no differences were observed in the phosphorylation of FLT3, STAT5, AKT, and ERK and in expression of STAT5 target genes Pim1 and Pim2 in cells expressing SOCS1-T2A-FLT3-ITD and FLT3-ITD (Figure 2A), suggesting that the major signaling pathways activated by FLT3-ITD were not affected by SOCS1 coexpression.
Coexpression of SOCS1 and FLT3-ITD. (A) Simultaneous equimolar coexpression of SOCS1 and FLT3-ITD does not affect FLT3-ITD–mediated signaling pathways. Western blot analysis of retrovirally transduced stable 32D cells expressing FLT3-ITD or SOCS1-T2A-FLT3-ITD or control (empty vector). Membranes were incubated with indicated phospho-specific antibodies and were reprobed for the nonphospho–specific-antibodies and β-actin to ensure equal loading. Two independent bulk cultures (1 and 2) of SOCS1-T2A-FLT3-ITD were used to exclude effects clonality. (B) Proliferation assay by measurement of DNA synthesis using [3H]thymidine incorporation. The stable 32D cells expressing the indicated constructs were analyzed for their proliferation by [3H]thymidine incorporation as described under “Methods.” Each data point represents the mean of [3H]thymidine incorporation of quadruplicates, with SD as error bars. (C) 32D cells stably expressing either empty vector control, SOCS1-T2A-FLT3-ITD, or FLT3-ITD were stimulated with either IFN-α (1000 U/mL) and IFN-γ (100 ng/mL) for 10 minutes or left unstimulated. Lysates were resolved by SDS-PAGE and immunoblotted for phospho-STAT1 and SOCS1, as indicated. β-actin served as a loading control. (D-E) 32D cells transduced with control, SOCS1, FLT3-ITD, and SOCS1-T2A-FLT3-ITD retroviruses were monitored for GFP-positivity (percentage) every 24 hours by FACS and cultured without IL-3 (D) or with IL-3 (E). Time is plotted on the x-axis, with the percentage of GFP positivity from 3 independent experiments, with SD as error bars, plotted on y-axis (*P < .05).
Coexpression of SOCS1 and FLT3-ITD. (A) Simultaneous equimolar coexpression of SOCS1 and FLT3-ITD does not affect FLT3-ITD–mediated signaling pathways. Western blot analysis of retrovirally transduced stable 32D cells expressing FLT3-ITD or SOCS1-T2A-FLT3-ITD or control (empty vector). Membranes were incubated with indicated phospho-specific antibodies and were reprobed for the nonphospho–specific-antibodies and β-actin to ensure equal loading. Two independent bulk cultures (1 and 2) of SOCS1-T2A-FLT3-ITD were used to exclude effects clonality. (B) Proliferation assay by measurement of DNA synthesis using [3H]thymidine incorporation. The stable 32D cells expressing the indicated constructs were analyzed for their proliferation by [3H]thymidine incorporation as described under “Methods.” Each data point represents the mean of [3H]thymidine incorporation of quadruplicates, with SD as error bars. (C) 32D cells stably expressing either empty vector control, SOCS1-T2A-FLT3-ITD, or FLT3-ITD were stimulated with either IFN-α (1000 U/mL) and IFN-γ (100 ng/mL) for 10 minutes or left unstimulated. Lysates were resolved by SDS-PAGE and immunoblotted for phospho-STAT1 and SOCS1, as indicated. β-actin served as a loading control. (D-E) 32D cells transduced with control, SOCS1, FLT3-ITD, and SOCS1-T2A-FLT3-ITD retroviruses were monitored for GFP-positivity (percentage) every 24 hours by FACS and cultured without IL-3 (D) or with IL-3 (E). Time is plotted on the x-axis, with the percentage of GFP positivity from 3 independent experiments, with SD as error bars, plotted on y-axis (*P < .05).
It is well established that FLT3-ITD expression confers IL-3 independence to 32D and BaF/3 cell lines.10 We therefore used these cells to analyze effects of FLT3-ITD activity on cytokine signaling. To analyze proliferation, [3H]thymidine incorporation was measured in the presence or absence of IL-3, IFN-α, IFN-γ, and the FLT3 kinase inhibitor PKC412: SOCS1 did not inhibit FLT3-ITD–mediated DNA synthesis and proliferation (Figure 2B). Importantly, in the absence of FLT3-ITD kinase activity (+PKC412), coexpression of SOCS1 severely impaired IL-3–induced proliferation of 32D cells (Figure 2B, IL3 + PKC412). Furthermore, in the presence of INF-α or IFN-γ, coexpression of SOCS1 and FLT3-ITD significantly enhanced proliferation of 32D cells compared with FLT3-ITD alone (Figure 2B right columns).
Next, we next analyzed the effect of FLT3-ITD–induced SOCS1 expression on IFN-α– and IFN-γ–mediated signaling as measured by STAT1 activation. In response to IFN-α and IFN-γ, STAT1 activation is decreased in FLT3-ITD–expressing cells compared with controls (possibly because of the SOCS1 induction by FLT3-ITD). STAT1 activation is abolished by exogenous SOCS1 expression (SOCS1-T2A-FLT3-ITD; Figure 2C).
In competitive proliferation assays, we performed retroviral transductions with our 4 constructs (control, SOCS1, FLT3-ITD, and SOCS1-T2A-FLT3-ITD) and compared the percentage of GFP-positive (ie, transduced) versus GFP-negative (nontransduced) cells in the absence of IL-3 (Figure 2D) and in the presence of IL-3 (Figure 2E). As expected, in the absence of IL-3, the control and the SOCS1-transduced 32D cells rapidly died (diminished GFP expression; Figure 2D). In contrast, 32D cells transduced with FLT3-ITD or SOCS1-T2A-FLT3-ITD rapidly grew at a similar rate to a homogeneous GFP+ population within 2 days. This demonstrates that SOCS1 coexpression has no influence on FLT3-ITD–induced cell proliferation or transformation (Figure 2D). In the presence of IL-3, 32D cells expressing SOCS1 rapidly died and left no detectable GFP+ cells (Figure 2E). In contrast, the percentage of GFP positivity remained constant in controls and in FLT3-ITD– and SOCS1-T2A-FLT3-ITD–transduced cells (Figure 2E). This implies that although SOCS1 expression inhibited IL-3–dependent proliferation of 32D cells, the FLT3-ITD–dependent proliferation was resistant to SOCS1-mediated inhibition.
SOCS1 coexpression with FLT3-ITD confers resistance to IFN-α and IFN-γ in primary murine bone marrow
Because SOCS proteins are known for their inhibitory function in cytokine signaling, we analyzed their effects in murine bone marrow cells in the context of FLT3-ITD. In particular, we investigated whether proproliferative or antiproliferative cytokines (such as IFN-α and -γ) exert their growth regulatory functions when FLT3-ITD is expressed. This is important because normal and malignant hematopoietic cells in the bone marrow constantly integrate simultaneous, often opposing, cytokine signals.
