Key Points
Loss of TET2 accelerates the degree of malignancy of MPNs in combination with JAK2V617F.
Loss of TET2 sustains MPNs in combination with JAK2V617F.
Abstract
Acquired mutations of JAK2 and TET2 are frequent in myeloproliferative neoplasms (MPNs). We examined the individual and cooperative effects of these mutations on MPN development. Recipients of JAK2V617F cells developed primary myelofibrosis–like features; the addition of loss of TET2 worsened this JAK2V617F-induced disease, causing prolonged leukocytosis, splenomegaly, extramedullary hematopoiesis, and modestly shorter survival. Double-mutant (JAK2V617F plus loss of TET2) myeloid cells were more likely to be in a proliferative state than JAK2V617F single-mutant myeloid cells. In a serial competitive transplantation assay, JAK2V617F cells resulted in decreased chimerism in the second recipients, which did not develop MPNs. In marked contrast, cooperation between JAK2V617F and loss of TET2 developed and maintained MPNs in the second recipients by compensating for impaired hematopoietic stem cell (HSC) functioning. In-vitro sequential colony formation assays also supported the observation that JAK2V617F did not maintain HSC functioning over the long-term, but concurrent loss of TET2 mutation restored it. Transcriptional profiling revealed that loss of TET2 affected the expression of many HSC signature genes. We conclude that loss of TET2 has two different roles in MPNs: disease accelerator and disease initiator and sustainer in combination with JAK2V617F.
Introduction
Myeloproliferative neoplasms (MPNs) are clinically characterized by the chronic overproduction of differentiated peripheral blood cells and a gradual expansion of malignant intramedullary/extramedullary hematopoiesis. Mutations in Janus kinase 2 (JAK2),1-4 myeloproliferative leukemia protein,5 and the recently reported calreticulin6,7 are considered to play a “driver” role in MPNs. JAK2 mutations (eg, JAK2V617F and JAK2 exon 12 mutations) are the most frequent of these; almost all polycythemia vera (PV) patients and about half of essential thrombocythemia (ET) and primary myelofibrosis (PMF) patients have these mutations.1-4,8,9 JAK2V617F mutations induce the phosphorylation of Janus kinases and signal transducers and activators of transcription (STATs) in cytokine-dependent cell lines without cytokine stimulation, and contribute to autonomous cell growth.1,4 JAK2V617F leads to MPNs in all transgenic10-12 or knockin mouse13-16 models, and is thought to play a central role in MPN development.
In addition to the above driver mutations, mutations in epigenetic regulators such as TET2, DNMT3A, ASXL1, EZH2, and IDH1/2 have been reported.17 Of these, TET2, a methylcytosine dioxygenase that converts 5-methylcytosine to 5-hydroxymethylcytosine (5-hmC),18-20 is mutated in chronic-phase MPNs, specifically in 10% to 17% of PV, 4% to 11% of ET, and 8% to 25% of PMF.21-23 TET2 is also mutated in 13% to 32% of blastic-phase MPNs and is involved in the leukemic transformation of MPNs.21,24-27 Mutations of TET2 result in loss of function; in fact, mutations of TET2 caused decreased levels of 5-hmC in MPN patients.19 TET2 knockout and knockdown lead to subtle phenotypes in MPNs, although they strongly increase the in-vivo repopulating capacity of hematopoietic stem cells (HSCs).20,28-31
Exome sequencing of DNA from many MPN patients revealed that the median number of mutations per patient was 6.5 in PV, 6.5 in ET, and 13.0 in PMF.6 Despite this mutation analysis study in MPN patients and the above-mentioned mouse studies, it still seems unclear whether a single gene mutation is sufficient for the initiation of human MPNs. To identify the mechanism underlying the initiation and development of MPNs, we focused on JAK2V617F and loss of TET2—the former as a representative of driver gene mutations and the latter as a representative of mutations in epigenetic regulators—and examined the influence of single or double mutations on stem/progenitor behavior, clonal evolution, and disease phenotype in MPNs.
Methods
Mice
JAK2V617F transgenic mice (JAK2V617F-TG)12 and TET2 heterozygous knockdown (TET2trap/+) mice31 were previously generated and reported. They were backcrossed at least 8 times onto C57BL/6 mice (B6-CD45.2). The TET2trap/trap mice frequently died during the neonatal period on the B6-CD45.2 background; therefore, we used their fetal liver (FL) cells (FLs) for the transplantation assays. B6 mice congenic for the CD45.1 locus (B6-CD45.1) were purchased from Sankyo Laboratory Service.
We crossed male JAK2V617F-TET2trap/+ mice with female TET2trap/+ mice, and obtained the following 4 types of E14.5 fetuses for the transplantation assays: JAK2WT-TET2WT (WT; wild-type), JAK2WT-TET2trap/trap (TET2KD), JAK2V617F-TET2WT (JAK2V617F), and JAK2V617F-TET2trap/trap (double-mutant). Animal studies were performed in accordance with the local University of Miyazaki Ethics Committee.
Transplantation assays
We intravenously transplanted 1 × 106 of each of the E14.5 FL cell types (B6-CD45.2) into lethally irradiated 8-week-old B6-CD45.1 mice. Transplanted recipients were sequentially analyzed in terms of complete blood cell counts, cell surface markers, and histologic examination of bone marrow (BM) and spleen. In competitive serial transplantation assays, we mixed 1 × 106 of each of the FL cell types and 5 × 106 of WT BM cells (B6-CD45.1), and transplanted them into mice. At 16 weeks after the first transplantations, second transplantations were carried out by transferring 1 × 106 BM cells from the first recipients.
FACS analysis
Fluorescence-activated cell sorter (FACS) analysis was performed using a MACSQuant Analyzer (Miltenyi Biotec) as previously described.32 FACS antibodies are listed in the supplemental Methods found on the Blood Web site. For cell-sorting experiments, lineage-positive cells were depleted with MACS beads (Miltenyi Biotec), and lineage−Sca-1+c-Kit+ (LSK) cells (LSKs) were sorted on a FACSAria II (BD Biosciences). Data were analyzed with FlowJo software (Tree Star).
Colony replating assay
Fifty thousand whole BM cells were plated in methylcellulose (StemCell Technologies) according to the manufacturer’s instructions. After culture for 1 week, colonies were counted, and single-cell suspensions (5 × 104 cells) of colonies were subsequently replated under identical conditions. Each week, replating was repeated in the same way.
Histologic examination
Tissue samples were fixed in formalin, paraffin embedded, and sectioned for hematoxylin and eosin staining or Gomori silver staining according to standard protocols. Megakaryocytes in BM at 6 to 8 weeks posttransplantation were quantitatively assessed in size by measuring the diameter of the cytoplasm. For immunohistologic evaluation, after autoclave pretreatment, tissue samples were stained by Ki-67 primary antibody (Abcam) and biotinylated secondary antibody. Standard Ultra-Sensitive ABC Peroxidase Staining kit (Pierce) and 3,3′-diaminobenzidine (Vector Laboratories) were used for detection and visualization.
DNA damage, cell cycle, and apoptotic status of LSKs
Mice were given bromodeoxyuridine (BrdU)-containing water (1 mg/mL) for 7 days. BM cells were analyzed using the Apoptosis, DNA Damage, and Cell Proliferation kit (BD Biosciences). CD45.2-LSKs were gated, and the proportion of DNA damaged, cycling, and apoptotic cells was assessed by phosphorylated histone H2AX (γH2AX), BrdU, and cleaved poly (ADP-ribose) polymerase positivity, respectively.
Microarray
RNA was isolated from 2 to 6 × 104 BM-LSKs obtained from recipients of the 4 types of FLs. The samples were amplified using the Ovation Pico WTA System V2 (NuGen). Two micrograms of purified, amplified complementary DNA were labeled with Cyanine 3 using the Genomic DNA Enzymatic Labeling Kit, and then hybridized on a Mouse GE 8x60K array, and the data were analyzed with GeneSpring (Agilent). Gene set enrichment analysis (GSEA) was performed across the complete list of genes ranked by signal-to-noise ratio according to their differential expression.33 All data are compliant with Minimum Information About a Microarray Experiment standards, and the raw data were deposited in the Gene Expression Omnibus database (accession number GSE62302).
Results
Loss of TET2 worsens JAK2V617F-induced MPNs
To compare the phenotypes of the single-mutation JAK2V617F-induced MPNs and the double-mutation JAK2V617F- and TET2KD-induced MPNs, considering the neonatal death of TET2KD mice and a necessity to exclude the influence of mutations in BM niche cells, we performed noncompetitive transplantation assays. We transplanted 4 types of E14.5 FLs, namely WT, TET2KD, JAK2V617F, and double-mutant, into lethally irradiated recipients. The fraction of LSKs in TET2KD-FLs and in double-mutant-FLs increased compared with that in WT-FLs, but the proportion of long-term HSCs (CD150+ LSKs) was comparable among the 4 types of E14.5 FLs (supplementary Figure 1).
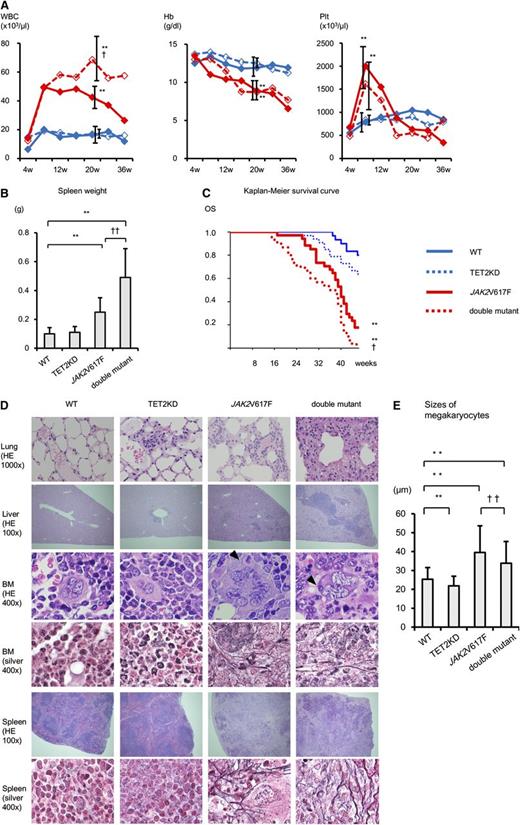
Compared with the recipients transplanted with WT cells, the recipients of TET2KD cells at 20 to 28 weeks after transplantation showed normal blood cell count, no splenomegaly, comparable overall survival duration, and minimal extramedullary hematopoiesis of the lung and liver, indicating that TET2KD cells developed a subtle clinical phenotype similar to chronic myelomonocytic leukemia–like MPNs (Figure 1). However, the recipients of JAK2V617F cells at 20 to 28 weeks after transplantation showed leukocytosis, anemia, thrombocytosis, splenomegaly, shorter survival duration, moderate extramedullary hematopoiesis, and fibrosis in BM and spleen, indicating that JAK2V617F cells induced clinically PMF-like MPNs. Consistent with this finding, megakaryocytes from recipients of JAK2V617F cells formed dense clusters and were significantly larger than those from recipients of WT cells. They also showed an abnormal nuclear-to-cytoplasmic ratio, hyperlobular nuclei, and emperipolesis. Furthermore, double-mutant cells showed not only the phenotype of JAK2V617F cell recipients but also prolonged leukocytosis, splenomegaly, and severe extramedullary hematopoiesis, with modestly shorter overall survival. Megakaryocytes in recipients of double-mutant cells were smaller than those in recipients of JAK2V617F cells (but still larger than those in recipients of WT cells), and showed additional atypical nuclear features such as hypolobulation. These results indicate that the combination of loss of TET2 and JAK2V617F worsened the disease compared with single-mutant JAK2V617F-induced MPNs.
Loss of TET2 function worsens JAK2V617F-induced MPNs. (A) The average complete blood cell counts of recipients every 4 weeks after transplantation (4-36 weeks) (n = 10). Compared with WT mice, JAK2V617F mice and double-mutant mice showed leukocytosis and anemia at 20 weeks and thrombocytosis at 8 weeks. Compared with JAK2V617F mice, double-mutant mice showed prolonged leukocytosis. (B) Spleen weight of recipient mice at 20 to 28 weeks after transplantation (n = 10). Compared with WT mice, JAK2V617F mice and double-mutant mice showed splenomegaly. Compared with JAK2V617F mice, double-mutant mice presented with more severe splenomegaly. (C) Kaplan-Meier survival curves of WT (n = 30), TET2KD (n = 33), JAK2V617F (n = 34), and double-mutant (n = 30) mice. The overall survival (OS) of JAK2V617F mice was worse than WT mice. Compared with JAK2V617F mice, double-mutant mice had modestly shorter survival duration. (D) Histologic analysis of recipient mice at 20 to 28 weeks after transplantation. Lung, liver, BM, and spleen stained with hematoxylin and eosin (HE); BM and spleen with Gomori silver stain. TET2KD mice show minimal extramedullary hematopoiesis of lung and liver. JAK2V617F mice show moderate extramedullary hematopoiesis, abnormal megakaryocytes, and fibrosis in BM and spleen. Double-mutant mice show severe extramedullary hematopoiesis, abnormal megakaryocytes, and fibrosis in BM. (E) Sizes of megakaryocytes in BM of recipients. In each group, the size of 100 megakaryocytes was measured. Megakaryocytes in JAK2V617F mice were significantly larger. Megakaryocytes in double-mutant mice were smaller than those in JAK2V617F mice but still larger than those in WT mice. Error bars represent standard deviation. *P < .05 and **P < .01 vs WT mice; †P < .05 and ††P < .01 vs JAK2V617F mice. Hb, hemoglobin; Plt, platelets; WBC, white blood cells.
Loss of TET2 function worsens JAK2V617F-induced MPNs. (A) The average complete blood cell counts of recipients every 4 weeks after transplantation (4-36 weeks) (n = 10). Compared with WT mice, JAK2V617F mice and double-mutant mice showed leukocytosis and anemia at 20 weeks and thrombocytosis at 8 weeks. Compared with JAK2V617F mice, double-mutant mice showed prolonged leukocytosis. (B) Spleen weight of recipient mice at 20 to 28 weeks after transplantation (n = 10). Compared with WT mice, JAK2V617F mice and double-mutant mice showed splenomegaly. Compared with JAK2V617F mice, double-mutant mice presented with more severe splenomegaly. (C) Kaplan-Meier survival curves of WT (n = 30), TET2KD (n = 33), JAK2V617F (n = 34), and double-mutant (n = 30) mice. The overall survival (OS) of JAK2V617F mice was worse than WT mice. Compared with JAK2V617F mice, double-mutant mice had modestly shorter survival duration. (D) Histologic analysis of recipient mice at 20 to 28 weeks after transplantation. Lung, liver, BM, and spleen stained with hematoxylin and eosin (HE); BM and spleen with Gomori silver stain. TET2KD mice show minimal extramedullary hematopoiesis of lung and liver. JAK2V617F mice show moderate extramedullary hematopoiesis, abnormal megakaryocytes, and fibrosis in BM and spleen. Double-mutant mice show severe extramedullary hematopoiesis, abnormal megakaryocytes, and fibrosis in BM. (E) Sizes of megakaryocytes in BM of recipients. In each group, the size of 100 megakaryocytes was measured. Megakaryocytes in JAK2V617F mice were significantly larger. Megakaryocytes in double-mutant mice were smaller than those in JAK2V617F mice but still larger than those in WT mice. Error bars represent standard deviation. *P < .05 and **P < .01 vs WT mice; †P < .05 and ††P < .01 vs JAK2V617F mice. Hb, hemoglobin; Plt, platelets; WBC, white blood cells.
Most myeloid cells with both JAK2V617F and loss of TET2 mutations are in the proliferative phase
Because the phenotype of recipients of double-mutant cells was more unfavorable than that of JAK2V617F cell recipients, we evaluated the proliferative activity of myeloid lineage cells from all 4 recipient groups at 20 to 28 weeks after transplantation. The proportion of Ki-67-positive myeloid cells in BM from recipients of TET2KD and that from recipients of JAK2V617F were greater than that from recipients of WT cells; this proportion was highest in recipients of double-mutant cells (Figure 2). The same pattern was observed for Ki-67-positive myeloid cells in the spleen. Double-mutant myeloid cells were more likely to be in a proliferative state compared with JAK2V617F myeloid cells in both BM and spleen.
JAK2V617F and TET2KD cooperatively drive cell proliferation. (A) Percentage of immunohistochemically Ki-67-positive myeloid cells in BM. TET2KD mice and JAK2V617F mice showed increased Ki-67 positivity relative to WT mice. Double-mutant mice showed significantly increased positivity relative to single-mutant mice. (B) Percentage of immunohistochemically Ki-67-positive cells in spleen. The pattern of results is similar to that observed in BM. Error bars represent standard deviation. *P < .05 and **P < .01 vs WT mice; †P < .05 vs JAK2V617F mice. (C) Representative immunohistochemical staining for Ki-67 in BM and spleen of recipients.
JAK2V617F and TET2KD cooperatively drive cell proliferation. (A) Percentage of immunohistochemically Ki-67-positive myeloid cells in BM. TET2KD mice and JAK2V617F mice showed increased Ki-67 positivity relative to WT mice. Double-mutant mice showed significantly increased positivity relative to single-mutant mice. (B) Percentage of immunohistochemically Ki-67-positive cells in spleen. The pattern of results is similar to that observed in BM. Error bars represent standard deviation. *P < .05 and **P < .01 vs WT mice; †P < .05 vs JAK2V617F mice. (C) Representative immunohistochemical staining for Ki-67 in BM and spleen of recipients.
The influence of JAK2V617F and TET2KD on hematopoietic stem/progenitor cells
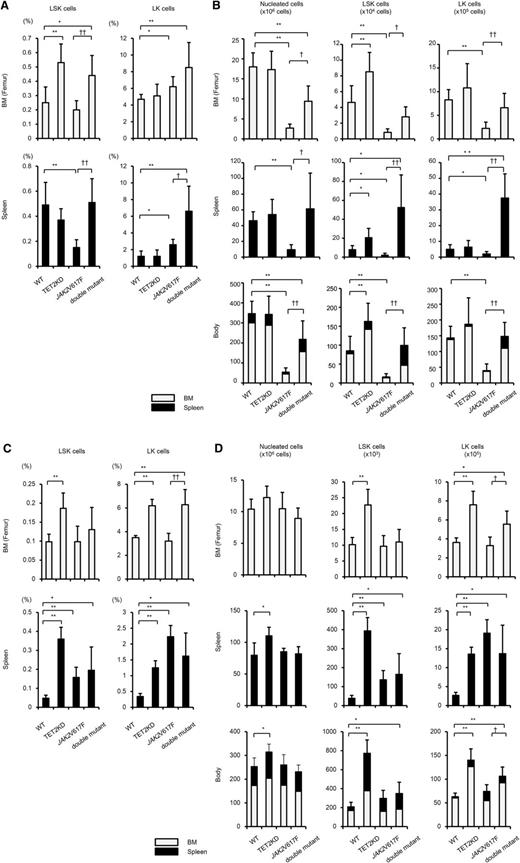
To characterize the impact of JAK2V617F-induced or double-mutant-induced MPNs in detail, we evaluated the frequencies and absolute numbers of LSKs and those of lineage−c-Kit+ (LK) cells (LKs) in the femur, spleen, and body of recipients using a noncompetitive transplantation assay (Figure 3).
The influence of JAK2V617F and TET2KD on hematopoietic stem/progenitor cells. The proportion (A,C) and absolute numbers of LSKs and LKs (B,D), and the absolute numbers of nucleated cells in the femur (1 per mouse; upper column), spleen (middle column), and body (bottom column) of recipients transplanted with 4 types of cells at 20 to 28 weeks (n = 5-9) (A-B) and at 6 to 8 weeks (n = 4) (C-D) after transplantation. Cell numbers in the body were calculated by summing estimated numbers in whole-body BM and in the spleen, as previously described.34,35 (A) Compared with WT mice, TET2KD mice and double-mutant mice showed increased BM-LSK frequencies, and JAK2V617F mice showed decreased spleen-LSK frequencies; JAK2V617F and double-mutant mice showed increased BM-LK frequencies and spleen-LK frequencies. (B) Compared with WT mice, JAK2V617F mice showed decreased nucleated cells in the femur, spleen, and body. Consequently, LSKs and LKs decreased in the femur, spleen, and body of recipients of JAK2V617F cells. Compared with JAK2V617F mice, double-mutant mice showed increased nucleated cells, LSKs, and LKs in the femur, spleen, and body, and double-mutant mice had comparable numbers of LSKs and LKs in the body as WT mice. (C) Compared with WT mice, TET2KD mice showed increased BM- and spleen-LSK frequencies, and JAK2V617F mice and double-mutant mice showed decreased spleen-LSK frequencies; TET2KD mice and double-mutant mice showed increased BM- and spleen-LK frequencies, and JAK2V617F mice showed increased spleen-LK frequencies. (D) Compared with WT mice, TET2KD mice showed increased nucleated cells in the spleen and body, and increased LSKs and LKs in the femur, spleen, and body; JAK2V617F mice showed increased LSKs and LKs in the spleen; double-mutant mice showed increased LSKs in the body and increased LKs in the femur, spleen, and body. Error bars represent standard deviation. *P < .05 and **P < .01 vs WT mice; †P < .05 and ††P < .01 vs JAK2V617F mice. In the bottom column, the gray bar represents the average cell number in whole BM, the black bar represents that in spleen, and the sum of both represents that in body.
The influence of JAK2V617F and TET2KD on hematopoietic stem/progenitor cells. The proportion (A,C) and absolute numbers of LSKs and LKs (B,D), and the absolute numbers of nucleated cells in the femur (1 per mouse; upper column), spleen (middle column), and body (bottom column) of recipients transplanted with 4 types of cells at 20 to 28 weeks (n = 5-9) (A-B) and at 6 to 8 weeks (n = 4) (C-D) after transplantation. Cell numbers in the body were calculated by summing estimated numbers in whole-body BM and in the spleen, as previously described.34,35 (A) Compared with WT mice, TET2KD mice and double-mutant mice showed increased BM-LSK frequencies, and JAK2V617F mice showed decreased spleen-LSK frequencies; JAK2V617F and double-mutant mice showed increased BM-LK frequencies and spleen-LK frequencies. (B) Compared with WT mice, JAK2V617F mice showed decreased nucleated cells in the femur, spleen, and body. Consequently, LSKs and LKs decreased in the femur, spleen, and body of recipients of JAK2V617F cells. Compared with JAK2V617F mice, double-mutant mice showed increased nucleated cells, LSKs, and LKs in the femur, spleen, and body, and double-mutant mice had comparable numbers of LSKs and LKs in the body as WT mice. (C) Compared with WT mice, TET2KD mice showed increased BM- and spleen-LSK frequencies, and JAK2V617F mice and double-mutant mice showed decreased spleen-LSK frequencies; TET2KD mice and double-mutant mice showed increased BM- and spleen-LK frequencies, and JAK2V617F mice showed increased spleen-LK frequencies. (D) Compared with WT mice, TET2KD mice showed increased nucleated cells in the spleen and body, and increased LSKs and LKs in the femur, spleen, and body; JAK2V617F mice showed increased LSKs and LKs in the spleen; double-mutant mice showed increased LSKs in the body and increased LKs in the femur, spleen, and body. Error bars represent standard deviation. *P < .05 and **P < .01 vs WT mice; †P < .05 and ††P < .01 vs JAK2V617F mice. In the bottom column, the gray bar represents the average cell number in whole BM, the black bar represents that in spleen, and the sum of both represents that in body.
At 20 to 28 weeks after transplantation, the frequency of LSKs in the femur was higher in recipients of TET2KD or double-mutant cells, and that in the spleen was lower in recipients of JAK2V617F cells compared with recipients of WT cells (Figure 3A). The frequency of LKs in both the femur and spleen was greater in recipients of JAK2V617F or double-mutant cells compared with recipients of WT cells. The most striking difference among the recipients of the 4 cell types was the decreased number of nucleated cells in recipients of JAK2V617F cells (Figure 3B). Consequently, in an estimation of the absolute number of stem/progenitor cells in the body,34,35 the recipients of TET2KD cells showed an increased number of LSKs but no increase in LKs compared with the recipients of WT cells. The recipients of JAK2V617F cells showed a decreased number of all-stage stem/progenitor cells (LSKs and LKs), and this decrement was restored in recipients of double-mutant cells (Figure 3B). These data suggest that TET2KD primarily affects the kinetics of early-stage stem/progenitor cells and increases their pool size, and that JAK2V617F diminishes all-stage stem/progenitor pool size. It should be noted, however, that recipients of JAK2V617F cells developed BM fibrosis at 20 to 28 weeks after transplantation (Figure 1D), with a decreased number of nucleated cells in both the femur and spleen. We performed the same types of experiments in recipients at 6 to 8 weeks after transplantation, and found that at this time point, BM fibrosis did not occur (data not shown).
In contrast, at 6 to 8 weeks after transplantation, recipients of JAK2V617F cells showed comparable nucleated cells both in the femur and spleen compared with recipients of WT cells, which culminated in comparable numbers of LSKs and LKs in the bodies of JAK2V617F-cell and WT-cell recipients (Figure 3C-D). Recipients of TET2KD and double-mutant cells at 6 to 8 weeks after transplantation showed a higher number of all-stage stem/progenitor cells in the body compared with those receiving WT cells.
These data indicate that although stem/progenitor pool size was not affected by JAK2V617F early after transplantation, it was diminished in later stages, accompanied by a decreased number of mononuclear cells and BM fibrosis. The cooperation between JAK2V617F and TET2KD results in dysregulated extramedullary migration, as well as maintenance of early-stage stem/progenitor cells over the long-term, along with abnormal proliferation of late-stage stem/progenitor cells and terminally differentiated cells in extramedullary sites.
Cooperation between JAK2V617F and TET2KD leads to increased self-renewal capacity in vitro
To identify the qualitative differences among HSCs with JAK2V617F or TET2KD single or double mutations, we performed colony replating assays of whole BM cells from the 4 types of FL-transplanted recipients. The proportions of LSKs and long-term HSCs in the femur at 20 to 28 weeks after transplantation were not comparable among the 4 types of mice, but the values in recipients of the 3 non-WT cell types were greater than 0.5-fold and less than twofold of those in recipients of WT cells (Figure 3A and supplemental Figure 2). Compared with WT cells, TET2KD cells showed increased colony numbers and had sequential colony replating capacity. The colony formation assay revealed reduced serial replating capacity in JAK2V617F cells, but this was restored in cells with compound JAK2V617F/TET2KD (Figure 4). In-vitro colony assay experiments suggested that JAK2V617F decreases the self-renewal capacity of HSCs, whereas the cooperation between JAK2V617F and TET2KD maintains and enhances it.
TET2 knockdown restores the JAK2V617F-induced impaired self-renewal capacity in vitro. Enumeration of colonies and serial replating capacity of 5 × 104 BM cells from recipients transplanted with 4 types of cells at 20 to 28 weeks after transplantation (n = 7-9). Compared to the BM cells from WT mice, those from JAK2V617F mice generated smaller numbers of colonies in vitro, whereas those from TET2KD mice generated larger numbers. Serial replating assays revealed restored serial replating capacity of cells by loss of TET2 in JAK2V617F BM cells. Error bars represent standard deviation. *P < .05 and **P < .01 vs WT mice; †P < .05 and ††P < .01 vs JAK2V617F mice.
TET2 knockdown restores the JAK2V617F-induced impaired self-renewal capacity in vitro. Enumeration of colonies and serial replating capacity of 5 × 104 BM cells from recipients transplanted with 4 types of cells at 20 to 28 weeks after transplantation (n = 7-9). Compared to the BM cells from WT mice, those from JAK2V617F mice generated smaller numbers of colonies in vitro, whereas those from TET2KD mice generated larger numbers. Serial replating assays revealed restored serial replating capacity of cells by loss of TET2 in JAK2V617F BM cells. Error bars represent standard deviation. *P < .05 and **P < .01 vs WT mice; †P < .05 and ††P < .01 vs JAK2V617F mice.
Both JAK2V617F and TET2KD are required to sustain MPNs over long periods
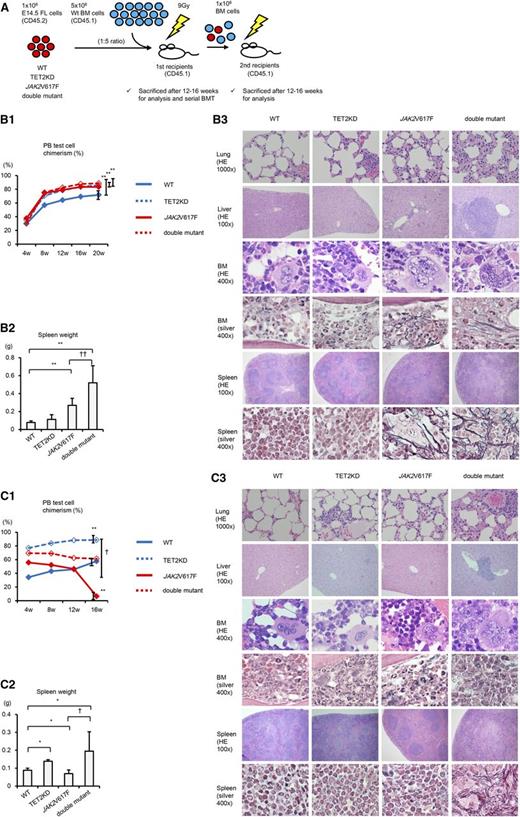
We next performed competitive and serial transplantation assays in which we transplanted the 4 cell types—WT, TET2KD, JAK2V617F, and double-mutant E14.5 FLs (B6-CD45.2)—and competitor WT BM cells (B6-CD45.1) in a 1:5 ratio into lethally irradiated recipients (B6-CD45.1), and then continued serial transplantations of recipient 1 × 106 BM cells into a second set of lethally irradiated recipients (B6-CD45.1) (Figure 5A). The proportion of CD45.2-LSKs from the BM of first recipient mice was almost the same in WT, JAK2V617F, and double-mutant mice, whereas the first recipients of TET2KD cells had a slightly higher LSK cell proportion compared with the recipients of WT cells (supplemental Figure 3).
Both JAK2V617F and TET2 knockdown are required to sustain MPNs over long periods. (A) Schematic depiction of competitive and serial transplantation assay. FLs (CD45.2, 1 × 106 cells) and competitor WT BM cells (CD45.1, 5 × 106 cells) were cotransplanted into lethally irradiated recipients (CD45.1), and serial BM transplantation was performed using 1 × 106 recipient BM cells after 16 weeks posttransplantation. (B) Assessment of donor chimerism in the peripheral blood (PB; B1), spleen weight (B2), and histologic analysis (B3) of the first recipients. (B1) Chimerism at 20 weeks was significantly higher in all JAK2V617F, TET2KD, and double-mutant mice compared with WT mice (n = 10, each group). (B2) Spleens in first recipient mice after 12 weeks transplanted with JAK2V617F and double-mutant cells were much heavier than those receiving WT cells (n = 8-10, each group). (B3) Compared with WT mice, TET2KD mice show very mild extramedullary hematopoiesis; JAK2V617F mice show moderate extramedullary hematopoiesis, abnormal megakaryocytes, and fibrosis in BM and spleen; double-mutant mice show severe extramedullary hematopoiesis, abnormal megakaryocytes, and fibrosis. Compared with JAK2V617F mice, double-mutant mice exhibit more severe splenomegaly and extramedullary hematopoiesis. Hematoxylin and eosin (HE) stain and Gomori silver stain. Error bars represent standard deviation. *P < .05 and **P < .01 vs WT; †P < .05 and ††P < .01 vs JAK2V617F. (C) Assessment of donor chimerism in the PB (C1), spleen weight (C2), and histologic analysis (C3) of the second recipients. (C1) Chimerism at 16 weeks was significantly higher in TET2KD and lower in JAK2V617F mice compared with WT mice (n = 4, each group). Double-mutant mice showed sustained chimerism. (C2) Spleens in second recipient mice 12 weeks after transplantation with TET2KD and double-mutant cells were much heavier, and those in JAK2V617F were much lighter than those in WT cells (n = 6-8, each group). (C3) Compared with WT mice, TET2KD mice show mild extramedullary hematopoiesis; JAK2V617F mice show almost normal histologic findings; double-mutant mice exhibit severe extramedullary hematopoiesis, abnormal megakaryocytes, and fibrosis in BM and spleen. HE stain and Gomori silver stain. Error bars represent standard deviation. *P < .05 and **P < .01 vs WT; †P < .05 and ††P < .01 vs JAK2V617F.
Both JAK2V617F and TET2 knockdown are required to sustain MPNs over long periods. (A) Schematic depiction of competitive and serial transplantation assay. FLs (CD45.2, 1 × 106 cells) and competitor WT BM cells (CD45.1, 5 × 106 cells) were cotransplanted into lethally irradiated recipients (CD45.1), and serial BM transplantation was performed using 1 × 106 recipient BM cells after 16 weeks posttransplantation. (B) Assessment of donor chimerism in the peripheral blood (PB; B1), spleen weight (B2), and histologic analysis (B3) of the first recipients. (B1) Chimerism at 20 weeks was significantly higher in all JAK2V617F, TET2KD, and double-mutant mice compared with WT mice (n = 10, each group). (B2) Spleens in first recipient mice after 12 weeks transplanted with JAK2V617F and double-mutant cells were much heavier than those receiving WT cells (n = 8-10, each group). (B3) Compared with WT mice, TET2KD mice show very mild extramedullary hematopoiesis; JAK2V617F mice show moderate extramedullary hematopoiesis, abnormal megakaryocytes, and fibrosis in BM and spleen; double-mutant mice show severe extramedullary hematopoiesis, abnormal megakaryocytes, and fibrosis. Compared with JAK2V617F mice, double-mutant mice exhibit more severe splenomegaly and extramedullary hematopoiesis. Hematoxylin and eosin (HE) stain and Gomori silver stain. Error bars represent standard deviation. *P < .05 and **P < .01 vs WT; †P < .05 and ††P < .01 vs JAK2V617F. (C) Assessment of donor chimerism in the PB (C1), spleen weight (C2), and histologic analysis (C3) of the second recipients. (C1) Chimerism at 16 weeks was significantly higher in TET2KD and lower in JAK2V617F mice compared with WT mice (n = 4, each group). Double-mutant mice showed sustained chimerism. (C2) Spleens in second recipient mice 12 weeks after transplantation with TET2KD and double-mutant cells were much heavier, and those in JAK2V617F were much lighter than those in WT cells (n = 6-8, each group). (C3) Compared with WT mice, TET2KD mice show mild extramedullary hematopoiesis; JAK2V617F mice show almost normal histologic findings; double-mutant mice exhibit severe extramedullary hematopoiesis, abnormal megakaryocytes, and fibrosis in BM and spleen. HE stain and Gomori silver stain. Error bars represent standard deviation. *P < .05 and **P < .01 vs WT; †P < .05 and ††P < .01 vs JAK2V617F.
As already reported,28-31 transplanted TET2KD cells resulted in higher peripheral blood chimerism in the first and second recipients (Figure 5B-C). Greater extramedullary hematopoiesis was also observed in these recipients compared with recipients transplanted with WT cells, but splenomegaly was very mild in only the second recipients. Mice transplanted with JAK2V617F cells developed MPNs characterized by higher chimerism, leukocytosis, thrombocytosis, moderate splenomegaly, moderate extramedullary hematopoiesis, abnormal megakaryocytes, and fibrosis in the first recipients (Figure 5B and supplemental Figure 4); however, JAK2V617F cells resulted in decreased chimerism in the second recipients. The second recipients of JAK2V617F cells did not develop MPNs with normal histologic findings in either spleen or BM and had favorable survival times, although they demonstrated leukopenia (Figure 5C and supplemental Figure 4). In marked contrast, both the first and second recipient mice transplanted with double-mutant cells developed MPNs characterized by splenomegaly, extramedullary hematopoiesis, abnormal megakaryocytes, and fibrosis, and the second recipients of double-mutant cells had shorter survival times (Figure 5 and supplemental Figure 4). Furthermore, the peripheral blood chimerism derived from double-mutant cells was sustained in the second recipients. These competitive serial transplantation assays suggest that JAK2V617F alone results in decreased HSC self-renewal capacity in vivo, does not confer a competitive growth advantage to HSCs over long-term follow-up, and has difficulty in sustaining MPNs against normal hematopoiesis over long periods. In addition, cooperation between JAK2V617F and TET2KD results in increased HSC self-renewal capacity in vivo, confers a competitive growth advantage to HSCs, and efficiently develops and sustains MPNs against normal hematopoiesis.
Loss of TET2 interferes with cell-cycle progression in JAK2V617F-LSKs and restores their HSC signature
To examine the mechanism responsible for both the decreased HSC self-renewal capacity caused by JAK2V617F and the compensation in this decrease by the addition of loss of TET2, we assessed DNA damage, cell-cycle status, and apoptosis in LSKs from the second recipients of the 4 types of cells at 16 weeks after transplantation (Figure 6A). We did not observe an increment of γH2AX positivity in JAK2V617F-LSKs, and there was no difference in degree of γH2AX positivity among the 4 types of LSKs. The proportion of actively cycling JAK2V617F-LSKs was greater than in WT-LSKs, and this was lowered to levels comparable to WT-LSKs by loss of TET2 (Figure 6A).
Loss of TET2 interferes with cell-cycle progression and gene expression in JAK2V617F-LSKs. (A) DNA damage, cell cycle, and apoptotic status of mutant LSKs assessed by flow cytometry. Bone marrow CD45.2-LSKs from second recipients at 16 weeks after transplantation in a competitive transplantation assay were gated, and the proportion of DNA damaged, cycling, and apoptotic LSKs was assessed by γH2AX, BrdU, and cleaved poly (ADP-ribose) polymerase (PARP) positivity, respectively. BrdU labeling was assessed after 7 days of in-vivo incorporation. There was no difference in the degree of γH2AX-positive cells among the 4 types of mice. In terms of cell-cycle status, JAK2V617F-LSKs showed greatly increased cell cycling relative to WT-LSKs, but this increase was attenuated by loss of TET2, and the percentage of actively cycling cells eventually declined to the same level as WT-LSKs. Finally, TET2KD cells showed decreased apoptosis. (B) Dendrogram constructed from unsupervised hierarchical clustering of data sets from 4 types of BM-LSKs using Pearson correlation. (C) GSEA demonstrating positive enrichment of the STAT5A target genes in both JAK2V617F-LSKs and double-mutant-LSKs. The normalized enrichment score (NES) from the overall gene expression profiles of LSKs and the false discovery rate q-value are indicated. (D) GSEA demonstrating negative enrichment of the HSC signature genes in JAK2V617F-LSKs and double-mutant-LSKs. Error bars indicate standard deviation of representative experiments performed in triplicate. *P < .05 vs WT; †P < .05 vs JAK2V617F.
Loss of TET2 interferes with cell-cycle progression and gene expression in JAK2V617F-LSKs. (A) DNA damage, cell cycle, and apoptotic status of mutant LSKs assessed by flow cytometry. Bone marrow CD45.2-LSKs from second recipients at 16 weeks after transplantation in a competitive transplantation assay were gated, and the proportion of DNA damaged, cycling, and apoptotic LSKs was assessed by γH2AX, BrdU, and cleaved poly (ADP-ribose) polymerase (PARP) positivity, respectively. BrdU labeling was assessed after 7 days of in-vivo incorporation. There was no difference in the degree of γH2AX-positive cells among the 4 types of mice. In terms of cell-cycle status, JAK2V617F-LSKs showed greatly increased cell cycling relative to WT-LSKs, but this increase was attenuated by loss of TET2, and the percentage of actively cycling cells eventually declined to the same level as WT-LSKs. Finally, TET2KD cells showed decreased apoptosis. (B) Dendrogram constructed from unsupervised hierarchical clustering of data sets from 4 types of BM-LSKs using Pearson correlation. (C) GSEA demonstrating positive enrichment of the STAT5A target genes in both JAK2V617F-LSKs and double-mutant-LSKs. The normalized enrichment score (NES) from the overall gene expression profiles of LSKs and the false discovery rate q-value are indicated. (D) GSEA demonstrating negative enrichment of the HSC signature genes in JAK2V617F-LSKs and double-mutant-LSKs. Error bars indicate standard deviation of representative experiments performed in triplicate. *P < .05 vs WT; †P < .05 vs JAK2V617F.
Next we performed gene expression analysis using BM-LSKs from recipients of the 4 types of FLs. There were close similarities in the whole-genome expression profiles between JAK2V617F-LSKs and double-mutant-LSKs (Figure 6B). Consistent with this finding, GSEA showed positive enrichment of the STAT5A target genes36 in both JAK2V617F-LSKs and double-mutant-LSKs (Figure 6C). In addition, pre–erythroid colony-forming unit signature genes37 were positively enriched in both JAK2V617F-LSKs and double-mutant-LSKs, but not in TET2KD-LSKs, suggesting that JAK2V617F expression induced a progenitor phenotype in LSKs (supplemental Figure 6A).
Although there was no significant enrichment of the HSC signature gene footprint38 in TET2KD-LSKs, it was negatively enriched in JAK2V617F-LSKs. Double-mutant-LSKs showed the same tendency as JAK2V617F-LSKs in terms of their HSC signature gene fingerprint, but the expression of individual genes differed between the two groups (Figure 6D and supplemental Figure 6B). Among 245 HSC signature genes, 100 were more highly expressed in double-mutant-LSKs than JAK2V617F-LSKs (supplemental Table 1).
Discussion
In this study, we used JAK2V617F transgenic and TET2 knockdown mouse models. The phenotype of the former closely resembles the clinical and pathological features of human PMF12 ; that of the latter has subtle features similar to chronic myelomonocytic leukemia–like MPNs with marked clonal hematopoiesis.31 Because previous studies reported that other JAK2V617F-TG or knockin mice developed PV- or ET-like features10,11,13-16 but not PMF-like features, we evaluated the amount of JAK2V617F protein in LKs and its messenger RNA in LSKs, both of which were estimated to be about 0.3- or 0.4-fold the levels in WT cells (supplemental Figure 5). JAK2 was present entirely in the cellular cytoplasm in both WT and JAK2V617F cells, although a small amount of JAK2 was observed in the nucleus in HEL cells. It may be difficult to account for the phenotypic difference between our JAK2V617F-TG and other transgenic or knockin mice based on the amount of JAK2V617F. The activity of the H-2Kb promoter, which was used in our model, was observed in all lineage hematopoietic cells but was relatively weak in erythroid and megakaryocyte lineages compared with Gr-1-positive cells and LSKs.39 The relatively strong expression of murine JAK2V617F complementary DNA in leukocytes might account for the phenotypic difference between our JAK2V617F-TG and those of others.
JAK2V617F mutation has frequently been observed in MPNs and is sufficient to produce an MPN phenotype in murine retroviral transplantation1,40-43 or transgenic10-12 /knockin mice.13-16,34 However, it is still debated whether JAK2V617F confers a competitive growth advantage in vivo. Several groups have reported that JAK2V617F-LSKs gradually outcompete JAK2WT-LSKs in the competitive transplantation assay, and mice transplanted with JAK2V617F-LSKs together with JAK2WT-LSKs develop PV over time,44,45 whereas Li et al reported that expression of JAK2V617F impaired HSC function and that the number of LSKs was reduced in JAK2V617F knockin mice.16 Furthermore, JAK2V617F-positive CD34+ cells from MPN patients have not been found to have a proliferative advantage over WT cells in immunodeficient mice.23,46
Our data are consistent with the previous studies by Li et al.16 When a sufficient number of JAK2V617F-positive FLs were transplanted alone into irradiated recipients, they developed MPNs that closely resembled human PMF, with decreased survival. Compared with recipients of WT cells, the absolute number of LSKs in the bodies of JAK2V617F cell recipients was comparable early after transplantation but decreased later, along with a decrement in nucleated cells and BM fibrosis. Although we could not definitively conclude whether the negative enrichment of HSC signature genes in JAK2V617F-BM-LSKs at 6 to 8 weeks after transplantation was due to the direct effect of JAK2V617F on stem/progenitor cells or to the secondary effect of BM fibrosis, this gene expression pattern might indicate that impaired HSC activity induced by JAK2V617F was at least partially due to the direct effect of JAK2V617F on HSCs. As mentioned before, these mice did not develop myelofibrosis at this point.
The disadvantage of JAK2V617F cells was apparent in competitive serial transplantation experiments. In the first recipients transplanted with JAK2V617F-FLs together with WT BM cells, JAK2V617F chimerism in peripheral blood cells gradually increased over time, reaching 80% at 16 weeks after transplantation, and the mice developed MPNs. JAK2V617F cells could not be sustained in the second recipient mice. JAK2V617F chimerism in peripheral blood reached 5% at 16 weeks after second transplantation. The second recipients transplanted with JAK2V617F cells demonstrated no splenomegaly, extramedullary hematopoiesis, or BM abnormalities, and these recipients’ survival was comparable to that of WT mice. Our data demonstrated that HSCs harboring JAK2V617F had a competitive advantage relative to WT HSCs soon after transplantation, but this advantage was lost over time. The cells then showed a subtle competitive disadvantage relative to WT HSCs, although this was under the stressful environment induced by transplantation. In-vitro sequential colony formation assays also supported the observations that JAK2V617F did not maintain HSC function over long periods. Like WT cells, JAK2V617F cells failed to generate subsequent colonies. Findings that support our hypothesis were recently reported in a study using human subjects. The MPNs of most advanced-phase PV and MF patients had lower JAK2V617F-positive clonal burdens in the HSC compartment compared with neutrophils, suggesting that the JAK2V617F mutation does not confer a significant growth advantage at the HSC level and that other genetic lesions may drive expansion of this population.47
BM cells harboring both JAK2V617F and loss of TET2 were not diminished and could be sustained in the second recipient mice, and they developed MPNs. Considering a recently reported myelodysplastic syndrome mouse model in which impaired self-renewal of ASXL1-deficient cells was compensated by concomitant loss of TET2,48 concurrent TET2 loss might restore the self-renewal defect induced by JAK2V617F.
Li et al reported that JAK2V617F induced senescence in HSCs, along with accumulation of DNA damage, a lower proportion of LSK cell cycling, and decreased apoptosis.16 In contrast to this report, we did not observe an increment in γH2AX in JAK2V617F-LSKs compared with WT-LSKs, and this was not affected by the addition of loss of TET2. The proportion of actively cycling JAK2V617F-LSKs was increased rather than decreased. Mullally et al49 and Hasan et al34 also reported that HSCs with JAK2V617F tended to be in the active cell-cycle phase. The increase in LSK cell cycling by JAK2V617F might exhaust HSCs and cause their impairment over the long-term. In addition, the attenuation of the HSC signature in JAK2V617F-LSKs as demonstrated by GSEA might account for the impaired HSC activity induced by JAK2V617F. JAK2V617F-LSKs had a STAT5A signature and skewed toward erythroid lineages. Double-mutant-LSKs also had the same tendency, whereas TET2KD-LSKs did not, indicating that the impact of JAK2V617F expression on LSKs was greater than that of loss of TET2 function.
As mentioned above, the HSC signature was not enriched in double-mutant-LSKs. This was unexpected, because double-mutant cells exhibited self-renewal activity and induced MPNs over the long-term in vivo. But the expression patterns of individual genes varied. Many HSC signature genes were downregulated in JAK2V617F-LSKs, but the expression of a significant number of them was restored in double-mutant-LSKs. JAK2V617F-induced impairments in HSC activity that are restored by loss of TET2 might be mediated by these HSC-specific genes.
The first recipients transplanted with JAK2V617F cells and competitor WT cells in a 1:5 ratio developed MPNs, but these cells were not sustained in the second recipients. At the hypothetical time point of MPN disease initiation, a single cell (or only a few cells) is likely to be responsible for MPN induction. It is probably difficult to develop and sustain MPNs over time when JAK2V617F alone occurs in a small portion of HSCs, because it does not confer long-term autonomous growth. MPNs may develop when both mutations occur. Loss of TET2 might induce clonal hematopoiesis mainly at the early-stage stem/progenitor cell level in vivo, and the cooperation of JAK2V617F and loss of TET2 might result in abnormal cell growth and efficient initiation of MPNs against normal hematopoiesis. Of course, MPN patients without TET2 mutations must have other gene mutations that confer a growth advantage on HSCs.
Loss of TET2 had an additional effect on MPNs harboring JAK2V617F: when it occurred in the context of JAK2V617F, the phenotype of double-mutant-induced MPNs became severe compared with JAK2V617F single-mutant-induced MPNs. The dual role of loss of TET2 in MPNs may account for the previous observation that there was no strict temporal order for the occurrence of TET2 and JAK2 mutations. Schaub et al reported that TET2 mutation occurred before JAK2V617F in some MPN patients who had both mutations, but the order was the opposite in other patients.50 In the former group, loss of TET2 might play a role in evolving clonal hematopoiesis, which is essential for MPN initiation; in the latter group, loss of TET2 might accelerate the degree of malignancy of already-developed MPNs. We also note the effect of TET2 mutation on MPN prognosis. TET2 mutation was reported not to predict shortened survival by an international collaborative study group with a large cohort.21 But as mentioned above, loss of TET2 has a dual role in MPNs. It is reasonable to speculate that loss of TET2 does not have any impact on prognosis when it occurs early in MPN oncogenesis, but it may predict shortened survival when it occurs late in already-developed MPNs. For the purposes of prognosis, it may be desirable to separate MPN patients harboring TET2 mutations into 2 groups based on the time when the mutation occurs. In the future, this approach should be taken for genetic prognosis markers in general, with gene mutations assessed in combination rather than individually.
The data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE62302).
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Matsushita, T. Shinmori, E. Torii, and S. Saito for their technical assistance, and Professor N. Arima and M. Hachiman for cell sorting assistance.
This work was supported in part by Grants-in-Aid for Scientific Research (grants 24641408, 24591401, and 26860738) from the Ministry of Education, Science, Sports, and Culture in Japan.
Authorship
Contribution: T.K., K. Shide, and T.Y. performed the research; K.M. performed the pathological examinations; G.S., K.A., and A.I. performed the western blot and gene set enrichment analyses; A. Kamiunten, M. Sekine, Y. Taniguchi, T. Hidaka, Y.K., H.S., T. Harada, Y. Tahara, M.Y., H.A., T.M., S.Y., H.I., M. Sueta, S.H., K.N., and A. Kitanaka analyzed and interpreted the data; and T.K., K. Shide, and K. Shimoda conceived the research; guided its design, analysis, and interpretation; and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kazuya Shimoda, Department of Gastroenterology and Hematology, Faculty of Medicine, University of Miyazaki, 5200 Kihara, Kiyotake, Miyazaki 889-1692, Japan; e-mail: kshimoda@med.miyazaki-u.ac.jp.