Key Points
We provide a functional analysis of IGF1R expression in primary human B-CLL.
Sorafenib reduces IGF1R expression in B-CLL.
Abstract
The receptor tyrosine kinase (RTK) insulin-like growth factor-1 receptor (IGF1R) is implicated in various tumor entities including chronic lymphocytic leukemia (CLL), but its functional significance in this disease remains poorly characterized. Here, we show that the IGF1R protein is overexpressed in various CLL subsets, suggesting a contribution to CLL pathology. Indeed, we show that IGF1R knockdown in primary human CLL cells compromised their viability. Likewise, IGF1R inhibition with 3 structurally distinct compounds induced apoptosis, even in the presence of protective stroma components. Furthermore, IGF1R inhibition effectively limited CLL development in Eμ-TCL1 transgenic mice and of primary human CLL xenografts. In agreement with its prosurvival function, IGF1R inhibition affected the phosphorylation and/or expression of multiple signaling proteins. The multikinase inhibitor sorafenib yielded similar effects on these signaling elements as IGF1R inhibitors. Indeed, IGF1R appears to be a direct sorafenib target because sorafenib decreased IGF1R expression and phosphorylation, counteracted insulin-like growth factor-1 (IGF-1) binding to CLL cells, and lowered the in vitro kinase activity of recombinant, purified IGF1R. Thus, we demonstrate that blockade of IGF1R-mediated signaling represents a novel mechanism of action for sorafenib in CLL. Importantly, IGF1R inhibitors compromise CLL viability in their microenvironment context, implicating this RTK as a promising therapeutic target.
Introduction
B-cell CLL, the most common adult leukemia in Western countries, is characterized by a progressive accumulation of clonal CD5+ B-lymphocytes in the peripheral blood, bone marrow (BM), and secondary lymphoid tissue.1,2 In addition to genetic determinants, microenvironmental crosstalk and B-cell antigen receptor (BCR) signaling play a major role in CLL pathogenesis.3-6 Despite encouraging advances in treatment, the disease remains incurable with standard therapy, warranting further efforts to identify novel therapeutic strategies to treat CLL. Recently, preclinical and early clinical data on the use of kinase inhibitors have sparked new hope in the treatment of CLL.7 Among these, inhibitors of key kinases of the BCR signaling pathway targeting spleen tyrosine kinase (SYK), phosphoinositide 3′-kinase (PI3K), and Bruton’s tyrosine kinase (BTK) appear to be highly active in relapsed refractory CLL, independent of high-risk disease markers such as del 17p.8-13 Furthermore, the crosstalk between CLL cells and the microenvironment, which is predominantly mediated via chemokine and integrine receptors, is well characterized and plays an important role in CLL cell survival.14 In this interplay, the role of receptor tyrosine kinases (RTKs) that are not directly involved in BCR signaling, but are able to bind autocrine or stroma cell–derived ligands to maintain CLL survival, is still poorly defined in CLL15,16 compared with other tumor entities.
The IGF1R (or CD221) is a RTK primarily activated by its cognate ligands, IGF-1 and II secreted by BM stromal cells, and, albeit at a much lower affinity, insulin.17 IGF1R primarily signals through insulin receptor substrate 1 (IRS-1) and Shc,18,19 which in turn activate the RAS-RAF-MEK-ERK kinase pathway that stimulates cellular proliferation, and the phosphinositide-3 kinase (PI3K)-Akt-mammalian target of rapamycin (mTOR) pathway that predominantly mediates cell survival.17 IGF1R is expressed in various solid tumor entities and hematologic malignancies including CLL.20 The implication of IGF1R in the development and progression of human cancer has led to its current evaluation in clinical trials as a potential therapeutic target for solid tumors. In contrast, the functional significance of IGF1R expression in CLL remains ill defined.
The multikinase inhibitor sorafenib (Nexavar; BAY43-9006), targeting RAF as well as platelet-derived growth factor receptor (PDGFR), KIT, FMS-like tyrosine kinase 3 (FLT3), and vascular endothelial growth factor receptor (VEGFR), has been approved for the treatment of renal cell carcinoma and hepatocellular carcinoma. Recent studies have shown that CLL cells might also be susceptible to this compound.15,16,21-23 However, little is known about its precise mode of action in CLL cells, in particular which signaling pathways affected by sorafenib might account for the observed cell death. The aim of the present study was to investigate the functional significance of IGF1R expression on CLL cells and to evaluate IGF1R as a potential target of sorafenib. Here, we report the enhanced surface expression of IGF1R in CLL compared with healthy donors. IGF1R inhibition induced apoptosis in CLL cells in vitro, independent of the presence of protective stromal cells, and in vivo. This identifies IGF1R as a promising target for therapeutic approaches and proposes IGF1R inhibitors for clinical assessment in treating CLL. Moreover, our results provide a novel mechanism of action for the multikinase inhibitor sorafenib in CLL cells by blocking IGF1R-mediated signaling.
Materials and methods
CLL samples
This study was approved by the Institutional Review Board of University Medical Center Freiburg (441/12). Peripheral blood samples were obtained with informed consent in accordance with the Declaration of Helsinki from B-CLL patients who were either untreated or had not been treated for at least 6 months (Table 1). All cases were characterized for IgVH mutational status,24,25 disease stage according to Rai criteria,26 history of treatment, and genetic aberrations. The 13q group contained only this genetic aberration and not 17p, 11q, or trisomy 12. Peripheral blood mononuclear cells (PBMCs) were separated by Ficoll gradient centrifugation. For CLL samples containing <90% tumor cells according to flow cytometric analysis with anti-CD19 (BioLegend) and anti-CD5 (BD Biosciences) (Beckman Coulter), CD19-B cells were isolated by negative selection (B-cell isolation kit II; Miltenyi Biotec). Cells were either fresh or cryopreserved in fetal calf serum (FCS)/10% dimethyl sulfoxide (DMSO) until use.
Patient characteristics
| No . | Sex . | Age . | Rai stage . | CD38 . | IgVH . | Previous therapies . | Genetic aberrations . |
|---|---|---|---|---|---|---|---|
| 1 | Male | 79 | I | neg | UM | A | del 17p13, complex karyotype |
| 2 | Male | 67 | III | neg | UM | A | del 17p13, complex karyotype |
| 3 | Female | 71 | IV | neg | UM | RB | del 17p13, complex karyotype |
| 4 | Female | 60 | IV | pos | M | Clb, ClbP | del 17p13, t trisomy12, del14q24.1-q32.33 |
| 5 | Male | 68 | II | pos | M | Clb, FC | del 17p13, trisomy 12 |
| 6 | Male | 62 | II | pos | UM | Clb, ClbP | del 17p13, complex karyotype |
| 7 | Male | 54 | II | neg | UM | RB, FCR | del 11q22, del 13q14 |
| 8 | Male | 78 | II | pos | UM | RB | del 13q14, del 11q14.3-23.3, gain 15q22.31-26.3 |
| 9 | Male | 72 | II | neg | UM | R-Clb, RB | 11q11.1-q23.3, del 13q14 |
| 10 | Male | 71 | III | neg | M | ClbP, R | del 11q22.3-q23.3, del 15q26.1-q26.2 |
| 11 | Female | 68 | 0 | ND | M | ClbP, RB | del 11q14.1-q23.3, complex karyotype |
| 12 | Male | 62 | IV | neg | UM | F, FCR | |
| 13 | Male | 67 | II | neg | UM | FCR, R-CHOP | del 11q22, trisomy 12, complex karyotype |
| 14 | Male | 49 | II | neg | UM | FC, RB | del 11q14.1-q23.3, complex karyotype |
| 15 | Male | 74 | 0 | ND | M | none | del 5q31.3, del 13q14, del 13q31.1, del 22q11.21 |
| 16 | Male | 49 | II | pos | UM | FCR | trisomy 12, trisomy 19 |
| 17 | Male | 62 | II | neg | M | Clb | trisomy 12, del 13q14, biclonal |
| 18 | Female | 77 | I | neg | M | F, B | Trisomy 12, trisomy 22 |
| 19 | Female | 79 | IV | pos | M | Clb | trisomy 12, del 13q14, biclonal |
| 20 | Male | 70 | I | pos | UM | FCR | trisomy 12 |
| 21 | Male | 71 | III | pos | UM | ClbP | del 13q14 |
| 22 | Male | 55 | II | neg | M | none | trisomy 12, del 13q14 |
| 23 | Female | 66 | II | pos | M | Clb | trisomy 12, del 13q14 |
| 24 | Male | 72 | IV | neg | M | ClbP | del 13q14 |
| 25 | Male | 79 | IV | neg | M | ClbP, COP | del 13q14 |
| 26 | Female | 84 | 0 | neg | M | Clb | del 13q14 |
| 27 | Male | 60 | II | pos | UM | ClbP | del 13q14 |
| 28 | Male | 46 | IV | neg | M | none | del 13q |
| 29 | Male | 67 | II | neg | M | none | del 13q14 |
| 30 | Male | 75 | 0 | neg | M | Clb | del 13q14 bi-allelic |
| 31 | Male | 69 | II | neg | M | none | del 13q14 |
| 32 | Male | 77 | 0 | neg | M | none | del 13q14 bi-allelic |
| 33 | Male | 74 | 0 | neg | UM | Clb | del 17p13, complex karyotype |
| 34 | Male | 59 | II | neg | UM | A | del 17p13, del 13q14 |
| 35 | Female | 72 | 0 | pos | M | ClbP | del 17p13 |
| 36 | Male | 61 | III | neg | M | ClbP | del 17p13, complex karyotype |
| 37 | Male | 78 | II | pos | M | none | trisomy 12 |
| 38 | Male | 72 | II | neg | UM | Clb, ClbP | trisomy 12 |
| 39 | Female | 47 | II | neg | M | none | del 13q14 |
| 40 | Male | 74 | 0 | neg | M | none | del 13q14 |
| No . | Sex . | Age . | Rai stage . | CD38 . | IgVH . | Previous therapies . | Genetic aberrations . |
|---|---|---|---|---|---|---|---|
| 1 | Male | 79 | I | neg | UM | A | del 17p13, complex karyotype |
| 2 | Male | 67 | III | neg | UM | A | del 17p13, complex karyotype |
| 3 | Female | 71 | IV | neg | UM | RB | del 17p13, complex karyotype |
| 4 | Female | 60 | IV | pos | M | Clb, ClbP | del 17p13, t trisomy12, del14q24.1-q32.33 |
| 5 | Male | 68 | II | pos | M | Clb, FC | del 17p13, trisomy 12 |
| 6 | Male | 62 | II | pos | UM | Clb, ClbP | del 17p13, complex karyotype |
| 7 | Male | 54 | II | neg | UM | RB, FCR | del 11q22, del 13q14 |
| 8 | Male | 78 | II | pos | UM | RB | del 13q14, del 11q14.3-23.3, gain 15q22.31-26.3 |
| 9 | Male | 72 | II | neg | UM | R-Clb, RB | 11q11.1-q23.3, del 13q14 |
| 10 | Male | 71 | III | neg | M | ClbP, R | del 11q22.3-q23.3, del 15q26.1-q26.2 |
| 11 | Female | 68 | 0 | ND | M | ClbP, RB | del 11q14.1-q23.3, complex karyotype |
| 12 | Male | 62 | IV | neg | UM | F, FCR | |
| 13 | Male | 67 | II | neg | UM | FCR, R-CHOP | del 11q22, trisomy 12, complex karyotype |
| 14 | Male | 49 | II | neg | UM | FC, RB | del 11q14.1-q23.3, complex karyotype |
| 15 | Male | 74 | 0 | ND | M | none | del 5q31.3, del 13q14, del 13q31.1, del 22q11.21 |
| 16 | Male | 49 | II | pos | UM | FCR | trisomy 12, trisomy 19 |
| 17 | Male | 62 | II | neg | M | Clb | trisomy 12, del 13q14, biclonal |
| 18 | Female | 77 | I | neg | M | F, B | Trisomy 12, trisomy 22 |
| 19 | Female | 79 | IV | pos | M | Clb | trisomy 12, del 13q14, biclonal |
| 20 | Male | 70 | I | pos | UM | FCR | trisomy 12 |
| 21 | Male | 71 | III | pos | UM | ClbP | del 13q14 |
| 22 | Male | 55 | II | neg | M | none | trisomy 12, del 13q14 |
| 23 | Female | 66 | II | pos | M | Clb | trisomy 12, del 13q14 |
| 24 | Male | 72 | IV | neg | M | ClbP | del 13q14 |
| 25 | Male | 79 | IV | neg | M | ClbP, COP | del 13q14 |
| 26 | Female | 84 | 0 | neg | M | Clb | del 13q14 |
| 27 | Male | 60 | II | pos | UM | ClbP | del 13q14 |
| 28 | Male | 46 | IV | neg | M | none | del 13q |
| 29 | Male | 67 | II | neg | M | none | del 13q14 |
| 30 | Male | 75 | 0 | neg | M | Clb | del 13q14 bi-allelic |
| 31 | Male | 69 | II | neg | M | none | del 13q14 |
| 32 | Male | 77 | 0 | neg | M | none | del 13q14 bi-allelic |
| 33 | Male | 74 | 0 | neg | UM | Clb | del 17p13, complex karyotype |
| 34 | Male | 59 | II | neg | UM | A | del 17p13, del 13q14 |
| 35 | Female | 72 | 0 | pos | M | ClbP | del 17p13 |
| 36 | Male | 61 | III | neg | M | ClbP | del 17p13, complex karyotype |
| 37 | Male | 78 | II | pos | M | none | trisomy 12 |
| 38 | Male | 72 | II | neg | UM | Clb, ClbP | trisomy 12 |
| 39 | Female | 47 | II | neg | M | none | del 13q14 |
| 40 | Male | 74 | 0 | neg | M | none | del 13q14 |
Complex karyotype: more than 4 aberrations.
A, alemtuzumab; B, bendamustin; Clb, chlorambucil; ClbP, chlorambucil 1 prednisone; COP, cyclophosphamide1 vincristine 1 prednisolone; F, fludarabine; FC, fludarabine 1 cyclophosphamide; FCR, fludarabine1cyclophosphamide1rituximab; M, mutated; ND, not done; R, rituximab; RB, rituximab 1 bendamustin; R-CHOP, rituximab 1 cyclophosphamide 1 doxorubicin1 vincristine 1 prednisolone; R-Clb, rituximab1chlorambucil; UM, unmutated.
Apoptosis assay
CLL cells were cultured in RPMI 1640 (Sigma-Aldrich) supplemented with 10% FCS, 100 U/mL penicillin, 0.1 mg/mL streptomycin at a concentration of 5 × 106 cells/mL in 24-well plates in the presence or absence of varying doses of sorafenib (10 μM); Plexxikon 4720 (10 µM); U0126 (10 µM); and the IGF1R inhibitors AG1024 (15 μM), picropodophyllin (PPP; 1 μM), or linsitinib (1 μM) (all from Selleckchem); or AS605240 (Tocris) for 24 hours. For CXCL12, ICAM or CD49d activation, cells were treated with 100 ng/mL CXCL12, ICAM, or CD49d (R&D Systems). For coculture experiments, M2-10B4 cells27 were cultured for 24 hours before CLL cells were added. For apoptosis analysis, cells were stained with the Annexin-V Apoptosis Detection kit I (BD Biosciences) and assayed by flow cytometry (Dako Cytomation). Results were analyzed with FlowJo 7.6 software (Tree Star, San Carlos, CA) and GraphPad Prism software.
IGF1R and insulin receptor surface expression
CLL cells or B cells isolated from healthy donors were isolated as described before, plated into 24-well plates at a concentration of 5 × 106 cells/mL, and stained for IGF1R and insulin receptor (IR) surface expression (PE anti-human CD221 [IGF1R], Clone 1H7/CD221 and PE-conjugated anti-human CD220 [IR], Clone B6.220; Biolegend) in the presence or absence of AG1024, linsitinib, PPP or sorafenib. Unspecific binding was measured by a PE mouse IgG1, κ (Clone 1H7/CD221; Biolegend) as an isotype control.
Immunoblotting
Healthy B cells and freshly isolated or freshly thawed viable frozen CLL cells were lysed and subjected to western blotting as described previously.28 A list providing all details on the used antibodies can be found in the supplementary data online.
Analysis of the IGF1R pathway after inhibitor treatment
To analyze the effect of IGF1R inhibitor and sorafenib treatment on the IGF1R pathway, CLL cells were plated into 24-well plates at a concentration of 5 × 106 cells/mL and supplemented with sorafenib, AG1024, PPP, or linsitinib, and lysates were generated at different time points (5 minutes, 24 hours) and analyzed by western blotting.28
siRNA-mediated IGF1R knockdown
For siRNA transfection, 7 × 106 primary CLL cells were resuspended in Nucleofector solution (Nucleofector kit V; Amaxa) containing 1 µM nontarget control siRNA (Dharmacon RNA Technologies) or IGF1R siRNA (Santa Cruz Biotechnologies) and transfected using the Amaxa Nucleofector program U-013. Subsequently, cells were plated in a 24-well plate in 1 mL of RPMI/10% FCS. Measurement of cell viability and lysates for immunoblotting were performed 72 hours after transfection.
IGF1R kinase assay
The IGF1R kinase assay (ADP-Glo Kinase Assay) was performed as described in the protocol (Promega) in the presence or absence of AG1024, PPP, or sorafenib.
Murine models and monitoring of tumors
A detailed description of the Eμ-TCL1 and NOD/SCID IL-2-Receptor γ chain−/− (NSG) xenograft models is provided in the supplementary data online.
Statistical analysis
Data are represented as the mean ± standard error of the mean (SEM). Comparisons between parameters were performed using a 2-tailed, paired Student t test. For all analyses, P < .05 was considered statistically significant. Three asterisks indicate a statistically significant difference of P < .0001, two asterisks indicates a statistically significant difference of P < .005), and a single asterisk indicates a statistically significant difference of P < .05.
Results
IGF1 and IGF1R are overexpressed in CLL
To obtain more insight into the role of IGF1R in CLL biology, we first assessed the expression levels of IGF1R (CD221) and the related IR (CD220) on CLL cells and on normal peripheral blood B cells from healthy donors. Interestingly, primary CLL cells displayed significantly increased levels of the RTK IGF1R on their surface (Figure 1A-B). In full agreement with a recent study,29 we observed an upregulation of the IR in our samples as well (Figure 1A). Because IGF1R is less ubiquitously expressed and emerges as a novel important target in tumor diseases,17 we wanted to explore its role in CLL in more detail. The prominent surface expression of IGF1R is also reflected by a western blot analysis of total cellular lysates, which also include IGF1R molecules residing within the endomembrane system (Figure 1B). In contrast to lysates from healthy control B cells, this western blot analysis also revealed the baseline phosphorylation of the IGF1R in most CLL samples, indicating its activation. Moreover, we observed an increased expression of Raf-1, PI3K, and phosphorylated Erk, the key molecules that are affected upon IGF1R signaling,19 in CLL samples compared with healthy B cells (supplemental Figure 1A). Interestingly, we also observed a differential IGF1R expression between samples from different patient subgroups. IGF1R was primarily expressed more strongly in samples of patients with high-risk genetic aberrations, such as del11q and del17p compared with del13q (Figure 1C).
IGF1R and IR expression are enhanced in primary human CLL cells compared with healthy B cells. (A) Healthy B cells or CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were stained for CD221 (IGF1R; left bar graph) and CD220 (IR; right bar graph), and expression was determined by flow cytometry (n = 10). (B) Healthy B cells or CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were lysed and analyzed for receptor expression by western blot analysis. Left: A representative example is shown. Right: Densitometric analysis of all samples (n = 20). (C) CLL B cells purified from freshly isolated or freeze-thawed PBMCs of different prognostic CLL subgroups with different genetic aberrations were lysed and compared for receptor expression by western blot analysis. Left: A representative example is shown. Right: Densitometric analysis of all samples (n = 12; mean ± SEM). (D) Healthy B cells or CLL B cells, NLCs, CD14+ cells, or T cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were lysed and analyzed for IGF-1 expression by western blot analysis. The densitometric analysis is derived from 12 different CLL samples (mean ± SEM). (E) Lymph nodes from healthy donors, CLL patients, breast cancer and prostate tissue underwent immunohistochemistry using a polyclonal rabbit antibody against IGF1R. One representative staining is shown for each group.
IGF1R and IR expression are enhanced in primary human CLL cells compared with healthy B cells. (A) Healthy B cells or CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were stained for CD221 (IGF1R; left bar graph) and CD220 (IR; right bar graph), and expression was determined by flow cytometry (n = 10). (B) Healthy B cells or CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were lysed and analyzed for receptor expression by western blot analysis. Left: A representative example is shown. Right: Densitometric analysis of all samples (n = 20). (C) CLL B cells purified from freshly isolated or freeze-thawed PBMCs of different prognostic CLL subgroups with different genetic aberrations were lysed and compared for receptor expression by western blot analysis. Left: A representative example is shown. Right: Densitometric analysis of all samples (n = 12; mean ± SEM). (D) Healthy B cells or CLL B cells, NLCs, CD14+ cells, or T cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were lysed and analyzed for IGF-1 expression by western blot analysis. The densitometric analysis is derived from 12 different CLL samples (mean ± SEM). (E) Lymph nodes from healthy donors, CLL patients, breast cancer and prostate tissue underwent immunohistochemistry using a polyclonal rabbit antibody against IGF1R. One representative staining is shown for each group.
To distinguish between an autocrine and/or paracrine IGF-1 contribution by the CLL micromilieu, we investigated IGF-1 expression in purified CD14+ macrophages, T cells, nurselike cells, CLL B cells, and healthy B cells. We could detect IGF-1 in all cell types except in healthy B cells. Interestingly, CLL cell lysates of patients with del13q showed a lower IGF-1 expression compared with protein extracted from cells of patients with high-risk genetic features (Figure 1D). In addition, immunohistochemical analysis of IGF1R expression in lymph nodes revealed an enhanced expression of IGF1R in most CLL cases compared with non-CLL samples. Breast cancer and prostate tissue samples were used as positive controls (Figure 1E).
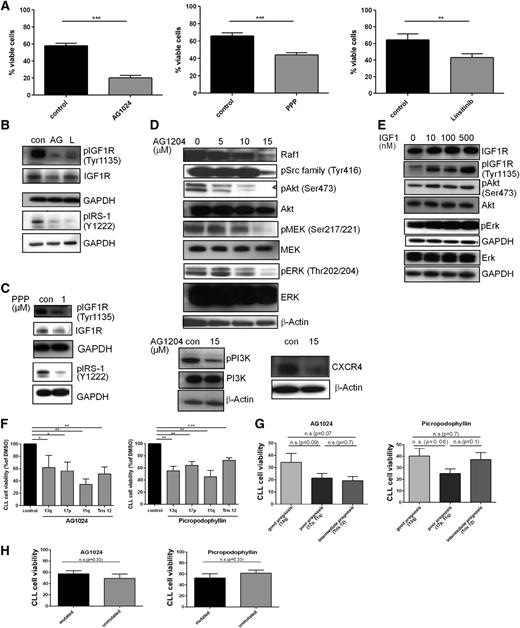
IGF1R inhibitors induce apoptosis in CLL via inhibition of the PI3K/Akt and Raf/Erk pathway independent of coculture with stromal cells
The functional significance of IGF1R overexpression is demonstrated by the observation that 3 structurally unrelated IGF1R inhibitors—AG1024 (also known as tyrphostin), PPP, and linsitinib—significantly reduced the cellular viability of primary CLL cells (Figure 2A) as well as their metabolic activity (supplemental Figure 4B) and reduced levels of phosphorylated IGF1R and IRS-1 (Figure 2B-C). AG1024, which showed the strongest effect on viability, caused a dose-dependent decrease in the levels of phosphorylated Src, PI3K, Akt, MEK, and ERK (Figure 2D), whereas healthy B cells were not affected (supplemental Figure 1B). Likewise, expression of Raf-1 and CXCR4 was also diminished (Figure 2D). PPP was shown to be selective for the IGF1R and does not inhibit the IR,30 whereas AG1024 targets both receptors.31 Treatment of primary CLL cells with recombinant human IGF-1 led to an enhanced IGF1R, Akt, and Erk phosphorylation, confirming that in CLL cells, IGF1R signaling proceeds via activation of the PI3/Akt and MAPK pathways (Figure 2E).
IGF1R inhibition is more efficient in CLL patient cells with a poor prognosis, and IGF1R engagement activates the PI3K and Erk pathways and its inhibition is associated with enhanced cell death and impaired downstream signaling. (A) CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were treated with a single dose of 15 µM AG1024, 1 µM PPP, or 1 µM linsitinib for 24 hours, and cell survival was determined by flow cytometry. Results are shown as mean ± SEM (n = 20). (B) CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were treated with a single dose of 15 µM AG1024 (AG) or 1 µM linsitinib (L) and immunoblotted for the expression of phosphorylated IGF1R and IRS-1 (n = 6). (C) CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were treated with a single dose of 1 µM PPP and were immunoblotted for the expression of phosphorylated IGF1R and IRS-1 (n = 4). (D) CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were treated with 5 to 15 µM AG1024 and underwent western blot analysis using the indicated antibodies. Results are represented as mean ± SEM (n = 10). Supplemental Figure 1C shows the associated densitometric analysis after treatment with 15 µM AG1024. (E) CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were treated with 10 to 500 nM rhIGF-1 and were immunoblotted for the expression of IGF1R, pIGF1R, pAkt, Akt, pERK, and Erk. A representative example from 4 independent experiments is shown. (F) CLL B cells from different patients were stratified according to different genetic aberrations and were compared for their responsiveness toward the IGF1R inhibitors AG1024 or PPP. Results are represented relative to DMSO-treated controls (n = 10). (G) CLL B cells from different patients were stratified according to distinct prognostic CLL subgroups and were compared for their responsiveness toward the IGF1R inhibitors AG1024 or PPP (mean ± SEM, n = 10). (H) CLL B cells with different mutational statuses were compared in their responsiveness toward the IGF1R inhibitors AG1024 or PPP (n = 10).
IGF1R inhibition is more efficient in CLL patient cells with a poor prognosis, and IGF1R engagement activates the PI3K and Erk pathways and its inhibition is associated with enhanced cell death and impaired downstream signaling. (A) CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were treated with a single dose of 15 µM AG1024, 1 µM PPP, or 1 µM linsitinib for 24 hours, and cell survival was determined by flow cytometry. Results are shown as mean ± SEM (n = 20). (B) CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were treated with a single dose of 15 µM AG1024 (AG) or 1 µM linsitinib (L) and immunoblotted for the expression of phosphorylated IGF1R and IRS-1 (n = 6). (C) CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were treated with a single dose of 1 µM PPP and were immunoblotted for the expression of phosphorylated IGF1R and IRS-1 (n = 4). (D) CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were treated with 5 to 15 µM AG1024 and underwent western blot analysis using the indicated antibodies. Results are represented as mean ± SEM (n = 10). Supplemental Figure 1C shows the associated densitometric analysis after treatment with 15 µM AG1024. (E) CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were treated with 10 to 500 nM rhIGF-1 and were immunoblotted for the expression of IGF1R, pIGF1R, pAkt, Akt, pERK, and Erk. A representative example from 4 independent experiments is shown. (F) CLL B cells from different patients were stratified according to different genetic aberrations and were compared for their responsiveness toward the IGF1R inhibitors AG1024 or PPP. Results are represented relative to DMSO-treated controls (n = 10). (G) CLL B cells from different patients were stratified according to distinct prognostic CLL subgroups and were compared for their responsiveness toward the IGF1R inhibitors AG1024 or PPP (mean ± SEM, n = 10). (H) CLL B cells with different mutational statuses were compared in their responsiveness toward the IGF1R inhibitors AG1024 or PPP (n = 10).
Dissection of the responsiveness of different CLL subtypes to IGF1R inhibitors revealed a differential inhibitor response. Although IGF1R was more highly expressed in most samples of patients with high-risk genetic aberrations, such as del11q or del17p compared with del13q (Figure 1C) as described before, CLL cells showed no significant difference in their responsiveness toward the IGF1R inhibitors AG1024 or PPP (Figure 2F) but showed a trend for better responsiveness of CLL cells with high-risk genetic features (poor and intermediate prognosis) toward IGF1R inhibitors (Figure 2G). This observation is in line with our IGF-1 and IGF1R expression analysis (Figure 1C-D). Interestingly, patients with del11q showed the best responsiveness toward AG1024, which may be a result of their enhanced IR expression29 and the fact that AG1024 inhibits both receptors—the IGF1R and IR.31 Furthermore, CLL cells with mutated or unmutated BCR showed no different responsiveness to IGF1R inhibitor treatment (Figure 2H). Thus, primary human CLL cells from all subsets were significantly affected in their viability by IGF1R inhibitors, even those from tumors with a 13q deletion, which displayed the lowest increase in IGF1R overexpression (Figure 1C).
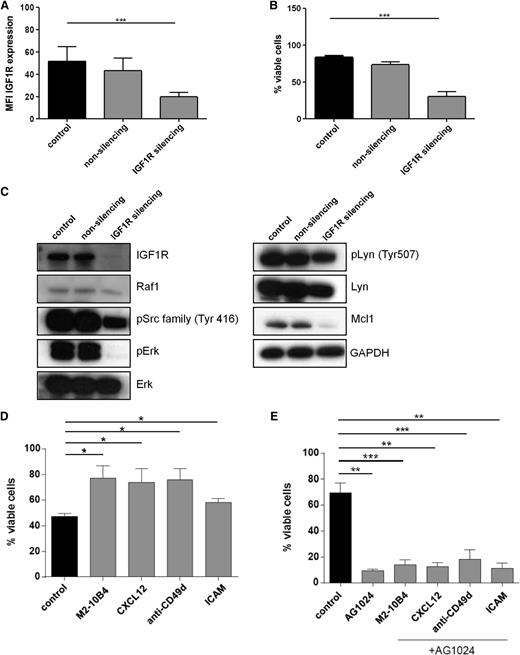
To further establish the functional relevance of IGF1R expression, we transfected primary human CLL cells with either nonsilencing or IGF1R-specific siRNAs (Figure 3A-B). In accordance with the proapoptotic effects of AG1024 on CLL cell viability, the marked reduction of IGF1R expression on the cell surface (Figure 3A) was associated with a significant decrease of cellular survival (Figure 3B). Thus, inhibition of either IGF1R expression or activity impairs the survival of CLL cells under tissue culture conditions. Similar to CLL cells treated with IGF1R inhibitors (Figure 2D), loss of this RTK by siRNA led to a marked reduction of the Erk phosphorylation (Figure 3C). Furthermore, loss of IGF1R expression was accompanied by a strong reduction in the antiapoptotic Mcl-1 protein (Figure 3C). Because we observed a reduced CXCR4 expression and phosphorylation after IGF1R inhibitor treatment (Figure 2D), and CLL cells receive essential growth and survival signals from their microenvironment via their contacts with stromal cells and chemokines such as CXCL12, the ligand of CXCR4,32 we next asked whether these factors were able to modulate the effect of AG1024 in CLL cells. To this end, freshly isolated CLL cells were cultivated in the presence of the murine stromal cell line M2-10B4, the chemokine CXCL12, ICAM, or antibody-mediated activation of integrin-α4 (CD49). Again, AG1024 counteracted the protective effect of these microenvironmental factors (Figure 3D-E).
siRNA-mediated knockdown of IGF1R leads to enhanced cell death of primary human CLL cells and is associated with downregulation of pErk. IGF1R inhibition overcomes prosurvival effects of the microenvironment. (A) CLL B cells were transfected with IGF1R-specific siRNA and analyzed by flow cytometry for receptor expression (mean ± SEM, n = 5). (B) CLL B cells were transfected with IGF1R-specific siRNA and analyzed by flow cytometry for cell survival (mean ± SEM, n = 5). (C) Downregulation of IGF1R and implicated downstream targets were analyzed by immunoblotting. A representative example from 5 independent experiments is shown. (D) CLL B cells were cocultured with the stromal cell line M2-10B4 or treated with CXCL12, anti-CD49d, or ICAM for 24 hours, and cell survival was determined by flow cytometry (mean ± SEM, n = 4). (E) CLL B cells were treated with a single dose of 15 µM AG1024 in the absence or presence of stroma cells, CXCL12, anti-CD49d, or ICAM for 24 hours, and cell survival was determined by flow cytometry (mean ± SEM, n = 4).
siRNA-mediated knockdown of IGF1R leads to enhanced cell death of primary human CLL cells and is associated with downregulation of pErk. IGF1R inhibition overcomes prosurvival effects of the microenvironment. (A) CLL B cells were transfected with IGF1R-specific siRNA and analyzed by flow cytometry for receptor expression (mean ± SEM, n = 5). (B) CLL B cells were transfected with IGF1R-specific siRNA and analyzed by flow cytometry for cell survival (mean ± SEM, n = 5). (C) Downregulation of IGF1R and implicated downstream targets were analyzed by immunoblotting. A representative example from 5 independent experiments is shown. (D) CLL B cells were cocultured with the stromal cell line M2-10B4 or treated with CXCL12, anti-CD49d, or ICAM for 24 hours, and cell survival was determined by flow cytometry (mean ± SEM, n = 4). (E) CLL B cells were treated with a single dose of 15 µM AG1024 in the absence or presence of stroma cells, CXCL12, anti-CD49d, or ICAM for 24 hours, and cell survival was determined by flow cytometry (mean ± SEM, n = 4).
IGF1R inhibition in TCL1 transgenic mice and NOD/SCID common γ chain–deficient (NSG) mice
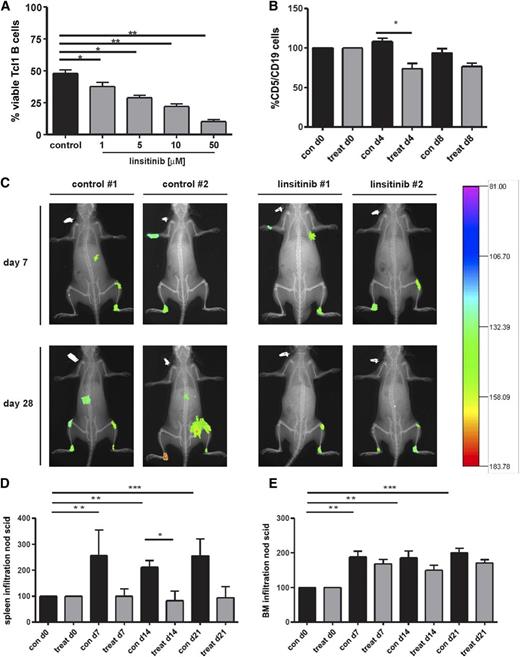
Because linsitinib is an orally active and well-tolerated compound33,34 and is tested in several clinical trials, we next evaluated its effect on the viability of B cells from Eµ-Tcl1 transgenic mice. This murine CLL model develops a monoclonal B-cell lymphocytosis resembling the course and phenotype of IgH-unmutated human CLL.35,36 Indeed, linsitinib induced cell death of CD5/CD19 double-positive peripheral blood cells in vitro in a dose-dependent manner (Figure 4A) and a small but significant decrease in murine Tcl-1 cells in vivo (Figure 4B). Importantly, linsitinib displayed an in vivo efficacy in NOD SCID mice transplanted with human CLL cells. NOD SCID mice treated with linsitinib showed an impaired CLL establishment accompanied by reduced tumor size and photon emission intensity (Figure 4C). Furthermore, during the experimental course after CLL cell engraftment, spleen infiltration was significantly enhanced in control mice, whereas linsitinib-treated mice showed no enhancement in spleen infiltration (Figure 4D). Likewise, BM infiltration was significantly enhanced in control mice, whereas linsitinib-treated mice showed an impaired BM infiltration compared with untreated mice (Figure 4E). We also observed a nonsignificant increase in CD45+ cells into the peripheral blood after 4 days of linsitinib and ibrutinib treatment, similar to the lymphocytosis observed in Tcl1 transgenic mice after ibrutinib treatment.37 However, this lymphocytosis disappeared after 7 days of inhibitor treatment (supplemental Figure 2B).
Oral administration of the IGF1R inhibitor linsitinib decreases the amount of CD5/CD19+ cells of Eµ-Tcl1 transgenic mice in vivo and reduces tumor engraftment, spleen, and BM infiltration in NOD/SCID common γ chain–deficient (NSG) mice. (A) B cells (CD5+/CD19+) purified from the spleens of Eµ-Tcl1 transgenic mice were treated with 1 to 50 µM linsitinib for 24 hours, and cell survival was determined by flow cytometry (mean ± SEM, n = 10). (B) Female and male Eµ-Tcl1 transgenic mice were randomized into 2 groups of 5 animals each and treated with 25 mg/kg linsitinib by oral gavage once per day for 8 days. The amount of CD5+/CD19+ cells was assessed at days 0, 4, and 8 by collecting tail vein blood and staining for CD5 and CD19. Results are represented relative to vehicle-treated control mice (n = 5 per group). (C) NSG mice were injected with 1 × 108 B-CLL patient-derived PBMCs intravenously and 1 × 108 PBMCs intraperitoneally. Seven days thereafter, mice were randomized into 2 groups of 6 animals each, treated with ClinOleic 20% (Baxter) (10 mL/kg per day orally on days 7-19) or linsitinib (25 mg/kg per day orally on days 7-19; Selleckchem), and tumor size and intensity was measured at different time points. After antibody injection, mice were anesthetized by isoflurane inhalation and images were taken using a Kodak in vivo imaging system (Kodak Image Station in vivo FX). In addition, animals were radiographed and the 2 pictures merged for optimal localization of the fluorescent region. Two representative images for the control and treatment groups are shown (n = 6 per group). (D-E) NSG mice were injected with 1 × 108 B-CLL patient-derived PBMCs intravenously and 1 × 108 PBMCs intraperitoneally. Seven days thereafter, mice were randomized into 2 groups of 6 animals each and treated with ClinOleic 20% (Baxter) (10 mL/kg per day orally on days 7-19) or linsitinib (25 mg/kg per day orally on days 7-19; Selleckchem), and cell engraftment was assessed in the spleen (D) and BM (E). Tumor cell growth repression was calculated as the reduction of human tumor cells compared with untreated mice (n = 6 per group).
Oral administration of the IGF1R inhibitor linsitinib decreases the amount of CD5/CD19+ cells of Eµ-Tcl1 transgenic mice in vivo and reduces tumor engraftment, spleen, and BM infiltration in NOD/SCID common γ chain–deficient (NSG) mice. (A) B cells (CD5+/CD19+) purified from the spleens of Eµ-Tcl1 transgenic mice were treated with 1 to 50 µM linsitinib for 24 hours, and cell survival was determined by flow cytometry (mean ± SEM, n = 10). (B) Female and male Eµ-Tcl1 transgenic mice were randomized into 2 groups of 5 animals each and treated with 25 mg/kg linsitinib by oral gavage once per day for 8 days. The amount of CD5+/CD19+ cells was assessed at days 0, 4, and 8 by collecting tail vein blood and staining for CD5 and CD19. Results are represented relative to vehicle-treated control mice (n = 5 per group). (C) NSG mice were injected with 1 × 108 B-CLL patient-derived PBMCs intravenously and 1 × 108 PBMCs intraperitoneally. Seven days thereafter, mice were randomized into 2 groups of 6 animals each, treated with ClinOleic 20% (Baxter) (10 mL/kg per day orally on days 7-19) or linsitinib (25 mg/kg per day orally on days 7-19; Selleckchem), and tumor size and intensity was measured at different time points. After antibody injection, mice were anesthetized by isoflurane inhalation and images were taken using a Kodak in vivo imaging system (Kodak Image Station in vivo FX). In addition, animals were radiographed and the 2 pictures merged for optimal localization of the fluorescent region. Two representative images for the control and treatment groups are shown (n = 6 per group). (D-E) NSG mice were injected with 1 × 108 B-CLL patient-derived PBMCs intravenously and 1 × 108 PBMCs intraperitoneally. Seven days thereafter, mice were randomized into 2 groups of 6 animals each and treated with ClinOleic 20% (Baxter) (10 mL/kg per day orally on days 7-19) or linsitinib (25 mg/kg per day orally on days 7-19; Selleckchem), and cell engraftment was assessed in the spleen (D) and BM (E). Tumor cell growth repression was calculated as the reduction of human tumor cells compared with untreated mice (n = 6 per group).
Pharmacologic dissection of pathways involved in IGF1R signaling
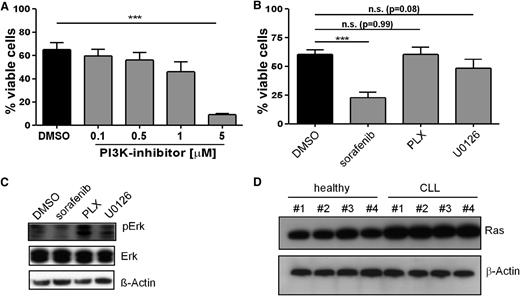
Our observation that loss of IGF1R activity (Figure 2) or expression (Figure 3) was associated with a marked reduction in phosphorylated PI3K, Akt, MEK, and ERK levels (Figure 2D) prompted us to assess the effect of PI3K, in addition to that of Raf and MEK inhibitors alone, on the viability and signaling pathways in primary human CLL cells (Figure 5). This was of particular interest because inhibitors of both pathways were shown to be effective in diminishing CLL cell survival,13,15,16,38 and the finding of IGF1R as a prominent target acting upstream of both cascades explains the enhanced activation of the PI3K pathway and underlines the advantage of using inhibitors of this RTK.
Effect of sorafenib, PI3K, Raf, and MEK inhibitors on the viability and signaling pathways in primary human CLL cells. (A) CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were treated with 0.1 to 5 µM of the PI3K inhibitor AS605240 for 24 hours, and cell survival was determined by flow cytometry (mean ± SEM, n = 4). (B) CLL B cells were treated with a single dose of 10 µM sorafenib, Plexxikon 4720 (PLX), or the Mek-Inhibitor U01206 for 24 hours, and cell survival was determined by flow cytometry (mean ± SEM, n = 10). (C) CLL B cells were treated with a single dose of 10 µM sorafenib, PLX, or U01206 and immunoblotted for Erk phosphorylation and expression. One representative result from 4 independent experiments is shown. (D) Healthy B cells or CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were lysed and immunoblotted for Ras expression. Four representative samples from 8 donor-derived lysates from each group are shown. n.s., not statistically significant.
Effect of sorafenib, PI3K, Raf, and MEK inhibitors on the viability and signaling pathways in primary human CLL cells. (A) CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were treated with 0.1 to 5 µM of the PI3K inhibitor AS605240 for 24 hours, and cell survival was determined by flow cytometry (mean ± SEM, n = 4). (B) CLL B cells were treated with a single dose of 10 µM sorafenib, Plexxikon 4720 (PLX), or the Mek-Inhibitor U01206 for 24 hours, and cell survival was determined by flow cytometry (mean ± SEM, n = 10). (C) CLL B cells were treated with a single dose of 10 µM sorafenib, PLX, or U01206 and immunoblotted for Erk phosphorylation and expression. One representative result from 4 independent experiments is shown. (D) Healthy B cells or CLL B cells purified from freshly isolated or freeze-thawed PBMCs from CLL patient samples were lysed and immunoblotted for Ras expression. Four representative samples from 8 donor-derived lysates from each group are shown. n.s., not statistically significant.
As shown in Figure 5A, treatment of CLL cells with the PI3K inhibitor AS605240 caused a marked reduction in cellular viability. Next, we assessed the effects of the Raf inhibitors sorafenib and PLX4720, as well as the MEK inhibitor U0126, on survival (Figure 5B). Sorafenib, a multikinase inhibitor targeting Raf-kinases and several proangiogenic RTKs,39 induces rapid cell death in primary CLL cells, even in the presence of stromal cells.15,16,21-23 In full agreement with these studies, 10 µM sorafenib, a concentration comparable with the plasma levels of this drug in patients,21,40 killed more than 50% of freshly isolated CLL cells, whereas the more specific B-Raf (PLX4720) and MEK (U0126) inhibitors had no significant effects on viability (Figure 5B). We attribute the failure of the B-Raf and MEK inhibitor to its well-described paradoxical MEK/ERK activation in cells lacking BRAF mutations41 and the narrow target spectrum of U0126, respectively. Indeed, only sorafenib and the MEK inhibitor reduced ERK phosphorylation (Figure 5C), whereas PLX4720 treatment led even to a slightly enhanced ERK phosphorylation as it has been described for various solid tumor types lacking BRAF mutations and containing increased Ras-GTP levels.41,42 In that regard, we show that primary CLL cells also express higher levels of Ras proteins as detected by a pan-Ras antibody (Figure 5D).
Sorafenib induces apoptosis in CLL cells by inhibiting several prosurvival signaling pathways including IGF1R-mediated signaling
Having shown that sorafenib impairs the viability of primary human CLL cells, we next addressed the influence of this multikinase inhibitor on various signaling pathways. Interestingly, 10 µM sorafenib not only reduced ERK phosphorylation, and consequently Mcl-1 expression as it would be expected from the existing literature,21 but it also caused a drastic reduction of IGF1R, IRS-1, PI3K, Akt, and Src-family kinases phosphorylation (Figure 6A-B), the same mediators that were also affected by IGF1R inhibition (Figures 2B-D and 6A-B). According to the present knowledge, sorafenib exerts its effects via inhibition of Raf and VEGFR, because CLL cells are not sensitive to inhibition of other familiar sorafenib targets.15 Notably, inhibition of VEGFR21 or Raf, or MEK alone, has a very low proapoptotic effect on CLL cell viability15 and is not sufficient to completely explain the sorafenib-mediated effects. Having established IGF1R inhibitors as novel potential compounds for CLL therapy, together with the observation that sorafenib inhibited IGF1R and PI3K/Akt signaling in addition to the MAPK pathway, we next asked whether sorafenib targets the IGF1R as well. Indeed, sorafenib and the IGF1R inhibitor AG1024 had little effect on the surface expression of the IR, but caused a significant reduction of the IGF1R (Figure 6C-D). This loss of IGF1R is also reflected by an IGF-1–binding assay demonstrating that the number of biologically active IGF1R molecules is diminished by sorafenib or IGF1R inhibitors, as was reflected in the impaired capacity of CLL cells to bind biotinylated IGF-1 after treatment with either sorafenib or IGF1R inhibitors (Figure 6E). This finding suggests that loss of IGF1R activity, in a similar way as has been described for Raf-1,43 is accompanied by the downregulation of the protein. Furthermore, our data strongly suggest that the IGF1R itself is inhibited by the multikinase inhibitor sorafenib. Indeed, the in vitro kinase activity of purified IGF1R was strongly affected by sorafenib, albeit not as efficiently, as with the specific inhibitors AG1024 and linsitinib (Figure 6F).
Sorafenib targets the same molecules as IGF1R inhibitors, reduces IGF1R expression, counteracts IGF-1 binding, and inhibits the in vitro kinase activity of recombinant human IGF1R. (A) CLL B cells were treated with 10 µM sorafenib and immunoblotted for the expression of pPI3K, PI3K, pAkt, Akt, pErk, Erk, pSrc, CXCR4, and Mcl1. A representative example from 8 independent experiments is shown. (B) CLL B cells were treated with 10 µM sorafenib or 15 µM AG1024 and immunoblotted for the expression of pIGF1R, IGF1R, pIRS1, Mcl1, and pCXCR4. A representative example from 10 independent experiments is shown. (C) CLL B cells were treated with a single dose of 10 µM sorafenib or 15 µM AG1024 for 24 hours, and IR expression was determined by flow cytometry (n = 10). (D) CLL B cells were treated with a single dose of 10 µM sorafenib or 15 µM AG1024 for 24 hours, and IGF1R expression was determined by flow cytometry (n = 10). (E) CLL B cells treated with DMSO, sorafenib, linsitinib, AG1024, or PPP were treated with biotinylated IGF-1, and binding of IGF-1 to CLL cells was determined by flow cytometry (n = 3). (F) Sorafenib inhibits the in vitro kinase activity of recombinant human IGF1R. Two IGF1R inhibitors (AG1024 and linsitinib) were used as a positive control. In this assay, the IGF1R activity is measured by luminescence-based detection of adenosine dipsosphate produced during the kinase reaction (n = 3).
Sorafenib targets the same molecules as IGF1R inhibitors, reduces IGF1R expression, counteracts IGF-1 binding, and inhibits the in vitro kinase activity of recombinant human IGF1R. (A) CLL B cells were treated with 10 µM sorafenib and immunoblotted for the expression of pPI3K, PI3K, pAkt, Akt, pErk, Erk, pSrc, CXCR4, and Mcl1. A representative example from 8 independent experiments is shown. (B) CLL B cells were treated with 10 µM sorafenib or 15 µM AG1024 and immunoblotted for the expression of pIGF1R, IGF1R, pIRS1, Mcl1, and pCXCR4. A representative example from 10 independent experiments is shown. (C) CLL B cells were treated with a single dose of 10 µM sorafenib or 15 µM AG1024 for 24 hours, and IR expression was determined by flow cytometry (n = 10). (D) CLL B cells were treated with a single dose of 10 µM sorafenib or 15 µM AG1024 for 24 hours, and IGF1R expression was determined by flow cytometry (n = 10). (E) CLL B cells treated with DMSO, sorafenib, linsitinib, AG1024, or PPP were treated with biotinylated IGF-1, and binding of IGF-1 to CLL cells was determined by flow cytometry (n = 3). (F) Sorafenib inhibits the in vitro kinase activity of recombinant human IGF1R. Two IGF1R inhibitors (AG1024 and linsitinib) were used as a positive control. In this assay, the IGF1R activity is measured by luminescence-based detection of adenosine dipsosphate produced during the kinase reaction (n = 3).
Discussion
PTKs such as SYK, PI3K, and BTK are emerging as new targets in CLL because their involvement in BCR-dependent signaling pathways plays a critical role in CLL cell survival.7 However, based on experiences with other targeted therapy compounds such as imatinib or vemurafenib in CML44 and melanoma,42,45 respectively, it will be important to identify additional targets to identify synthetic lethal combinations by targeting 2 or more signaling hubs that are critical for tumor growth and survival, and to efficiently counteract the development of therapy resistance. Here, we report for the first time the functional significance and surface overexpression of the IGF1R protein and its baseline phosphorylation in human CLL cells (Figure 1B). It should be noted that the IGF1R was previously shown to be expressed in human CLL at the mRNA level and that these cells produce IGF-1 themselves, thereby driving their survival in an autocrine manner.20 We also extend these findings by showing that IGF1R overexpression is found in all 4 karyotypically defined CLL subsets (Figure 1C). The molecular mechanisms responsible for the observed IGF1R overexpression in CLL represent the aspect of future studies. Nevertheless, on the basis of insights gained from other RTKs, it can be speculated that dysregulation of IGF1R expression can occur at the pretranscriptional level (eg, by epigenetic events) or at the transcriptional level by miRNA networks. Likewise, oncogenic alterations preventing IGF1R internalization and/or degradation may be responsible for this phenomenon. Indeed, our observation that IGF1R inhibition by specific inhibitors for this RTK or sorafenib treatment causes a downregulation of the receptor at the protein level argues in favor of the latter possibility but does not rule out a potential regulatory layer at the transcriptional level. It is also conceivable that distinct pathogenetic mechanisms for IGF1R overexpression are found in the 4 CLL subsets.
Importantly, because this RTK is increasingly implicated in the development and progression of human cancer, in particular for solid tumors,17 its overexpression in CLL highlights the possibility to exploit this RTK as a pharmacologic target in this leukemia entity. Indeed, we demonstrate for the first time that CLL cells activate the PI3/Akt and MAPK pathways upon IGF-1 stimulation and that IGF1R inhibition with 3 structurally distinct compounds inhibits both pathways and induces apoptosis in primary human CLL cells with different genetic abnormalities. Thus, IGF1R inhibition could potentially represent a potential therapeutic approach for all CLL patients, including those with an unfavorable prognosis.
Remarkably, IGF1R inhibition even caused cell death in the presence of protective stroma microenvironment components. Importantly, the inhibitor-induced effects on the viability of primary human CLL cells were recapitulated by siRNA-mediated knockdown, indicating that the effects of IGF1R inhibitors are mediated by the inhibition of this RTK and not by a potential “off-target” effect of these drugs. We further extended our studies to 2 in vivo CLL models in which we demonstrated an efficacy for linsitinib, an orally active compound that is already established as successfully inhibiting the growth of xenografts33,34 and represents the only IGF1R inhibitor that is both commercially available and currently tested in different clinical trials (clinicaltrials.gov and Scagliotti and Novello46 ). Importantly, NOD SCID mice treated with linsitinib showed an obvious reduction in tumor size and intensity and BM and spleen infiltration (Figure 4C-E).
In addition, we identified the IGF1R pathway as a novel target of sorafenib in CLL cells. This finding is of particular interest, first because sorafenib is well characterized in clinical application and second because sorafenib potently induces apoptosis in primary CLL cells corresponding to distinct karyotypic subsets, even in the presence of the protective microenvironment.15,16,21-23 However, the target kinases of sorafenib responsible for this proapoptotic effect remain ill-defined for CLL. Recent publications concerning sorafenib treatment in CLL summarized that sorafenib exerts its effects via the inhibition of Raf and VEGFR, because CLL cells are not sensitive to the inhibition of KIT, PDGFR, and FLT3, but they remain sensitive to the inhibition of RAF and VEGFR.15 In agreement with previous data,15,16,21 sorafenib-induced cell death in primary human CLL cells was followed by downregulation of pErk (Figures 5C and 6A). Surprisingly, but also in agreement with previous studies,47 we did not observe an enhanced cell death after treatment with PLX4720, the tool compound for vemurafenib,48 or after treatment with the MEK inhibitor U0126. These observations can be explained in light of recent literature. PLX4720 treatment leads to a so-called “paradoxical Erk phosphorylation” compared with DMSO, sorafenib, or Mek inhibitor–treated cells (Figure 5C). This is caused by the inhibition of wild-type B-Raf in CLL cells in the presence of increased Ras-GTP levels.41 Although described for many other cancer types,49 this is to the best of our knowledge the first description of a paradoxical ERK activation by a Raf inhibitor in leukemia cells. The observed paradoxical ERK activation and PLX4720 inhibitor resistance could be potentially explained by the high Ras expression levels in CLL cells, as we observed compared with healthy B cells (Figure 5D). Second, the failure of U0126 to induce cell death in CLL cells despite achieving a strong reduction in ERK phosphorylation like sorafenib argues against an essential role of this MAPK pathway in CLL cell survival. In light of these data and because sorafenib efficiently compromised the viability of CLL cells in vitro (Figure 5B), the question arose of which additional targets contribute to its proapoptotic effect. Given the broad target spectrum of sorafenib, we hypothesized that this drug targets the IGF1R and leads to apoptosis via extensive inhibition of the PI3K/Akt pathway. This hypothesis was driven by our observation that sorafenib downregulated the same targets, especially PI3K, and Akt, which were also affected by AG1204 treatment (Figure 6A-B). Notably, sorafenib treatment not only decreased the expression of IGF1R and the phosphorylation of IGF1R and IRS-1, the first mediator of IGF1R signal transduction (Figure 6B), but it also counteracted IGF-1 binding to primary human CLL cells (Figure 6E). Although we never considered or investigated this compound as a target of sorafenib before, we could show that it moderately lowered IGF receptor kinase activity in a cell-free–based kinase assay (Figure 6F). This is the first description or mechanistic evidence that sorafenib-induced cell death is partly mediated via inhibition of IGF1R.
Furthermore, we have shown that not only indirect PI3K inhibition by sorafenib and IGF1R inhibitors, but also direct inhibition by AS 605240 compromises cellular survival. This finding extends previous reports by Longo et al, demonstrating that the PI3K/Akt/Mcl-1 pathway mediates antiapoptotic signals50 and is of additional relevance because PI3K inhibitors are currently evaluated in clinical CLL trials (clinicaltrials.gov). Our data identified IGF1R overexpression as one potential mediator of PI3K hyperactivation, which may explain the success of currently tested PI3K inhibitors.13,38 The finding of IGF1R as a prominent overexpressed target acting upstream of the PI3K/Akt and MAPK pathways explains the enhanced expression of both pathways and underlines the advantage of using inhibitors of this RTK.
In summary, our results provide a novel mechanism of action for the multikinase inhibitor sorafenib in CLL cells by blocking IGF1R-mediated signaling. Given the increasing consideration of sorafenib in the treatment of hematologic neoplasias, such as CLL and AML, this finding is of potential clinical relevance. Most importantly, we have shown that IGF1R inhibition by itself induced apoptosis in CLL cells in vitro and in vivo. IGF1R inhibitors not only reduced PI3K and Akt phosphorylation, but also reduced expression of Raf and phosphorylated Erk and Src, thus enabling CLL cells to overcome paradoxical Erk activation. These data identify IGF1R as a promising target for future therapeutic approaches and suggest the further (pre-)clinical assessment of IGF1R inhibitors for CLL therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The Eμ-TCL1-tg breeder mice were obtained from C. Croce (Ohio State University, Columbus, OH).
This work was supported by the Deutsche Krebshilfe Verbund “Receptor signaling and comparative genomics in CLL” (grant 108935) and by the Deutsche Krebshilfe (grant 109605). Work in the laboratory of T.B. is supported by the Emmy-Noether-Program (BR-3662-1/1) of the DFG and the Centre for Biological Signalling Studies EX294 BIOSS, funded by the Excellence Initiative of the German federal and state governments.
Authorship
Contribution: N.Y. designed and performed all experiments unless indicated otherwise, analyzed data, and wrote the manuscript; R.Ü. biotinylated IGF-1 and gave technical advice; J.S. performed the NSG mouse experiment; D.P. analyzed genetic aberrations of patient samples; R.Ü., J.S., D.P., C.D., M.B., H.J., and H.V. discussed data and contributed to experimental design and manuscript writing; K.A. contributed to experimental design and performed the immunohistochemical analysis of IGF1R expression; and T.B. and K.Z. supervised the study, designed experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Katja Zirlik, University Medical Center Freiburg, Department of Hematology/Oncology, Hugstetter Strasse 55, 79106 Freiburg, Germany; e-mail: katja.zirlik@uniklinik-freiburg.de.
References
Author notes
T.B. and K.Z. are co-senior authors.