For this purpose, primary bone marrow cells were transduced with control GFP, SOCS1, FLT3-ITD, and SOCS1-T2A-FLT3-ITD, and GFP-positive (ie, transduced) cells were FACS-sorted.
First, we measured the expression of endogenous and exogenous SOCS1 mRNA levels (Figure 3A). SOCS1- and FLT3-ITD–expressing bone marrow show similar amounts of SOCS1 mRNA expression, and a very high level of SOCS1 mRNA expression was observed in SOCS1-T2A-FLT3-ITD–expressing bone marrow (Figure 3A). Bone marrow cells expressing FLT3-ITD or SOCS1-T2A-FLT3-ITD showed equal FLT3 protein expression, but—as expected—they differed in the amount of (endogenous and exogenous) SOCS1 protein expressed (Figure 3B). Next, the above-mentioned bone marrow cells were cultured in methylcellulose-based colony-forming assays in the presence of a cocktail of proproliferative cytokines (SCF, IL-3, and IL-6; Figure 3C left) or the antiproliferative cytokines IFN-α (Figure 3C middle) and IFN-γ (Figure 3C right). As expected, in the presence of SCF, IL-3, and IL-6, SOCS1-expressing bone marrow formed significantly less number of colonies compared with control; in contrast, cells expressing FLT3-ITD or SOCS1-T2A-FLT3-ITD formed comparable number of colonies (Figure 3C left).
Coexpression of SOCS1 and FLT3-ITD shields colony growth from inhibition by interferon α and γ. Primary murine bone marrow was retrovirally transduced with empty vector control, SOCS1, FLT3-ITD, or SOCS1-T2A-FLT3-ITD and was sorted for GFP coexpression. (A) After RNA extraction and reverse transcription, quantitative PCR was performed to determine endogenous and exogenous levels of SOCS1, normalized to RNA polymerase IIa. (B) Cell lysates were resolved by SDS-PAGE and immunoblotted for (total) FLT3, SOCS1, GFP, and β-actin (loading control). Exogenous SOCS1 (right lane) slightly larger in size because of few additional amino acids resulted from T2A cleavage. (C) Sorted GFP–positive bone marrow cells were washed and plated at a density of 2000 cells/mL in triplicates in methylcellulose medium containing either proproliferative growth factors (IL-3, SCF, and IL-6) or IFN-α (300 U/mL) or IFN-γ (100 ng/mL). Colony numbers were counted after 12 days, and mean colony number from triplicates from 2 independent experiments were plotted, with SD as error bars. (D) Sorted, FLT3-ITD–expressing bone marrow cells were transduced with either SOCS1-specific shRNAs1–5 or a nonspecific shRNA and selected in the presence of puromycin. Puromycin-resistant clones were analyzed for SOCS1 protein expression, parental bone marrow expressing FLT3-ITD (not transduced with shRNA vectors) was used as a control, and β-actin was detected as a loading control. (E) Sorted and puromycin-resistant (FLT3-ITD and shRNA expressing clones 3 and 4) bone marrow cells or FLT3-ITD–expressing control cells were washed and plated at a density of 2000 cells/mL in triplicates in methylcellulose medium containing either proproliferative growth factors (IL-3, SCF, and IL-6) or IFN-α (300 U/mL) or IFN-γ (100 ng/ mL). Colony numbers were counted after 12 days, and mean colony number from triplicates are plotted, with SD as error bars (*P < .05).
Coexpression of SOCS1 and FLT3-ITD shields colony growth from inhibition by interferon α and γ. Primary murine bone marrow was retrovirally transduced with empty vector control, SOCS1, FLT3-ITD, or SOCS1-T2A-FLT3-ITD and was sorted for GFP coexpression. (A) After RNA extraction and reverse transcription, quantitative PCR was performed to determine endogenous and exogenous levels of SOCS1, normalized to RNA polymerase IIa. (B) Cell lysates were resolved by SDS-PAGE and immunoblotted for (total) FLT3, SOCS1, GFP, and β-actin (loading control). Exogenous SOCS1 (right lane) slightly larger in size because of few additional amino acids resulted from T2A cleavage. (C) Sorted GFP–positive bone marrow cells were washed and plated at a density of 2000 cells/mL in triplicates in methylcellulose medium containing either proproliferative growth factors (IL-3, SCF, and IL-6) or IFN-α (300 U/mL) or IFN-γ (100 ng/mL). Colony numbers were counted after 12 days, and mean colony number from triplicates from 2 independent experiments were plotted, with SD as error bars. (D) Sorted, FLT3-ITD–expressing bone marrow cells were transduced with either SOCS1-specific shRNAs1–5 or a nonspecific shRNA and selected in the presence of puromycin. Puromycin-resistant clones were analyzed for SOCS1 protein expression, parental bone marrow expressing FLT3-ITD (not transduced with shRNA vectors) was used as a control, and β-actin was detected as a loading control. (E) Sorted and puromycin-resistant (FLT3-ITD and shRNA expressing clones 3 and 4) bone marrow cells or FLT3-ITD–expressing control cells were washed and plated at a density of 2000 cells/mL in triplicates in methylcellulose medium containing either proproliferative growth factors (IL-3, SCF, and IL-6) or IFN-α (300 U/mL) or IFN-γ (100 ng/ mL). Colony numbers were counted after 12 days, and mean colony number from triplicates are plotted, with SD as error bars (*P < .05).
In the presence IFN-α and IFN-γ, no colony growth was observed in control and SOCS1-overexpressing bone marrow, whereas FLT3-ITD expression led to colony growth (Figure 3C middle and right). Importantly, bone marrow transduced with SOCS1-T2A-FLT3-ITD formed significantly more colonies compared with FLT3-ITD alone (Figure 3C middle and right). Although previous reports have shown no increased replating activity of FLT3-ITD in primary bone marrow,28 we tested whether SOCS1 coexpression would alter this; here, we observed no significant differences in replating efficiency between SOCS1-T2A-FLT3-ITD and FLT3-ITD (supplemental Figure 1).
To examine the requirement of SOCS1 in FLT3-ITD–mediated colony growth, we performed knockdown experiments with shRNA for SOCS1 in primary murine bone marrow expressing FLT3-ITD. First, we identified 2 sequences (3 and 4) that consistently led to loss of SOCS1 protein expression in primary cells, compared with control or nonspecific shRNA (Figure 3D). In a second step, we performed colony assays of FLT3-ITD–expressing bone marrow cells selected to express either knockdown sequences 3 or 4. As shown in Figure 3E, SOCS1 knockdown perturbs colony growth under both pro- and antiproliferative conditions (Figure 3E). Taken together, our results demonstrate that (1) SOCS1 on its own functions as a negative growth regulator of colony formation; (2) when coexpressed with FLT3-ITD, SOCS1 protects colony growth from IFN-α and IFN-γ inhibition; and (3) SOCS1 knockdown reveals a dependency of SOCS1 in FLT3-ITD–mediated colony growth and transformation.
SOCS1 accelerates the onset of FLT3-ITD–induced MPD
FLT3-ITD has been shown to lead to the development of an MPD and acute lymphocytic leukemia of B- and T-cell origin in a murine bone marrow transplantation model29–31 and either a myeloproliferative or lymphoid disease in a transgenic mouse model.32 Here, we investigated the role of SOCS1 in FLT3-ITD–mediated leukemogenesis in a bone marrow transplantation model. Shown is the combined analysis of 2 independent experiments.
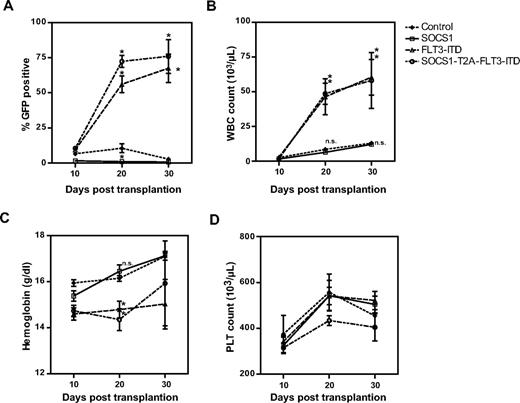
Retrovirally transduced bone marrow cells were transplanted into recipient mice, and the peripheral blood parameters of all animals were monitored at 10-day intervals (Figure 4). We determined the expression of GFP by flow cytometry (Figure 4A), measured white blood cell (WBC) counts (Figure 4B), hemoglobin levels (Figure 4C), and platelet counts (Figure 4D). As shown in Figure 4A and Table 1, the expression of GFP in the peripheral blood of mice that received the control vector remained constant over time, whereas GFP signal was lost by day 20 in mice transplanted with SOCS1-expressing bone marrow. This clearly demonstrates a competitive growth disadvantage of SOCS1-expressing (ie, GFP-positive) bone marrow cells in the latter group. In contrast, the percentage of GFP-positive cells in mice that were transplanted with FLT3-ITD– or SOCS1-T2A-FLT3-ITD–expressing bone marrow cells significantly increased from day 10 to day 30, with an increase of 9.4% to 67.5% and 10% to 76%, respectively (Figure 4A). Similarly, WBC counts of FLT3-ITD mice (with a mean of 2.7 × 103/μL on day 10 to 60.5 × 103/μL on day 30) and SOCS1-T2A-FLT3-ITD mice (with a mean of 1.7 × 103/μL on day 10 to 58.2 × 103/μL on day 30) showed a significant increase compared with control mice (Figure 4B; Table 1). Both the increase of GFP-positive cells and WBCs in the FLT3-ITD and the SOCS1-T2A-FLT3-ITD mice suggest a biologic advantage in these cell populations. Interestingly, analysis of GFP-positive cells on day 20 showed significantly higher GFP+ cells in the SOCS1-T2A-FLT3-ITD group compared with the FLT3-ITD group. Conversely, hemoglobin content in FLT3-ITD and SOCS1-T2A-FLT3-ITD groups were significantly lower at day 20 compared with the control or SOCS1 mice, who showed the signs of leukemia (Figure 4C-D; Table 1); however, there was no statistical differences with respect to platelet counts.
Posttransplantation follow-up of mice for engraftment and development of leukemia. All transplanted mice (as described under “Transplantation of murine bone marrow”) were followed at regular intervals of 10 days for the peripheral blood cell counts (Haemavet) and percentage of GFP-positive cells (flow cytometry). The mean values from each different group of mice were plotted as percentage GFP (A), WBC count (× 1000/μL; B), hemoglobin content (grams per deciliter; C) and platelet count (PLT; × 1000/μL; D) versus time in days after transplantation (*P < .05).
Posttransplantation follow-up of mice for engraftment and development of leukemia. All transplanted mice (as described under “Transplantation of murine bone marrow”) were followed at regular intervals of 10 days for the peripheral blood cell counts (Haemavet) and percentage of GFP-positive cells (flow cytometry). The mean values from each different group of mice were plotted as percentage GFP (A), WBC count (× 1000/μL; B), hemoglobin content (grams per deciliter; C) and platelet count (PLT; × 1000/μL; D) versus time in days after transplantation (*P < .05).
Peripheral blood analysis of transplanted mice at the time of death
| . | No. mice (n) . | Phenotype . | WBC count, 103/μL . | Hemoglobin content, g/dL . | Platelets, 103/μL . | Differentiated myeloid cells (%)* . | Blasts, % . | GFP+ (%) . |
|---|---|---|---|---|---|---|---|---|
| Control | 7 | None | 6.9 (4.6-11.2) | 16 (11.4-17.5) | 499 (335-1203) | 20 (14-31) | 0 | 5 (0.9-6.1) |
| SOCS1 | 10 | None | 6.2 (4.5-16.6) | 14.9 (12.9-16.9) | 256 (131-2612) | 26 (17-33) | 0 | 0.7 (0-2.2) |
| FLT3-ITD | 18 | 12 MPD | 108 (31.4-190.0) | 13.1 (9.6-14.3) | 695 (464-858) | 74 (61-94) | 5 (0-9) | 92 (9.2-96.0) |
| 6 ALL | 90.2 (8.2-146.0) | 13.6 (11.0-16.6) | 497.5 (282-760) | 24 (11-33) | 76 (51-94) | 88.9 (39.3-93.0) | ||
| SOCS1-T2A-FLT3-ITD | 22 | 11 MPD | 51.7 (11.1-80.6) | 13.7 (10.4-17.3) | 421 (227-539) | 71 (58-95) | 6 (0-15) | 66.0 (61.2-91.0) |
| 11 ALL | 56.5 (3.4-133.7) | 14.2 (11.0-17.0) | 422 (312-627) | 23 (14-38) | 81 (22-95) | 88.0 (21.6-97.6) |
| . | No. mice (n) . | Phenotype . | WBC count, 103/μL . | Hemoglobin content, g/dL . | Platelets, 103/μL . | Differentiated myeloid cells (%)* . | Blasts, % . | GFP+ (%) . |
|---|---|---|---|---|---|---|---|---|
| Control | 7 | None | 6.9 (4.6-11.2) | 16 (11.4-17.5) | 499 (335-1203) | 20 (14-31) | 0 | 5 (0.9-6.1) |
| SOCS1 | 10 | None | 6.2 (4.5-16.6) | 14.9 (12.9-16.9) | 256 (131-2612) | 26 (17-33) | 0 | 0.7 (0-2.2) |
| FLT3-ITD | 18 | 12 MPD | 108 (31.4-190.0) | 13.1 (9.6-14.3) | 695 (464-858) | 74 (61-94) | 5 (0-9) | 92 (9.2-96.0) |
| 6 ALL | 90.2 (8.2-146.0) | 13.6 (11.0-16.6) | 497.5 (282-760) | 24 (11-33) | 76 (51-94) | 88.9 (39.3-93.0) | ||
| SOCS1-T2A-FLT3-ITD | 22 | 11 MPD | 51.7 (11.1-80.6) | 13.7 (10.4-17.3) | 421 (227-539) | 71 (58-95) | 6 (0-15) | 66.0 (61.2-91.0) |
| 11 ALL | 56.5 (3.4-133.7) | 14.2 (11.0-17.0) | 422 (312-627) | 23 (14-38) | 81 (22-95) | 88.0 (21.6-97.6) |
Mice transplanted with bone marrow expressing FLT3-ITD or SOCS1-T2A-FLT3-ITD were sacrificed when moribund, whereas mice transplanted with bone marrow expressing control GFP or SOCS1 were sacrificed at the end of the experiment at day 120. Peripheral blood analysis included WBC count, hemoglobin content, platelets, percentage of differentiated myeloid cells and blasts quantified by microscopic quantification of blood smears, and the percentage of GFP expression analyzed by FACS. Values are presented as median (range).
Includes neutrophils and macrophages.
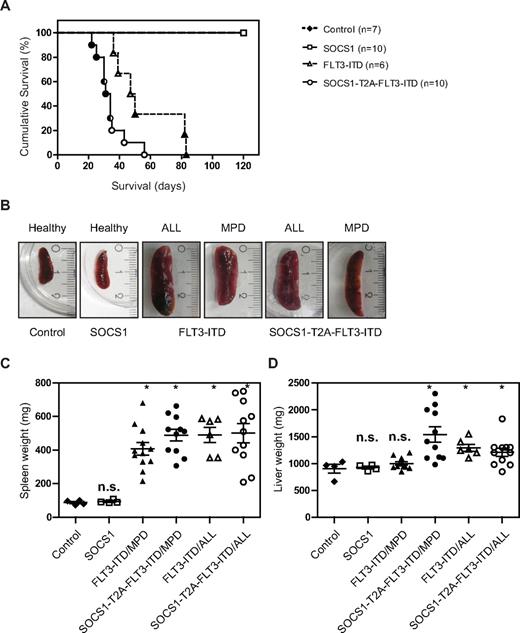
The Kaplan-Meier analysis of the 2 independent experiments are shown separately in Figure 5A and supplemental Figure 2. Both experiments reveal a statistically significant difference in latency of disease, with the SOCS1-T2A-FLT3-ITD group having a significantly shorter latency compared with the FLT3-ITD group (P < .01 and P < .0002 by log-rank Mantel-Cox test, respectively).
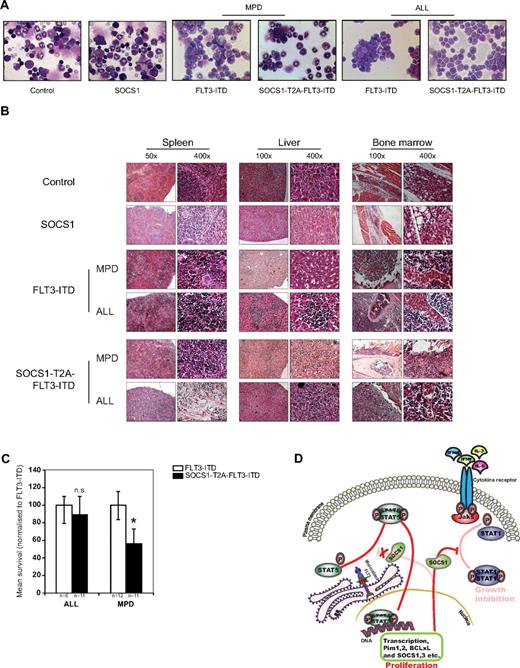
SOCS1 cooperates with FLT3-ITD–induced disease in a murine bone marrow transplant model. (A) Kaplan-Meyer plot displays the survival after transplantation. All mice that received transplants of control or SOCS1-transduced bone marrow survived the 120 days of follow up. All animals from FLT3-ITD and SOCS1-T2A-FLT3-ITD died before the end of the experiment (120 days). Open circles/triangles represent ALL phenotype, filled circles/triangles represent MPD phenotype, and half-filled circles/triangles represent both events. (B) Representative microphotographs of spleens at the time of death. Spleens from control and SOCS1 mice (from 120 days after transplantation) or from FLT3-ITD and SOCS1-T2A-FLT3-ITD moribund mice indicate normal and enlarged spleens, respectively. (C-D) Analysis of spleen weight (C) and liver weight (D) at death or at the end of experiment (120 days). The mice that received FLT3-ITD and SOCS1-T2A-FLT3-ITD transplants suffered from splenomegaly compared with controls (C). In addition, mice transplanted with SOCS1-T2A-FLT3-ITD/MPD suffered from significantly increased hepatomegaly, compared with FLT3-ITD/MPD (D; * P < .05).
SOCS1 cooperates with FLT3-ITD–induced disease in a murine bone marrow transplant model. (A) Kaplan-Meyer plot displays the survival after transplantation. All mice that received transplants of control or SOCS1-transduced bone marrow survived the 120 days of follow up. All animals from FLT3-ITD and SOCS1-T2A-FLT3-ITD died before the end of the experiment (120 days). Open circles/triangles represent ALL phenotype, filled circles/triangles represent MPD phenotype, and half-filled circles/triangles represent both events. (B) Representative microphotographs of spleens at the time of death. Spleens from control and SOCS1 mice (from 120 days after transplantation) or from FLT3-ITD and SOCS1-T2A-FLT3-ITD moribund mice indicate normal and enlarged spleens, respectively. (C-D) Analysis of spleen weight (C) and liver weight (D) at death or at the end of experiment (120 days). The mice that received FLT3-ITD and SOCS1-T2A-FLT3-ITD transplants suffered from splenomegaly compared with controls (C). In addition, mice transplanted with SOCS1-T2A-FLT3-ITD/MPD suffered from significantly increased hepatomegaly, compared with FLT3-ITD/MPD (D; * P < .05).
In the experiment depicted in Figure 5A, control and SOCS1 mice survived until the end of the experiment (day 120), and mice in the FLT3-ITD group died with a median survival of 48.5 days (range, 36-83 days). In the SOCS1-T2A-FLT3-ITD group, latency was shortened to a median survival of 32.5 days (range, 22-56 days).
All the mice from the 2 independent experiments that received FLT3-ITD or SOCS1-T2A-FLT3-ITD bone marrow developed either an MPD or acute lymphoblastic leukemia (B-ALL). In total, 12 of 18 mice with FLT3-ITD developed an MPD, whereas 6 of 18 developed a B-ALL. In the SOCS1-T2A-FLT3-ITD group, the phenotypes were more evenly distributed (11 MPD, 11 ALL).
Spleen, liver, and bone marrow infiltration in FLT3-ITD– or SOCS1-T2A-FLT3-ITD–transplanted mice
All moribund mice transplanted with either FLT3-ITD or SOCS1-T2A-FLT3-ITD show enlarged spleens (Figure 5B). Spleen weights of FLT3-ITD and SOCS1-T2A-FLT3-ITD mice were significantly higher (P < .05) compared with controls, irrespective of MPD or ALL phenotype (Figure 5C). Likewise, there was a significant increase (P < .05) in liver weights in the SOCS1-T2A-FLT3-ITD (ALL and MPD) and FLT3-ITD/ALL groups; on the contrary, there was no significant enlargement of livers in FLT3-ITD/MPD mice (Figure 5D), demonstrating a less aggressive phenotype compared with SOCS-T2A-FLT3-ITD/MPD mice.
Representative bone marrow cytospins of FLT3-ITD– and SOCS1-T2A-FLT3-ITD–transplanted mice showed either an MPD phenotype or a lymphoid blast infiltration compared with SOCS1 and control animals (Figure 6A).
SOCS1 coexpression with FLT3-ITD accelerates the development of MPD. (A) Representative bone marrow cytospins from 120-day posttransplantation control and SOCS1 or from FLT3-ITD and SOCS1-T2A-FLT3-ITD moribund mice at the time of death, indicating MPD and ALL, respectively. (B) H&E staining of tissues (spleen, liver, and bone marrow) from control and SOCS1 mice and leukemic FLT3-ITD and SOCS1-T2A-FLT3-ITD mice, indicating MPD and ALL, respectively. Data are representative moribund FLT3-ITD and SOCS1-T2A-FLT3-ITD mice that died of MPD or ALL. (C) Comparison of mean survival (normalized to FLT3-ITD) from 2 independent transplantation experiments. Similar latency in ALL and significantly different latency in the MPD phenotype are shown. (D) Mechanistic model for the cooperation of SOCS1 in FLT3-ITD–mediated leukemogenesis. As we have described recently, constitutively active oncogenic FLT3-ITD directly phosphorylates STAT5 from the endoplasmic reticulum.21,22 On phosphorylation, STAT5 dimerizes and translocates into nucleus and induces transcription of STAT5 target genes that includes SOCS1. FLT3-ITD–induced SOCS1 expression neither affects FLT3-ITD–mediated proliferative signals nor STAT5 activation, whereas it terminates cytokine-dependent signals. Thereby, SOCS proteins cooperate with FLT3-ITD by “shielding” the cell from cytokine control (eg, IFNα, IFN-γ, IL-3, and IL-6; *P < .05).
SOCS1 coexpression with FLT3-ITD accelerates the development of MPD. (A) Representative bone marrow cytospins from 120-day posttransplantation control and SOCS1 or from FLT3-ITD and SOCS1-T2A-FLT3-ITD moribund mice at the time of death, indicating MPD and ALL, respectively. (B) H&E staining of tissues (spleen, liver, and bone marrow) from control and SOCS1 mice and leukemic FLT3-ITD and SOCS1-T2A-FLT3-ITD mice, indicating MPD and ALL, respectively. Data are representative moribund FLT3-ITD and SOCS1-T2A-FLT3-ITD mice that died of MPD or ALL. (C) Comparison of mean survival (normalized to FLT3-ITD) from 2 independent transplantation experiments. Similar latency in ALL and significantly different latency in the MPD phenotype are shown. (D) Mechanistic model for the cooperation of SOCS1 in FLT3-ITD–mediated leukemogenesis. As we have described recently, constitutively active oncogenic FLT3-ITD directly phosphorylates STAT5 from the endoplasmic reticulum.21,22 On phosphorylation, STAT5 dimerizes and translocates into nucleus and induces transcription of STAT5 target genes that includes SOCS1. FLT3-ITD–induced SOCS1 expression neither affects FLT3-ITD–mediated proliferative signals nor STAT5 activation, whereas it terminates cytokine-dependent signals. Thereby, SOCS proteins cooperate with FLT3-ITD by “shielding” the cell from cytokine control (eg, IFNα, IFN-γ, IL-3, and IL-6; *P < .05).
Histopathologic examination of organs from FLT3-ITD and SOCS1-T2A-FLT3-ITD mice demonstrate blast infiltrations into spleen and liver, with destruction of the natural organ architecture (Figure 6B). Furthermore, there was a clear increase in bone marrow cellularity (with consequent loss of bone marrow adipocytes) in both groups of mice compared with controls (Figure 6B). The detailed FACS analysis of infiltrated cells from spleen and bone marrow is shown in Table 2. FLT3-ITD and the SOCS1-T2A-FLT3-ITD mice that developed MPD have infiltration of either GFP+/Gr1+ or GFP+/MPO+ cells (and often an aberrant CD19 coexpression of weak intensity) in the bone marrow and in the spleen, whereas FLT3-ITD and SOCS1-T2A-FLT3-ITD B-ALL mice show GFP+/CD19+ blasts in the bone marrow and spleen (Table 2).
Bone marrow and splenocyte analysis of transplanted mice at the time of death
| . | No. mice, phenotype . | Bone marrow . | Splenocytes . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % GFP+ . | % CD19+ . | % GR1+ . | % B220+ . | % MPO+ . | % CD117+ . | % GFP+ . | % CD19+ . | % GR1+ . | % CD117+ . | ||
| Control | 7 None | 1.4 (0.7-6.7) | 19.9 (7.7-27.2) | 66.8 (41.6-82.1) | 24.9 (9.9-45.3) | 0.5 (0.2-2.9) | 12.8 (4.0-22.6) | 2.3 (0.8-11.4) | 42.6 (23.6-53.0) | 18.7 (8.8-24.2) | 4.4 (1.1-9.1) |
| SOCS1 | 10 None | 0.3 (0.0-1.4) | 33.4 (12.1-43.1) | 58.5 (40.6-85.3) | 34.4 (19.9-49.2) | 7.2 (0.3-18.5) | 0.3 (0.0-1.4) | 56.2 (24.1-61.4) | 11.4 (1.4-25.9) | 3.2 (0.2-4.3) | |
| FLT3-ITD | 12 MPD | 91.3 (21.8-97,6) | 94.7* (8.2-98.6) | 0.0 (0.0-66.4) | 73.9 (11.2-84.4) | 12.4 (6.4-20.0) | 0.3 (0.0-6.2) | 82.6 (45.4-95) | 93.9* (1.9-97.6) | 0.0 (0.0-66.7) | 0.2 (0.0-7.4) |
| 6 ALL | 90.6 (36.6-94.0) | 96.8 (69.7-97.5) | 0.0 (0.0-1.8) | 75.8 (38.9-83.5) | 7.2 (6.7-10.0) | 0.2 (0.1-1.5) | 76.2 (26.1-84.9) | 94.2 (13.3-96.2) | 0.1 (0.0-6.9) | 0.1 (0.1-7.5) | |
| SOCS1-T2A-FLT3-ITD | 11 MPD | 78.9 (22.6-95.2) | 92.8* (5.1-99.2) | 0.0 (0.0-56.2) | 87.0 (3.1-93.4) | 10.2 (8.7-78.0) | 0.2 (0.0-2.5) | 63.9 (47.8-92.4) | 94.8* (2.6-98.8) | 0.2 (0.0-38.9) | 0.6 (0.1-2.6) |
| 11 ALL | 89.2 (39.8-96.9) | 94.7 (74.6-99.1) | 0.1 (0.0-19.3) | 80.7 (12.9-93.8) | 4.2 (2.9-6.9) | 0.2 (0.0-3.8) | 89.3 (24.2-94.3) | 94.8 (87.4-98.8) | 0.2 (0.0-7.5) | 0.5 (2.5-6.0) | |
| . | No. mice, phenotype . | Bone marrow . | Splenocytes . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % GFP+ . | % CD19+ . | % GR1+ . | % B220+ . | % MPO+ . | % CD117+ . | % GFP+ . | % CD19+ . | % GR1+ . | % CD117+ . | ||
| Control | 7 None | 1.4 (0.7-6.7) | 19.9 (7.7-27.2) | 66.8 (41.6-82.1) | 24.9 (9.9-45.3) | 0.5 (0.2-2.9) | 12.8 (4.0-22.6) | 2.3 (0.8-11.4) | 42.6 (23.6-53.0) | 18.7 (8.8-24.2) | 4.4 (1.1-9.1) |
| SOCS1 | 10 None | 0.3 (0.0-1.4) | 33.4 (12.1-43.1) | 58.5 (40.6-85.3) | 34.4 (19.9-49.2) | 7.2 (0.3-18.5) | 0.3 (0.0-1.4) | 56.2 (24.1-61.4) | 11.4 (1.4-25.9) | 3.2 (0.2-4.3) | |
| FLT3-ITD | 12 MPD | 91.3 (21.8-97,6) | 94.7* (8.2-98.6) | 0.0 (0.0-66.4) | 73.9 (11.2-84.4) | 12.4 (6.4-20.0) | 0.3 (0.0-6.2) | 82.6 (45.4-95) | 93.9* (1.9-97.6) | 0.0 (0.0-66.7) | 0.2 (0.0-7.4) |
| 6 ALL | 90.6 (36.6-94.0) | 96.8 (69.7-97.5) | 0.0 (0.0-1.8) | 75.8 (38.9-83.5) | 7.2 (6.7-10.0) | 0.2 (0.1-1.5) | 76.2 (26.1-84.9) | 94.2 (13.3-96.2) | 0.1 (0.0-6.9) | 0.1 (0.1-7.5) | |
| SOCS1-T2A-FLT3-ITD | 11 MPD | 78.9 (22.6-95.2) | 92.8* (5.1-99.2) | 0.0 (0.0-56.2) | 87.0 (3.1-93.4) | 10.2 (8.7-78.0) | 0.2 (0.0-2.5) | 63.9 (47.8-92.4) | 94.8* (2.6-98.8) | 0.2 (0.0-38.9) | 0.6 (0.1-2.6) |
| 11 ALL | 89.2 (39.8-96.9) | 94.7 (74.6-99.1) | 0.1 (0.0-19.3) | 80.7 (12.9-93.8) | 4.2 (2.9-6.9) | 0.2 (0.0-3.8) | 89.3 (24.2-94.3) | 94.8 (87.4-98.8) | 0.2 (0.0-7.5) | 0.5 (2.5-6.0) | |
Mice transplanted with bone marrow expressing FLT3-ITD or SOCS1-T2A-FLT3-ITD were sacrificed when moribund, whereas mice transplanted with bone marrow expressing control GFP or SOCS1 were sacrificed at the end of the experiment at day 120. Bone marrow and spleen cells were analyzed for GFP positivity to monitor infiltration of transplants and for surface expression of lineage specific markers CD19 (immature B cells), B220 (B-cell marker), Gr1 (granulocytes), and CD117 (KIT receptor). Bone marrow cells also were stained for MPO (granulocytic marker). Values are presented as median (range).
Weak CD19 coexpression.
Further analysis of median survival times by genotype of transplant and phenotype of disease revealed a surprising correlation: SOCS1 coexpression in SOCS1-T2A-FLT3-ITD–expressing mice did not significantly affect the latency of the B-ALL phenotype compared with FLT3-ITD mice, whereas latency of the MPD phenotype was significantly shorter in SOCS1-T2A-FLT3-ITD mice compared with FLT3-ITD mice (P < .05; Figure 6C).
Based on these data, we propose a model in which mutant FLT3-ITD up-regulates SOCS family proteins to counteract cytokine signaling to escape from differentiation and antiproliferative control. By abrogating these external signals, the proproliferative and survival signals are dictated by oncogenic FLT3-ITD (Figure 6D).
Discussion
Aberrant activation of oncogenic receptor tyrosine kinases and deregulation of downstream STAT signaling cascades have been described for all hematologic malignancies studied to date. However, the exact mechanism of transformation is only partially understood. In particular, it remains unclear how transformed cells escape the tight regulatory cytokine networks that normally control the cell cycle, survival, and lineage choice of hematopoietic stem and progenitor cells.33
Several biologic differences between FLT3-ITD– and FL-stimulated FLT3-WT have been described to date, including the striking ability of FLT3-ITD to cause colony growth in semisolid methylcellulose—as an in vitro surrogate for transformation—but FL-stimulated FLT3-WT does not.10,11,34–38 Importantly, FLT3-ITD (but not ligand-activated FLT3-WT) activates STAT5 and induces expression of its target genes.11 By analyzing global phosphoproteomic changes induced by FLT3-ITD, we have recently found that inappropriate activation of FLT3-ITD in the biosynthetic compartment (endoplasmic reticulum) causes aberrant STAT5 activation.22 However, contribution of STAT5 target genes in FLT3-ITD–mediated transformation remained unclear. We have now performed a systematic expression analysis of the STAT5 target genes CIS, SOCS1, SOCS2, and SOCS3 (Figure 1). These experiments consistently demonstrated elevated expression of SOCS family members in human FLT3-ITD–positive AML cell lines in a kinase-dependent manner (Figure 1A-B), in murine lymphoid and myeloid cell lines overexpressing FLT3-ITD (Figure 1D-E) and in retrovirally transduced primary murine bone marrow (Figure 1F). Importantly, the analysis of human AML samples has revealed a significant increase of SOCS1 (but not SOCS3) expression in AML samples with FLT3-ITD compared with samples with FLT3-WT (Figure 1G-H). Importantly, increased SOCS1 protein expression also was shown (Figure 1C). These data demonstrate a consistent pattern of increased SOCS1 expression in the presence of FLT3-ITD, and to a lesser degree, of other SOCS family members. Similar observations were made in TEL-Jak2–39 and v-Abl–overexpressing cell lines.40
We have previously shown that FLT3-ITD phosphorylates STAT5 directly, independently of Jak kinase family members.21 We therefore analyzed the biologic effects of SOCS1 overexpression in the presence or absence of FLT3-ITD using a T2A expression construct that allows for equimolar expression of both SOCS1 and FLT3-ITD in the same target cell.26 In the 32D cell line model, SOCS1 overexpression protected FLT3-ITD from the antiproliferative effects of IFN-α and IFN-γ (Figure 2B). FLT3-ITD kinase activity was inhibited by PKC412 that was partially rescued by IL-3 and attributes the PKC412 effect specific to FLT3 kinase inhibition. SOCS1 overexpression abrogated the IFN-mediated STAT1 phosphorylation (Figure 2C), because SOCS1 is known to inhibit STAT1 activation downstream of IFN-α, IFN-β, and IFN-γ.41,42 In addition, IFN-α– and IFN-γ–induced STAT1 activation was diminished in 32D cells expressing FLT3-ITD alone, presumably because of low level expression of endogenous SOCS1 (Figure 2C).
As shown previously,21 FLT3-ITD was resistant to SOCS1 overexpression, whereas expression of SOCS1 alone caused a rapid loss of cells that cannot be rescued by IL-3 treatment (Figure 2D-E). These experiments determined the percentage of GFP-positive cells over several days, with GFP-negative cells serving as controls under identical growth conditions. In the absence of IL-3, 32D cells expressing FLT3-ITD grow independently of IL-3 irrespective of the presence of SOCS1, showing that FLT3-ITD growth is resistant to SOCS1 (Figure 2D). After stimulation with IL-3, cells expressing FLT3-ITD grow for 2 reasons: their growth is stimulated by the oncogene FLT3-ITD and the proproliferative cytokine IL-3 in this IL-3–dependent 32D cell line, explaining why the percentage of GFP-positive cells slowly increases over time (Figure 2 open triangles). When SOCS1 is overexpressed in addition to FLT3-ITD (SOCS1-T2A-FLT3-ITD) in the presence of IL3, the overexpressed SOCS1 inhibits IL-3 signaling, abolishing the growth advantage seen in FLT3-ITD–expressing cells.
In addition, in the absence of FLT3-ITD, we could not obtain 32D cells stably expressing SOCS1 (data not shown), consistent with similar observation made in the IL-3–dependent myeloid cell line FDC-P1.43 Furthermore, coexpression of SOCS1 with FLT3-ITD did not alter constitutively activated signaling pathways compared with FLT3-ITD alone, as measured by phospo-AKT, phospho-ERK, and phospho-STAT5 levels (Figure 2A).
To address the role of SOCS1 in a more physiologic setting, further experiments were performed in primary murine bone marrow cells (Figures 3,Figure 4,Figure 5–6). As seen by others for M-CSF43 and G-CSF,44 overexpression of SOCS1 led to smaller (data not shown) and fewer colonies under proproliferative conditions with IL-3, IL-6, and SCF, compared with controls (Figure 3C). In the presence of FLT3-ITD, however, coexpression of SOCS1 did not affect colony growth under these conditions (Figure 3C left). Importantly, under the conditions with IFN-α and IFN-γ, coexpression of SOCS1 with FLT3-ITD led to significantly more colonies compared with FLT3-ITD alone (Figure 3C middle and right). Because FLT3-ITD induces SOCS1 expression (Figure 3A-B), colony formation was observed in the presence of IFN-α and IFN-γ; however, when SOCS1 is overexpressed (SOCS1-T2A-FLT3-ITD), colony formation is further enhanced in a dose-dependent manner (Figure 3C). Because SOCS1 has been shown to be the major inhibitor of IFN-γ signaling,41 these data suggest that coexpression of (either endogenous or exogenous) SOCS1 protects FLT3-ITD from the antiproliferative and possibly deleterious effects of IFN-α and IFN-γ. These findings contrast to previous reports that have demonstrated that SOCS1 can inhibit transformation by TEL-Jak2 and by oncogenic VAV through their ubiquitination and proteosomal degradation,16,45,46 suggesting that SOCS1 can act as a substrate-specific recognition component of the E3 ubiquitin ligase complex.47
It had been shown previously that FLT3-ITD overexpression in primary bone marrow does not increase replating efficiency.48 We postulated that the cytokine escape mechanism in SOCS1-overexpressing cells may contribute to self-renewal of hematopoietic stem cells; however, replating assays showed no significant differences between FLT3-ITD alone and SOCS1/FLT3-ITD conditions (supplemental Figure 1). Importantly, knockdown experiments for SOCS1 revealed an important role for SOCS1 in FLT3-ITD–mediated colony growth under cytokine stimulation (Figure 3D-E), further underlining the physiologic role of SOCS1 in this context.
We next asked whether coexpression of SOCS1 would affect the transforming phenotype of FLT3-ITD in vivo and made again use of our FLT3-ITD and SOCS1-T2A-FLT3-ITD constructs, which leads to similar FLT3-ITD protein levels (Figure 3B). It has been shown previously that in bone marrow transplantation models and in knockin models FLT3-ITD leads to the development of an MPD or lymphoid disease.29,30,32,48
As shown in the posttransplantation follow-up, mice transplanted with either FLT3-ITD– or SOCS1-T2A-FLT3-ITD–expressing bone marrow revealed significantly higher GFP-positive cells and higher WBC counts, with a tendency toward lower hemoglobin and platelet levels (Figure 4A-D). In addition, the biologic advantage of GFP-positive cells seemed the most pronounced in the SOCS1-T2A-FLT3-ITD arm. On the contrary, as expected, SOCS1-expressing cells rapidly disappeared from the peripheral blood, demonstrating a pronounced biologic disadvantage (Figure 4A). The survival data demonstrate a clearly more aggressive phenotype in those mice coexpressing both SOCS1 and FLT3-ITD, with a shorter latency in MPD development (Figure 6C) and increased liver weight compared with FLT3-ITD alone (Figure 5D). Disease was manifested in peripheral blood, spleen, liver, and bone marrow (Tables 1–2). The mice in both groups developed either MPD or ALL with a 100% penetrance, whereas mice transplanted with SOCS1-transduced cells did not develop any disease.
The determination of the MPD phenotype was based on a careful morphologic assessment of peripheral blood, bone marrow (Figure 6A), and tissue sections of spleen, liver, and bone marrow (Figure 6B), as recommended by Kogan et al49 FACS analysis revealed an MPO-positive MPD, with a surprisingly low Gr1 expression and a weak (but consistent) CD19 coexpression. The reasons for this are unknown and require further investigation. However, lymphoid characteristics of myeloid diseases have been observed by numerous groups in several mouse models.50,51
Previous reports on FLT3-ITD have mostly used the MSCV-IRES-GFP retroviral backbone. Several groups have used pMY retroviruses developed by T. Kitamura. These differ in their 5′ long terminal repeat and have 2 enhancer elements (whereas MSCV-IRES-GFP has only 1). Using this retrovirus, Nakajima et al31 have observed a B-ALL phenotype. We speculate (although not formerly proven) that the B-ALL phenotype observed may be associated with the use of pMY vectors.
Previously, in a similar study, coexpression of SOCS1 prolonged the latency of disease by TEL-Jak2 in vivo in a bone marrow transplant model. This was mainly attributed to the degradation of TEL-Jak2 and inhibition Jak kinase activity, leading to diminished ERK activation by exogenously expressed SOCS1.46 In contrast, in our model we neither observed degradation of FLT3-ITD nor diminished ERK activation by SOCS1 coexpression. Thus, SOCS1 functions in a context-dependent manner. This is because FLT3-ITD directly activates STAT5 and does not activate Jak kinases, a prerequisite for SOCS1-mediated degradation.21 When we carefully analyzed the median survival of phenotypes, the decreased median survival in the SOCS1 coexpressing group was exclusively found in the mice that developed the MPD phenotype but not by the ALL phenotype. This suggests that SOCS1 might play a particularly important role in the myeloid compartment. Taken together, our data provide a proof of principle that induction of SOCS proteins by FLT3-ITD is a mechanism to escape the external cytokine control and contributes to leukemogenesis. Our model reconciles the seemingly contradictory findings of increased expression of SOCS proteins with tumor suppressor function in the context of the oncogenic kinase FLT3-ITD (Figure 6D). We predict this may be a general mechanism applicable to many other oncogenes that activate the STAT5 pathway and for which Jak kinase activity is dispensable for the transformation, such as breakpoint cluster region-ABL and TEL-PDGFβR.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marion Rensinghoff, Beate Lindtner, Astrid Eichler, and Ramona Goelzer for technical assistance; Olesya Vakrusheva, Benjamin August, and Claudia Oancea for help in performing animal experiments; and Martin Ruthardt, Reinhard Henschler, Michael Rieger, and Rolf Marschalek for insightful discussions.
The authors thank the German Cancer Aid (Deutsche Krebshilfe) for financial support (108699 and 108688). The Center for Protein Research is funded by a generous donation from the Novo Nordisk Foundation.
Authorship
Contribution: P.N.G.R., C.C., H.S., and C.H.B. conceptualized the idea and designed the research; P.N.G.R., B.S., S.S., and C.H.B. performed the experiments; P.N.G.R., B.S., C.C., M.G., C.M.-T., W.E.B., H.S., and C.H.B. interpreted the results and discussed the data; and P.N.G.R. and C.H.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of SAL group members please see the online supplemental Appendix.
Correspondence: Christian H. Brandts or Hubert Serve, Department of Medicine, Hematology/Oncology, Goethe University, Theodor-Stern-Kai 7, D-60590 Frankfurt, Germany; e-mail: brandts@em.uni-frankfurt.de or serve@em.uni-frankfurt.de.
References
Author notes
P.N.G.R. and B.S. contributed equally to the work.

![Figure 2. Coexpression of SOCS1 and FLT3-ITD. (A) Simultaneous equimolar coexpression of SOCS1 and FLT3-ITD does not affect FLT3-ITD–mediated signaling pathways. Western blot analysis of retrovirally transduced stable 32D cells expressing FLT3-ITD or SOCS1-T2A-FLT3-ITD or control (empty vector). Membranes were incubated with indicated phospho-specific antibodies and were reprobed for the nonphospho–specific-antibodies and β-actin to ensure equal loading. Two independent bulk cultures (1 and 2) of SOCS1-T2A-FLT3-ITD were used to exclude effects clonality. (B) Proliferation assay by measurement of DNA synthesis using [3H]thymidine incorporation. The stable 32D cells expressing the indicated constructs were analyzed for their proliferation by [3H]thymidine incorporation as described under “Methods.” Each data point represents the mean of [3H]thymidine incorporation of quadruplicates, with SD as error bars. (C) 32D cells stably expressing either empty vector control, SOCS1-T2A-FLT3-ITD, or FLT3-ITD were stimulated with either IFN-α (1000 U/mL) and IFN-γ (100 ng/mL) for 10 minutes or left unstimulated. Lysates were resolved by SDS-PAGE and immunoblotted for phospho-STAT1 and SOCS1, as indicated. β-actin served as a loading control. (D-E) 32D cells transduced with control, SOCS1, FLT3-ITD, and SOCS1-T2A-FLT3-ITD retroviruses were monitored for GFP-positivity (percentage) every 24 hours by FACS and cultured without IL-3 (D) or with IL-3 (E). Time is plotted on the x-axis, with the percentage of GFP positivity from 3 independent experiments, with SD as error bars, plotted on y-axis (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/120/8/10.1182_blood-2010-08-301416/4/m_zh89991291920002.jpeg?Expires=1770361271&Signature=Gy8Cya8~u4xfAwZ3-l-6zq1bwgXmJN8B6oUhVvMHFXEWLn637IW8Xk7iHoFujS-G~Nmv3KxFsnxfbP49mxKFbuNfPFjnPvowYWm-SHoO6iLK~b0ulO1Nerl9M1l7jtkK4VKTX1Rae-StGOjvARd2qfY9~B6dvfoyHjp4QAUTkOpGBAO4LrQC-lHqsXiEX2cPkAgNHd4Ihl3RzZolOpXkTFEbU4Dl6YykBKtBgPnpxrQxYJcElGtPGqTPYP3X2oIVqe3r95hjkj~7eXbp4~9GOenFNZf9aIJ5a0eZavwSJAYDKvcH5XD8PEaFIv-aA8nntcnmqjsGc69DI32Gqo61nA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal