Key Points
T follicular helper cells and germinal center B cells are increased and strongly correlate with the development of cGVHD in a murine model.
Blocking mAbs for IL-21, ICOS, and CD40L are potential novel therapeutics for cGVHD.
Abstract
Chronic graft-versus-host disease (cGVHD) is a leading cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation. Having shown that germinal center (GC) formation and immunoglobulin deposition are required for multiorgan system cGVHD and associated bronchiolitis obliterans syndrome (BOS) in a murine model, we hypothesized that T follicular helper (Tfh) cells are necessary for cGVHD by supporting GC formation and maintenance. We show that increased frequency of Tfh cells correlated with increased GC B cells, cGVHD, and BOS. Although administering a highly depletionary anti-CD20 monoclonal antibody (mAb) to mice with established cGVHD resulted in peripheral B-cell depletion, B cells remained in the lung, and BOS was not reversed. BOS could be treated by eliminating production of interleukin-21 (IL-21) by donor T cells or IL-21 receptor (IL-21R) signaling of donor B cells. Development of BOS was dependent upon T cells expressing the chemokine receptor CXCR5 to facilitate T-cell trafficking to secondary lymphoid organ follicles. Blocking mAbs for IL-21/IL-21R, inducible T-cell costimulator (ICOS)/ICOS ligand, and CD40L/CD40 hindered GC formation and cGVHD. These data provide novel insights into cGVHD pathogenesis, indicate a role for Tfh cells in these processes, and suggest a new line of therapy using mAbs targeting Tfh cells to reverse cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) is a major obstacle following allogeneic hematopoietic stem cell transplantation.1,2 Clinically representative models have increased our understanding of acute GVHD, but the dearth of relevant cGVHD murine models has limited our ability to interrogate its underlying pathophysiology.3,4 However, recent work with a novel murine model of multiorgan cGVHD that highlights lung pathology with the development of bronchiolitis obliterans syndrome (BOS) has provided new insight into research on cGVHD.5,6
Even though the exact mechanism of cGVHD is unknown, B cells and pathogenic antibody production are clearly implicated in both human and mouse models. Patients diagnosed with cGVHD had elevated soluble B-cell activating factor and increased proportions of pre-germinal center (GC) B cells and post-GC plasmablasts.7 Furthermore, male patients who received grafts from female donors had an increase in antibody response to H-Y minor histocompatibility antigens, which correlated with cGVHD.8 In addition, we have shown that B cells are required to induce cGVHD and associated BOS in this clinically relevant murine model.5 Not only was the presence of B cells necessary but the development of tissue fibrosis was dependent on secretion of class-switched antibody. These data suggest that B-cell activation and maturation is necessary for cGVHD progression.
The ability of B cells to create high-affinity antibodies is dependent on the GC reaction and extrafollicular B cells. Once B cells recognize cognate antigen, they can undergo somatic hypermutation and class switching with the aid of CD4 T cells in the B-T cell junction within secondary lymphoid organs. T cells are required to provide survival signals to B cells that are rapidly making random mutations to the complementary determining regions in the immunoglobulin (Ig) genes. This results in the negative selection of poor-affinity antibodies, while selecting for those B cells with mutations that increase antibody affinity. B cells that produce high-affinity class-switched antibodies are able to activate immune responses and, in the case of cGVHD, cause severe damage to the target tissues by activating complement or antibody-dependent cell-mediated cytotoxicity.
We sought to investigate the role of T follicular helper (Tfh) cells in the genesis of cGVHD in order to develop new interventions. Previously, we defined the role of antibody production by bone marrow (BM)-derived B-cell progeny in the initiation and maintenance of cGVHD in this clinically relevant murine model.5 The ability of B cells to produce class-switched antibodies and the need for lymphotoxin β receptor signaling in the GC was highlighted, clearly defining the importance of GC maturation during cGVHD. Tfh cells are a subset of CD4+ T cells that are located in the B-cell follicle and express the transcription factor Bcl6 along with high levels of the chemokine receptor CXCR5 and programmed cell death protein-1 (PD-1).9 These cells support the generation of GCs by providing signaling through interleukin-21 (IL-21), inducible T-cell costimulator (ICOS), and CD40.10-13 Having previously shown that B-cell production of class-switched antibody is necessary for cGVHD, we hypothesized that maintenance of the GC by Tfh cells is necessary for the progression of cGVHD and associated BOS. Genetic deletions and interventional therapies were used to study the imporance of Tfh cell signaling of GC B cells during murine cGVHD.
Materials and methods
Mice
C57Bl/6 (B6; H2b) mice were purchased from the National Cancer Institute. B10.BR (H2k), CXCR5−/−, and ICOS−/− B6 knockout (KO) mice were purchased from Jackson Laboratories. B6 IL-21−/− and IL-21 receptor (IL-21R)−/− KO mice were bred at the University of Minnesota animal facility. Mice were housed in a specific pathogen-free facility and used with the approval of the University of Minnesota institutional animal care facility.
Bone marrow transplant and therapeutic intervention
B10.BR recipients were conditioned with cytoxan on days −3 and −2 (120 mg/kg per day intraperitoneally). On day 1, recipients received total-body irradiation by x-ray (8.3 Gy). B6 donor BM was T-cell depleted with anti-Thy1.2 monoclonal antibody (mAb) followed by rabbit complement. T cells were purified from spleens by incubation with phycoerythrin-labeled anti-CD19 (eBioscience), followed by anti-phycoerythrin beads and depletion with a magnetic column (Miltenyi-Biotec). On day 0, recipients received 10 × 106 T-cell–depleted BM cells with or without allogeneic spleen cells (0.75 × 106 to 1 × 106) or purified splenic T cells (0.1 × 106), as indicated. Weights of individual mice were recorded each week. Where indicated, recipients in cGVHD groups were given anti-CD20 (250 μg per animal every 2 weeks; clone 18B12, IgG2a; kindly provided by Biogen Idec), anti-IL-21 (250 μg per animal twice a week; clone Ch268.5.1, mIgG1, kindly provided by Novo Nordisk), anti-ICOS (200 μg per animal three times a week; clone 7E.17G9.G1, rIgG2b), or anti-CD40L (200 μg per animal twice a week; clone MR1, hamster IgG), or irrelevant isotype antibody as previously reported.14-16
Pulmonary function tests
Pulmonary function tests (PFTs) were performed as described.6 Briefly, anesthetized mice were weighed, and lung function was assessed by whole-body plethysmography using the Flexivent system (Scireq) and was analyzed by using the Flexivent software version 5.1.
Frozen tissue preparation
All organs harvested were embedded in Optimal Cutting Temperature compound, snap-frozen in liquid nitrogen, and stored at −80° centigrade. Lungs were inflated by infusing 1 mL of optimum cutting temperature compound:phosphate-buffered saline (3:1) intratracheally prior to harvest.
Trichrome staining
Cryosections (6 μm) were fixed for 5 minutes in acetone and stained with Masson’s trichrome staining kit (Sigma) for detection of collagen deposition. Collagen deposition was quantified on trichrome-stained sections as a ratio of area of blue staining to area of total staining by using the Adobe Photoshop CS3 analysis tool (Adobe Systems), as previously described.5
Immunofluorescence
For Ig deposition, 6-μm cryosections were fixed with acetone and then blocked with horse serum and streptavidin-biotin blocking kit (Vector) and stained with fluorescein isothiocyanate (FITC)-labeled anti-mouse-Ig (BD Pharmingen) or goat-anti-mouse-IgG2c followed by FITC-donkey-anti-goat Ig (Jackson ImmunoResearch). Antibody deposition was quantified by area of Ig staining per 100-μm section. Fixed 6-µm spleen cryosections were stained with rhodamine-peanut agglutinin (Vector Laboratories), anti-PD-1 FITC, anti-GL7 FITC, anti-CD4 biotin or anti-CD19 biotin followed by Cy5-conjugated strepavidin to detect the presence of B cells and T cells in the GC. Confocal images were acquired on an Olympus FluoView500 Confocal Laser Scanning Microscope at ×200, analyzed by using FluoView3.2 software (Olympus), and processed with Adobe Photoshop CS3, version 9.0.2.
Flow Cytometry
For flow cytometric analysis of Tfh cells and GC B cells, single-cell suspensions from spleens or the peritoneal cavity were obtained and labeled with anti-CD4, anti-CXCR5, anti-PD-1, anti-CD19, anti-GL7, anti-CD38, anti-IgM, anti-IgD, anti-CD23, or anti-CD5 (eBioscience). Cells were analyzed on a BD LSRFortessa cell analyzer.
Statistics
Group comparisons of cell counts and flow cytometry data were analyzed by Student t test.
Results
Increased Tfh cell frequency during cGVHD
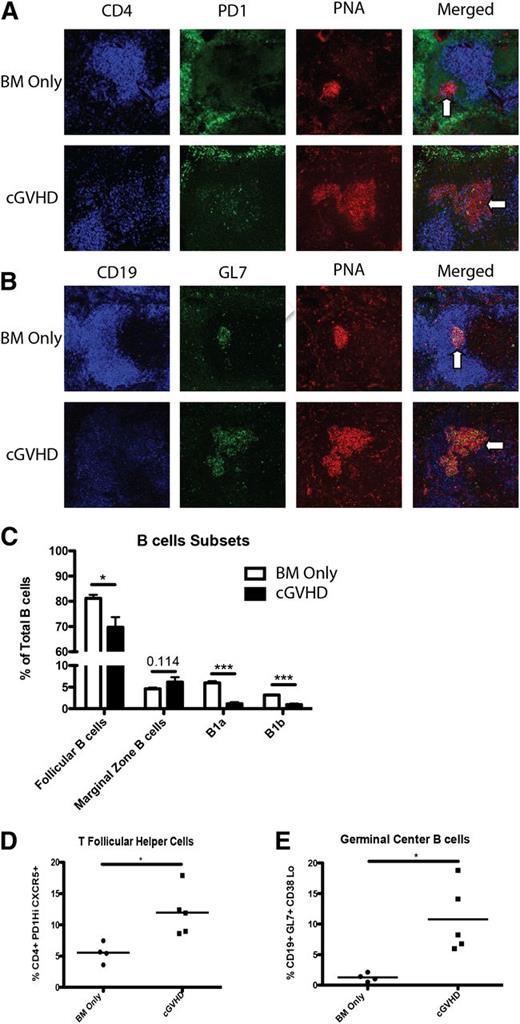
Previously, we demonstrated that mice with cGVHD have larger and more frequent GCs in the spleen compared with BM only (non-cGVHD) mice.5 Therefore, we examined the presence of splenic Tfh cells in cGVHD mice because Tfh cell support is necessary for GC formation.17-19 Spleens harvested from mice with cGVHD demonstrated an increased presence of CD4+ cells in peanut agglutinin–positive GC areas compared with non-GVHD mice, co-localizing to regions of high PD-1 expression (Figure 1A). Localization of CD4+ cells to the GC by the chemical gradient chemokine ligand CXCL13 binding with its receptor CXCR5 is a hallmark of Tfh cells.20 During cGVHD, CD4 localization was associated with increased GC size and GC B cells, denoted by GL7 staining (Figure 1B; see also Figure 1E). Flow cytometry of purified splenocytes from BM only non-cGVHD and cGVHD mice showed a significant decrease in the proportion of B1a, B1b, CD23+, IgM+, and IgD+ follicular B cells (Figure 1C). Even though there are fewer follicular B cells, there is an increase in the frequency of B cells entering the GC. In addition, there was a twofold increase in the frequency of splenic Tfh cells, defined as PD-1hiCXCR5+ CD4 T cells in cGVHD mice compared with non-cGVHD mice (Figure 1D), which correlated with a twofold increase in the percentage of GL7+, Fas+, and CD38− GC B cells (Figure 1E).
Increased Tfh cells in cGVHD. B10.BR mice were transplanted with BM only or BM and splenic T cells from B6 donor mice and spleens were harvested on day 60. (A) Representative spleens harvested from BM only and BM plus spleen mice on day 60 showing CD4 (blue), PD-1 (green), and peanut agglutinin (PNA) (red) or (B) CD19 (blue), GL7 (green), or PNA (red). Arrows indicate GC areas. (C) Composition of B cells in the spleen and peritoneal cavity. (D) Frequency of Tfh cells and (E) GC B cells. *P < .05; ***P < .001.
Increased Tfh cells in cGVHD. B10.BR mice were transplanted with BM only or BM and splenic T cells from B6 donor mice and spleens were harvested on day 60. (A) Representative spleens harvested from BM only and BM plus spleen mice on day 60 showing CD4 (blue), PD-1 (green), and peanut agglutinin (PNA) (red) or (B) CD19 (blue), GL7 (green), or PNA (red). Arrows indicate GC areas. (C) Composition of B cells in the spleen and peritoneal cavity. (D) Frequency of Tfh cells and (E) GC B cells. *P < .05; ***P < .001.
Anti-CD20 therapy does not prevent the development of cGVHD
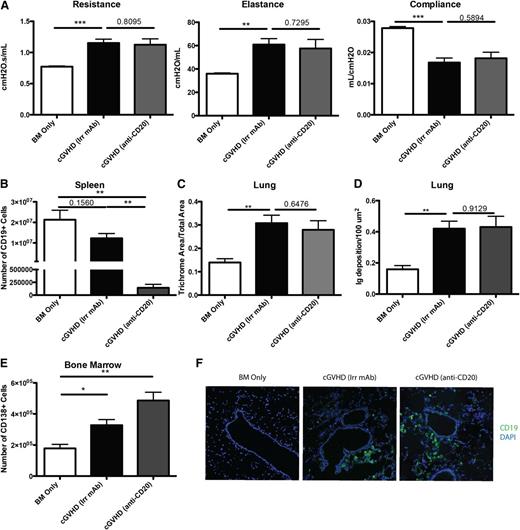
Rituximab therapy has been used as an attractive treatment for some patients with cGVHD to eliminate pathogenic B cells. We sought to determine the therapeutic effect of anti-CD20 in our mouse model of cGVHD, which is dependent on B-cell activation and GC reaction. B10.BR mice were transplanted with wild-type (WT) B6 BM cells and WT B6 T cells and allowed to develop cGVHD over 28 days, at which point mice were given anti-CD20 to deplete B cells. When analyzed on day 60, >98% of the splenic B cells were depleted (Figure 2B). However, mice still developed cGVHD. PFTs demonstrated decreased functional capacity of the lungs as evidenced by increased resistance and elastance and decreased compliance similar to irrelevant mAb–treated cGVHD mice (Figure 2A). Furthermore, anti-CD20–treated cGVHD mice had increased collagen deposition surrounding the bronchioles, consistent with BOS compared with BM only mice (Figure 2C). Tissues were analyzed for the deposition of IgG2c antibody. Compared with BM only controls and despite peripheral B-cell depletion by anti-CD20 mAb, cGVHD mice given anti-CD20 mAb had increased IgG2c, but not the isotype of anti-CD20 mAb (IgG1) (Figure 2D and supplemental Figure 1 available on the Blood Web site). We examined potential reasons why anti-CD20 therapy was not able to decrease development of BOS and cGVHD. First, plasma cells (CD138+ CD19lo), which do not express CD20, were significantly increased in the BM and spleen of mice treated with anti-CD20 mAb (Figure 2E and data not shown). This suggests that depletion therapy increases selection of CD20-deficient further differentiated antibody-producing cells. Next, anti-CD20 therapy was unable to deplete CD19+ B cells within the target organs, including the lung (Figure 2F). Finally, even though there was a significant depletion of total B cells, there was a threefold increase in the relative frequency of GC B cells in the B-cell compartment of mice treated with anti-CD20 therapy compared with irrelevant mAb–treated cGVHD control mice (P < .05; data not shown). The increase in frequency highlights the relative resistance of GC B cells to B-cell–depleting therapy.21 Thus, B-cell depletion therapy for treatment of active cGVHD had little effect on the progression of the disease likely because of the inability to deplete all GC B cells.
B-cell depletion by anti-CD20 therapy is not sufficient to prevent cGVHD and associated BOS. cGVHD was established in B10.BR mice transplanted with WT B6 BM and WT B6 spleen cells, and mice were treated with anti-CD20 or irrelevant mouse IgG1 antibody from day 28 to day 56. (A) PFTs for mice treated with irrelevant or anti-CD20 antibody. (B) Number of B cells purified from the spleens of mice on day 56. (C) Trichrome deposition and (D) Ig deposition in the lungs of mice harvested on day 56. (E) Number of CD138+ CD19 low plasma cells in the BM of D60 transplanted mice. (F) Representative images of frozen lung tissues stained with anti-CD19 FITC and 4,6 diamidino-2-phenylindole. Representative data from 2 experiments; n = 8 mice per group. *P < 0.5; **P < .01; ***P < .001.
B-cell depletion by anti-CD20 therapy is not sufficient to prevent cGVHD and associated BOS. cGVHD was established in B10.BR mice transplanted with WT B6 BM and WT B6 spleen cells, and mice were treated with anti-CD20 or irrelevant mouse IgG1 antibody from day 28 to day 56. (A) PFTs for mice treated with irrelevant or anti-CD20 antibody. (B) Number of B cells purified from the spleens of mice on day 56. (C) Trichrome deposition and (D) Ig deposition in the lungs of mice harvested on day 56. (E) Number of CD138+ CD19 low plasma cells in the BM of D60 transplanted mice. (F) Representative images of frozen lung tissues stained with anti-CD19 FITC and 4,6 diamidino-2-phenylindole. Representative data from 2 experiments; n = 8 mice per group. *P < 0.5; **P < .01; ***P < .001.
Trafficking to the B-cell follicle by the chemokine receptor CXCR5 is necessary in cGVHD
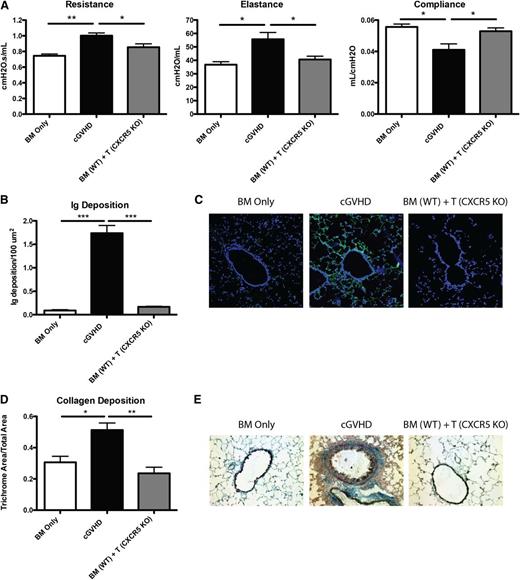
Trafficking of Tfh cells to the B-cell zone is dependent on the expression of the chemokine receptor CXCR5, which binds CXCL13 secreted by follicular dendritic cells. Without CXCR5, Tfh cells are unable to localize to the B-cell follicle and cannot interact with GC B cells. To determine the requirement for such Tfh cell migration during cGVHD, we transplanted mice with T cells deficient in CXCR5. Compared with positive control mice with cGVHD, which received WT BM and WT T cells, recipients of WT BM cells with CXCR5 KO T cells had PFTs that were significantly improved and comparable to BM-only controls (Figure 3A). In recipients given CXCR5 KO T cells, there was a significant decrease in the amount of Ig found in the lungs of these mice (Figure 3B-C), and collagen surrounding the bronchioles was significantly decreased as demonstrated by trichrome staining (Figure 3D-E). Together, these data demonstrate that CXCR5 expression on donor T cells is needed for cGVHD associated with BOS.
Chemokine receptor CXCR5 on donor mature T cells is necessary for cGVHD. B10.BR mice were transplanted with WT B6 BM and WT B6 T cells or B6 CXCR5 KO T cells. (A) Mice were evaluated for pulmonary function. (B) Lung sections were stained with FITC-conjugated anti-mouse Ig, and the area of Ig deposition was measured as shown in (C) representative images. (D) Lungs of mice were stained with trichrome, and the ratio of collagen stain to total stain was measured surrounding the bronchioles as shown in (E) representative images. Representative data from 3 experiments; n = 8 mice per group. *P < .05; **P < .01; ***P < .001.
Chemokine receptor CXCR5 on donor mature T cells is necessary for cGVHD. B10.BR mice were transplanted with WT B6 BM and WT B6 T cells or B6 CXCR5 KO T cells. (A) Mice were evaluated for pulmonary function. (B) Lung sections were stained with FITC-conjugated anti-mouse Ig, and the area of Ig deposition was measured as shown in (C) representative images. (D) Lungs of mice were stained with trichrome, and the ratio of collagen stain to total stain was measured surrounding the bronchioles as shown in (E) representative images. Representative data from 3 experiments; n = 8 mice per group. *P < .05; **P < .01; ***P < .001.
IL-21 is a required cytokine for the development of cGVHD
Tfh cells are able to drive GC reactions by multiple mechanisms, including cell-to-cell contact and secretion of key cytokines. IL-21 is a major cytokine produced by Tfh cells. Although IL-21 is not essential for GC formation, it is important for the production of memory B cells with high-affinity antibody production.22 Therefore, we investigated the role of IL-21 in cGVHD and its possible requirement for the production of class-switched IgG that is deposited in cGVHD organs. Mice were transplanted with WT BM and either no T cells or WT or IL-21 KO splenocytes. Recipients of IL-21 KO splenic T cells did not develop symptoms of BOS as demonstrated by PFTs (Figure 4A). A decrease in the GC sizes (Figure 4B) was also seen. Deposition of Ig in the tissues was restored to healthy control levels (Figure 4C), leading to a twofold decrease in collagen (Figure 4D) in recipients of IL-21 KO splenic T cells compared with cGVHD controls. There was a twofold decrease in frequency of splenic Tfh cells; moreover, the frequency of GC B cells was significantly decreased compared with the recipients of WT splenic T cells (Figure 4E-F).
IL-21 production from donor T cells is required for cGVHD pathology. B10.BR mice were transplanted with WT B6 BM and splenocytes from either WT B6 or B6 IL-21 KO mice. (A) Mice were evaluated for pulmonary function. (B) Lungs of mice were stained with trichrome, and the ratio of collagen stain to total stain was measured surrounding the bronchioles. (C) Lung sections were stained with FITC-conjugated anti-mouse Ig, and the area of Ig deposition was measured. (D) Whole spleen sections were stained with rhodamine-conjugated PNA, and the size of the PNA-positive sections was measured. Frequency of (E) Tfh and (F) GC B cells in the spleen of transplanted mice on day 60. Representative data from 3 experiments; n = 8 mice per group. *P < .05; **P < .01; ***P < .001.
IL-21 production from donor T cells is required for cGVHD pathology. B10.BR mice were transplanted with WT B6 BM and splenocytes from either WT B6 or B6 IL-21 KO mice. (A) Mice were evaluated for pulmonary function. (B) Lungs of mice were stained with trichrome, and the ratio of collagen stain to total stain was measured surrounding the bronchioles. (C) Lung sections were stained with FITC-conjugated anti-mouse Ig, and the area of Ig deposition was measured. (D) Whole spleen sections were stained with rhodamine-conjugated PNA, and the size of the PNA-positive sections was measured. Frequency of (E) Tfh and (F) GC B cells in the spleen of transplanted mice on day 60. Representative data from 3 experiments; n = 8 mice per group. *P < .05; **P < .01; ***P < .001.
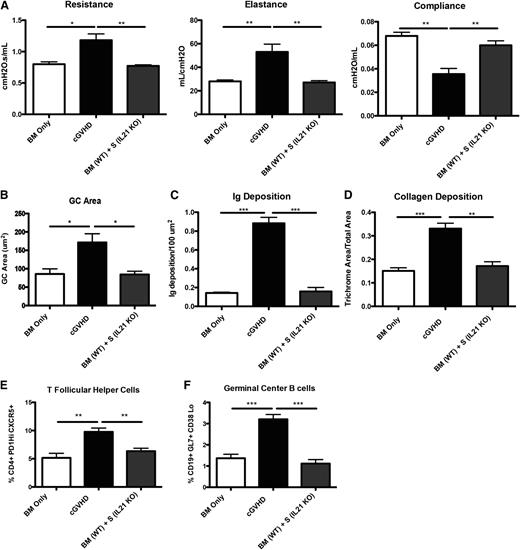
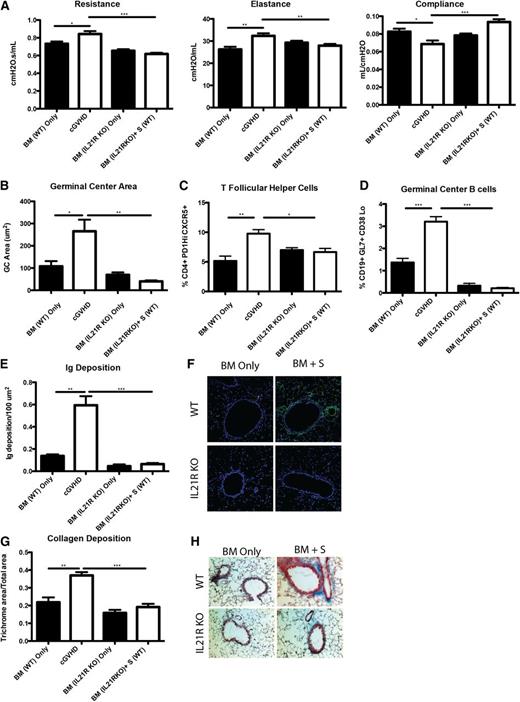
Profibrotic effects of IL-21 may contribute to cGVHD and BOS.23 To determine the extent to which the profibrotic vs B-cell activating functions of IL-21 accounted for improved PFTs, we transplanted mice with BM from WT or IL-21R KO donors with or without WT splenic T cells. Compared with recipients of WT BM and WT splenic T cells, mice transplanted with the IL-21R KO BM and WT splenic T cells had significantly improved PFTs (Figure 5A) and reduced GC area (Figure 5B) associated with reduced frequencies of PD-1hiCXCR5+ Tfh cells (Figure 5C) and GL7+CD38lo GC B cells (Figure 5D) that was not different from BM only no cGVHD controls. Concordant with these findings, Ig (Figure 5E-F) and collagen (Figure 5G-H) were detected at levels less than that found in BM only controls. Together these data demonstrate a requirement for both donor T-cell IL-21 production and donor BM IL-21R expression in generating Tfh cells, GCs, and cGVHD with BOS.
B cells need IL-21R for full maturation and progression of cGVHD. B10.BR recipients were transplanted with either WT BM or IL-21R KO BM and WT splenic (S) T cells. (A) Mice were evaluated for pulmonary function. (B) Whole spleen sections were stained with rhodamine-conjugated PNA, and the size of the PNA-positive sections was measured. Frequency of (C) Tfh and (D) GC B cells in the spleens of transplanted mice on D60. (E) Lung sections were stained with FITC-conjugated anti-mouse Ig, and the area of Ig deposition was measured, and (F) representative images. (G) Lungs of mice were stained with trichrome, and the ratio of collagen stain to total stain surrounding the bronchioles was measured with (H) representative images. Representative data from 3 experiments with n = 8 mice per group. *P < .05; **P < .01; ***P < .001.
B cells need IL-21R for full maturation and progression of cGVHD. B10.BR recipients were transplanted with either WT BM or IL-21R KO BM and WT splenic (S) T cells. (A) Mice were evaluated for pulmonary function. (B) Whole spleen sections were stained with rhodamine-conjugated PNA, and the size of the PNA-positive sections was measured. Frequency of (C) Tfh and (D) GC B cells in the spleens of transplanted mice on D60. (E) Lung sections were stained with FITC-conjugated anti-mouse Ig, and the area of Ig deposition was measured, and (F) representative images. (G) Lungs of mice were stained with trichrome, and the ratio of collagen stain to total stain surrounding the bronchioles was measured with (H) representative images. Representative data from 3 experiments with n = 8 mice per group. *P < .05; **P < .01; ***P < .001.
Blocking signaling from ICOS and CD40 has a therapeutic benefit in cGVHD
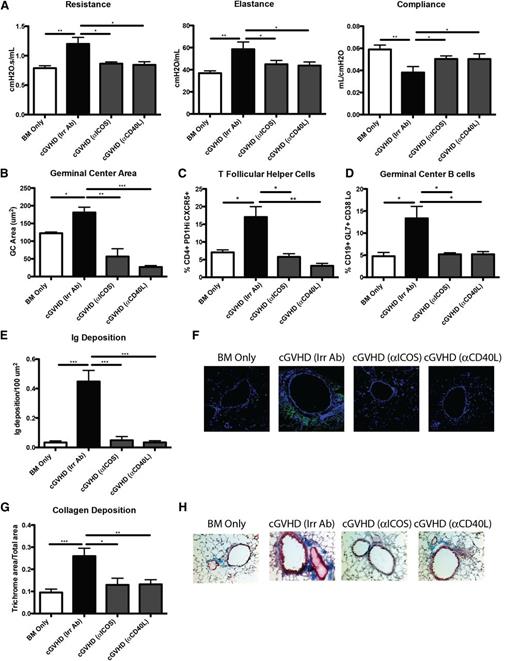
ICOS and CD40 signals are required for GC formation.13 ICOS is highly expressed on Tfh cells and is necessary for secretion of IL-21 following engagement with ICOS ligand–expressing B cells. CD40 is expressed on B cells in the GC, and signaling through CD40 by CD40 ligand–expressing T cells is necessary for the survival of B cells in the GC. Disruption of ICOS/ICOS ligand and CD40/CD40 ligand has been shown to decrease antibody formation in murine lupus models and to be important in the progression of acute GVHD.15,24 To determine whether ICOS and CD40 are necessary signals in cGVHD, mice with WT BM and splenic T cells were administered anti-ICOS– and anti-CD40 ligand–blocking mAbs beginning 28 days after transplantation, when cGVHD has been established.5 Mice given either anti-ICOS– or anti-CD40 ligand–blocking mAbs had PFTs comparable to those of BM only controls (Figure 6A). Mice treated with anti-ICOS or anti-CD40L had a 95% reduction in GC formation (Figure 6B). The decrease in overall size of GCs correlated with a threefold reduction in Tfh cells and GC B cells present in the spleen (Figure 6C-D). Furthermore, IgG2c deposition surrounding the bronchioles in the lung was decreased (Figure 6E-F). Finally, the mice treated with anti-ICOS or anti-CD40L mAb did not have increased collagen surrounding the bronchioles (Figure 6G-H). Consistent with anti-ICOS–blocking mAb data, PFTs in recipients of ICOS KO vs WT T cells indicated normalization compared with BM only controls (supplemental Figure 2). These data indicate that the ICOS and CD40 signaling in the GC is necessary for the development of cGVHD.
ICOS or CD40L costimulatory pathway blockade is sufficient for reducing GCs and preventing BOS. cGVHD was established in B10.BR mice transplanted with WT B6 BM and WT B6 T cells, and mice were treated with either anti-ICOS, anti-CD40L, or irrelevant rat antibody from day 28 to day 56. (A) PFT. (B) GC size was analyzed in situ by measuring the PNA-positive sections in transplanted spleens from day 56. Splenocytes were harvested from transplanted mice on day 56, made into a single-cell suspension, and analyzed for (C) Tfh cells or (D) GC B cells. (E) Amount of Ig deposited in the lungs of transplanted mice on day 56 with (F) representative images. (G) Quantified trichrome present in the lungs of mice on day 56 with (H) representative images. Representative data from 2 experiments; n = 8. *P < .05; **P < .01; ***P < .001.
ICOS or CD40L costimulatory pathway blockade is sufficient for reducing GCs and preventing BOS. cGVHD was established in B10.BR mice transplanted with WT B6 BM and WT B6 T cells, and mice were treated with either anti-ICOS, anti-CD40L, or irrelevant rat antibody from day 28 to day 56. (A) PFT. (B) GC size was analyzed in situ by measuring the PNA-positive sections in transplanted spleens from day 56. Splenocytes were harvested from transplanted mice on day 56, made into a single-cell suspension, and analyzed for (C) Tfh cells or (D) GC B cells. (E) Amount of Ig deposited in the lungs of transplanted mice on day 56 with (F) representative images. (G) Quantified trichrome present in the lungs of mice on day 56 with (H) representative images. Representative data from 2 experiments; n = 8. *P < .05; **P < .01; ***P < .001.
Therapeutic effect of IL-21 mAb in mice with cGVHD and BOS
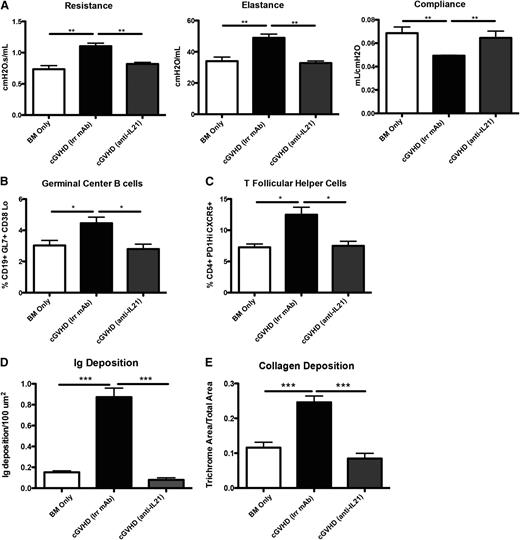
Blocking cytokine signaling was shown to be effective in many autoimmune diseases, including systemic lupus erythematous and rheumatoid arthritis.25,26 To determine whether the effects of neutralizing IL-21 would be a potential therapeutic for the progression of cGVHD and establishment of BOS, mice were transplanted with WT BM and WT splenic T cells and were treated with irrelevant or anti-IL-21 neutralizing mAb starting on day 28 after BM transplant. Mice treated with anti-IL-21 mAb demonstrated normalized pulmonary function comparable to that in BM only recipients (Figure 7A). Anti-IL-21 mAb was effective at decreasing the frequency of GC B cells and Tfh cells in the spleen (Figure 7B-C). By blocking IL-21 in the cGVHD mice, we were able to limit the GC reaction and Ig deposition in the lung (Figure 7D). Finally, there was no increase in collagen deposition in mice treated with anti-IL-21 mAb (Figure 7E). These data demonstrate a novel potential therapy for the treatment of cGVHD and associated BOS.
Anti-IL-21 mAb reverses BOS. cGVHD was established in B10.BR mice transplanted with WT B6 BM and WT B6 T cells, and mice were treated with either irrelevant mouse IgG1 or anti-IL-21. (A) PFTs from mice treated with either neutralizing anti-IL-21 mAb or irrelevant mAb. Splenocytes were isolated and analyzed for (B) GC B cells or (C) Tfh cells. (D) Ig and (E) ratio of trichrome-positive to total area surrounding bronchioles in trichrome stain. Representative data from 3 experiments; n = 8. *P < .05; **P < .01; ***P < .001.
Anti-IL-21 mAb reverses BOS. cGVHD was established in B10.BR mice transplanted with WT B6 BM and WT B6 T cells, and mice were treated with either irrelevant mouse IgG1 or anti-IL-21. (A) PFTs from mice treated with either neutralizing anti-IL-21 mAb or irrelevant mAb. Splenocytes were isolated and analyzed for (B) GC B cells or (C) Tfh cells. (D) Ig and (E) ratio of trichrome-positive to total area surrounding bronchioles in trichrome stain. Representative data from 3 experiments; n = 8. *P < .05; **P < .01; ***P < .001.
Discussion
In this study, we defined a novel role of Tfh cells in the activation of GC B cells in the production and deposition of pathologic Ig and collagen, ultimately causing BOS and multiorgan system cGVHD. Although cGVHD B cells are activated and primed for survival via B-cell activating factor and BCR-associated signaling pathways,27 the direct mechanism underpinning B-cell activation has yet to be clearly defined. By using a preclinical cGVHD model, our data strongly suggest that donor T-cell–derived Tfh cells are necessary for the activation of donor BM–derived B cells that differentiate into GC B cells, resulting in Ig deposition in BOS lesional tissue. Consistent with this notion, preliminary data indicate that recipients of either BM or T cells from bcl6 KO donors are unable to cause cGVHD (data not shown), pointing to the requirement for Tfh cells and GC B cells in cGVHD generation and/or maintenance under these conditions. The increase in GC size (Figure 1A-B) is independent of the number and frequency of follicular B cells present during cGVHD (Figure 1C) and, in fact, CD19+B220+CD5hi B1a cells (that produce broadly reactive natural IgM) and CD19+B220+CD5lo B1b B cells (that produce adaptive IgG antibody, especially to T-cell–independent type 2 antigens) are significantly decreased in cGVHD mice. Together, these data indicate that there is not a general increase in the B-cell population, but there is a selective increase in the frequency of GC B cells and GC number per area. Although splenic B-cell and GC populations were largely depleted by anti-CD20 mAb therapy for established cGVHD, there was no impact on cGVHD BOS (Figure 2). Possible explanations include inadequate B-cell depletion in cGVHD target organs (Figure 2F) and/or failure to deplete preformed plasma cells residing in the BM (Figure 2E). The increased activation state of GC B cells during cGVHD has the potential to make monoclonal cellular depletion therapies less effective, as seen in other autoimmune disorders.28,29
The production of class-switched antibodies requires the ability of T cells to localize to the GC and provide survival signals to B cells during cGVHD. Interruption of trafficking of T cells to the B-cell follicle by CXCR5 was sufficient for preventing GC response and preventing cGVHD (Figure 3). Expression of CXCR5 is a hallmark of Tfh cells, and without this essential chemokine receptor, GCs failed to increase posttransplant during conditions that otherwise were conducive to cGVHD generation. Di Carlo et al30 demonstrated that cardiac allo-rejection was highly associated with production of CXCL13 and increased GC formation.
Robust GC reactions during cGVHD were dependent on the production of IL-21 from donor T cells (Figures 4 and 5). Tfh cells are dependent on the antigen-presenting function of B cells in the GC for maintenance.31 In cGVHD, a decrease in IL-21 could potentially play multiple roles independent of Tfh cell involvement, including increase in induced T regulatory cells,14 possibly including T follicular regulatory cells. For example, IL-21 is important in the production of higher-affinity antibodies by stabilizing the expression of Bcl6.11 This is consistent with our findings that IL-21 signaling in B cells is necessary to produce Ig that is deposited in the lung and associated with BOS (Figure 5). Our data using IL-21 KO T cells, IL-21R KO BM cells, and neutralizing anti-IL-21 mAb collectively indicate that the IL-21/IL-21R pathway is a promising target for therapeutics. Together, these data suggest that naïve donor T cells differentiate into Tfh cells that support GC formation after alloactivation, resulting in the upregulation of CXCR5, ICOS, CD40L, and IL-21 production, which then work in concert to migrate to secondary lymphoid organs and drive GC B-cell differentiation and GC formation.
ICOS and CD40 signaling are required for the establishment of GCs and have been demonstrated to eliminate GC reactions upon blockade.32,33 The presence of Tfh cells and the stability of GC are contingent upon ICOS and CD40 expression33 and are necessary for the progression of cGVHD in our model (Figure 6 and supplemental Figure 2). ICOS could also be necessary for T-cell activation independent of GCs, as demonstrated when ICOS KO T cells are used in acute GVHD.15 By using either blocking anti-ICOS or anti-CD40 ligand mAbs, we have demonstrated that disease progression is prevented or even reversed, revealing that the maintenance of GCs is essential in cGVHD. Although data presented here suggest that ICOS, CD40, and IL-21 blockade are effective in treating cGVHD associated with the dampening of the Tfh cell response, alternative mechanisms may exist. For example, it has previously been demonstrated that anti-IL-21 can increase peripheral-derived T regulatory cells.14 The increase in T regulatory cells may dampen the GC response. Moreover, alloactivated T effector cells might directly contribute to tissue injury and work alone or in concert with Tfh cells to cause cGVHD. By blocking IL-21, ICOS, or CD40L signaling, T-cell proliferation, survival, or effector function may be compromised, resulting in reduced tissue injury and cGVHD, independent of the requirement for Tfh cells in the cGVHD process.
The use of this BOS murine model of cGVHD should be readily translatable to human cGVHD. The National Institutes of Health consensus group defined the diagnosis of BOS as a pathognomonic symptom of lung cGVHD.34 By using clinically relevant functional, histopathologic, and immunologic markers of cGVHD as readouts, we can confirm the cGVHD disease state. Moreover, Srinivasan et al5 demonstrated that the BOS model represents a multiorgan syndrome that is consistent with human cGVHD pathology. Finally, prior to interventions, we established cGVHD instead of taking a prophylactic approach. This is consistent with diagnosis and starting patients on treatment following allogeneic hematopoietic stem cell transplantation. However, it is important to point out that there are several potential adverse events associated with the proposed therapeutics. For example, blocking IL-21, ICOS, or CD40L with mAbs might increase either T-cell responsive infections or the rate of leukemia relapse as a result of dampening of peripheral immune surveillance mechanisms. Finally, our discovery that Tfh cells are upregulated in murine cGVHD warrants investigation of this cell population in the peripheral blood35 of cGVHD patients. If correlations are noted with cGVHD onset or severity, Tfh cells could be targeted, as we have done in this study, which may prove useful for treating or perhaps preventing cGVHD in the clinic.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Novo Nordisk and Drs. Dorthe Lundsgaard and Pallavur Sivakumar for providing neutralizing anti-IL-21 mAb and Jeff Browning for providing anti-CD20 antibody.
Supported in part by National Institutes of Health National Cancer Institute grant P01 CA142106-06A1, National Institutes of Allergy and Infectious Diseases grants P01 AI 056299 and T32 AI 007313, and National Heart, Lung, and Blood Institute grant T32 HL 00706237. The use of the confocal microscope was supported by grant S10 RR16851 from the National Center for Research Resources Shared Instrumentation.
Authorship
Contribution: R.F. designed and performed experiments, analyzed data, and wrote the paper; J.D., R.G.V., and D.K.R. performed experiments and analyzed data, designed experiments, and edited the paper; P.A.T. designed and performed experiments, analyzed data, and edited the paper; A.P.M., G.J.F., J.S.S., D.H.M., S.S., I.M., J.K., C.C., R.J.S., J.R., J.H.A., W.J.M., L.L., J.A.D., J.C.B., K.P.M., and G.R.H. discussed experimental design and analysis and edited the paper; and B.R.B. designed and analyzed experiments and edited the paper.
Conflict-of-interest disclosure: G.F. has patents and receives patent royalties on the PD-1 pathway from Bristol-Myers Squibb, Merck, Roche, EMD Serrono, Boehringer Ingelheim, Amplimmune, and CoStim Pharmaceuticals. G.F. is a scientific founder and scientific advisory board member of CoStim Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Bruce R. Blazar, University of Minnesota, 420 Delaware St SE, MMC 109, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal