Key Points
Over 30% of patients with unexplained cytopenias who do not meet diagnostic criteria for MDS carry MDS-associated somatic mutations.
Clonal cytopenias of undetermined significance are more common than MDS and show comparable variant allele frequencies and blood counts.
Abstract
Establishing a diagnosis in patients suspected of having a myelodysplastic syndrome (MDS) can be challenging and could be informed by the identification of somatic mutations. We performed a prospective study to examine the frequency and types of mutations encountered in 144 patients with unexplained cytopenias. Based on bone marrow findings, 17% were diagnosed with MDS, 15% with idiopathic cytopenias of undetermined significance (ICUS) and some evidence of dysplasia, and 69% with ICUS and no dysplasia. Bone marrow DNA was sequenced for mutations in 22 frequently mutated myeloid malignancy genes. Somatic mutations were identified in 71% of MDS patients, 62% of patients with ICUS and some dysplasia, and 20% of ICUS patients and no dysplasia. In total, 35% of ICUS patients carried a somatic mutation or chromosomal abnormality indicative of clonal hematopoiesis. We validated these results in a cohort of 91 lower-risk MDS and 249 ICUS cases identified over a 6-month interval. Mutations were found in 79% of those with MDS, in 45% of those with ICUS with dysplasia, and in 17% of those with ICUS without dysplasia. The spectrum of mutated genes was similar with the exception of SF3B1 which was rarely mutated in patients without dysplasia. Variant allele fractions were comparable between clonal ICUS (CCUS) and MDS as were mean age and blood counts. We demonstrate that CCUS is a more frequent diagnosis than MDS in cytopenic patients. Clinical and mutational features are similar in these groups and may have diagnostic utility once outcomes in CCUS patients are better understood.
Introduction
Myelodysplastic syndromes (MDS) are clonal bone marrow disorders characterized by inefficient and dysmorphic hematopoietic differentiation, cytopenias of the peripheral blood, and increased risk of transformation to acute myeloid leukemia (AML).1 Establishing a diagnosis of MDS in a cytopenic patient is often challenging as the bone marrow must demonstrate dysplasia in 10% or more of a myeloid cell lineage or a blast proportion of 5% or greater.2 Quantification of these features can be subjective and prone to wide interobserver variation even among expert hematopathologists.3,4 In cases that do not meet either bone marrow criteria, the presence of certain clonal karyotype abnormalities typical for MDS can serve as presumptive evidence of the diagnosis.2 Finally, other neoplasms and nonclonal causes of cytopenias must also be reasonably excluded. Many patients with otherwise unexplained cytopenias will fail to meet the diagnostic criteria for MDS and instead carry a designation of idiopathic cytopenias of undetermined significance (ICUS).5-7
The natural history of patients with ICUS is largely unknown and appears to be highly variable. Small studies indicate that some patients will go on to develop frank MDS or a related myeloid malignancy such as AML.8 Others may follow a more indolent course.9 Clear factors that predict progression have not been discovered. Just as certain chromosomal abnormalities can indicate the presence of clonal hematopoiesis and serve as presumptive evidence of MDS, it was hoped that somatic mutations might help identify clonal ICUS cases that could be considered to have MDS instead.8,10 This parallels how identification of specific recurrent mutations has redefined the diagnostic criteria for several myeloproliferative neoplasms (MPN). Targeted sequencing of 40 or so genes can identify 1 or more somatic mutations in over 80% of MDS cases.11,12 In this sense, targeted gene sequencing might be more useful than cytogenetics because <50% of patients with MDS will have a karyotype abnormality and the vast majority of patients with ICUS will have a normal karyotype.
Unfortunately, no somatically mutated gene is unique to MDS. Several genes, such as U2AF1, SRSF2, and ASXL1 are more frequently mutated in myelodysplastic conditions, but these mutations can also occur in MPN and AML.13-16 Other frequently mutated genes in MDS (eg, SF3B1, TP53, TET2, or DNMT3A) are not even unique to myeloid disorders as they can be found in lymphoid malignancies as well.17-20 In fact, somatic mutations indicative of clonal hematopoiesis have been identified in persons without clinically evident cytopenias.21-25 The prevalence of these mutations increases steadily with age, approaching 10% of the population in their 70s, and far exceeds the prevalence of MDS, MPN, and AML combined in this age group. This high rate of background mutations and the lack of specificity they have for MDS make it seem unlikely that somatic mutations can be used to clarify the distinction between MDS and ICUS.10,26 However, asymptomatic persons with age-related clonal hematopoiesis are at increased risk of developing a hematologic malignancy, particularly if the size of their detected clone is large.21,22 Whether these risks are higher in cytopenic patients with evidence of clonal hematopoiesis is unknown as are the incidence and outcomes of patients with clonal ICUS, which we will refer to as clonal cytopenias of undetermined significance or CCUS. Nor is it clear whether specific mutations or the total number of mutations might retain prognostic significance in CCUS, as they do in MDS. Despite the lack of evidence for this approach, some hematologists may already be using genetic sequencing results to make or confirm equivocal diagnoses of MDS in clinical practice.27 Additional information on the prevalence, nature, and risk of somatic mutations detected in cytopenic patients who do not meet the diagnostic criteria for MDS are needed to determine whether this practice is justified. To address these questions, we report the results of 2 studies, 1 prospective and 1 retrospective, examining the frequency and nature of somatic mutations in patients with ICUS, comparing them to patients with clearly defined MDS.
Methods
Study designs and patient sample selection
Prospective study.
A prospective registry study enrolled 144 consenting patients suspected of having MDS who were scheduled to undergo a bone marrow biopsy as part of their routine evaluation. Patients had a complete medical history and physical examination taken, as well as screening tests that included a complete blood count with differential, iron studies, reticulocyte count, erythropoietin level, vitamin B12, and folate levels. The bone marrow was evaluated by at least 2 hematopathologists using standard diagnostic practices including morphology review, flow cytometry, karyotype analysis, and fluorescence in situ hybridization (FISH). A third hematopathologist reviewed cases in which there was diagnostic discordance. Institutional review board approval was obtained for all sites that were part of the study. Eligibility was based on the bone marrow evaluation and patients were assigned into 1 of 3 study groups depending on the MDS diagnosis using the 2008 World Health Organization (WHO) criteria: positive (confirmed diagnosis of MDS), equivocal (MDS cannot be ruled in or out), or negative (no evidence of MDS). For all cases, assignment to the diagnostic group was performed centrally at Genoptix Medical Laboratory. Subjects were excluded if there was any other malignancy present in the sample. An earlier analysis of the prospective study data has been presented.28
Retrospective study.
A retrospective analysis of bone marrow samples submitted to Genoptix Medical Laboratory from January 2014 to June 2014 for the evaluation of persistent unexplained cytopenia(s) identified 249 patients with ICUS and 91 patients with International Prognostic Scoring System (IPSS)-stratified lower-risk MDS (ie, low or intermediate-1 risk). To be included, all patients were required to have a comprehensive bone marrow evaluation that included morphology review, flow cytometry, karyotype analysis, FISH, and myeloid gene sequencing. Cases were excluded if they had insufficient material for an analysis or an alternative diagnosis identified, such as multiple myeloma, AML, chronic lymphocytic leukemia, MPN, solid tumor, or higher-risk MDS. The diagnoses of ICUS and MDS were based on criteria from the 2008 WHO classification, with morphology reviewed by at least 2 hematopathologists for concurrence. A third hematopathologist reviewed cases in which there was diagnostic discordance. Subjects were classified into 1 of 3 groups: MDS, ICUS with mild dysplasia, and ICUS with no dysplasia. An earlier analysis of the retrospective study data has been presented.29 Demographic and clinical characteristics of the patients from both studies are detailed in supplemental Table 1 (see supplemental Data available on the Blood Web site).
DNA isolation and sequencing
Genomic DNA was extracted from the bone marrow aspirate samples using the EZ1 DNA Blood kit (Qiagen) in accordance with protocols approved by an institutional review board. Selected exons and flanking sequences of 22 myeloid genes (ASXL1, EZH2, ETV6, RUNX1, TP53, CBL, DNMT3A, IDH1, IDH2, JAK2, KIT, MPL, NPM1, NRAS, PHF6, SETBP1, SF3B1, SRSF2, TET2, U2AF1, ZRSR2, and FLT3 [tyrosine kinase domain only]) were amplified by multiplex polymerase chain reaction (supplemental Table 2). Libraries were created using a Fluidigm Access Array system (Fluidigm Corporation). Amplicon libraries were split to be sequenced on the Illumina MiSeq platform (Illumina) in duplicate.
Mutation calling
The lower limit of detection of this commercial assay was set at 5% mutant allele reads based on the high rate of orthogonal validation of variants at this threshold during clinical development. The minimum depth of coverage required to call a mutation was 500×. Alignment and variant calling were performed using NextGENe software (SoftGenetics, LLC). Variant calls were then compared between the duplicate samples and annotated using laboratory developed and validated software that queried a variety of databases containing known germ-line and somatic variants. Missense variants present in databases of polymorphisms (ExAC and dbSNP 142) at a population frequency of 1% or greater were excluded from further analysis.
Statistical methods
The mutations and demographic/clinical characteristics were compared across the 3 disease groupings for each study using conventional statistical methods. The potential associations between categorical variables such as mutation status and diagnostic groups were evaluated using χ2 tests. The relationship between continuous variables and diagnostic groups was investigated with analysis of variance tests; here, Tukey multiple comparisons were used to determine which variables underlie any significant results. All P values are 2-sided, and all statistical tests were undertaken with the R program (R Core Team, Vienna, Austria).
Results
Prospective evaluation of cytopenic patients and the incidence of ICUS and CCUS
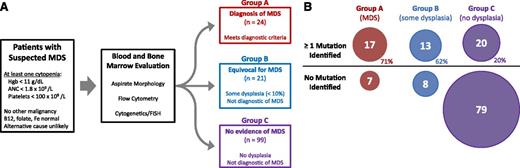
To determine the prevalence of somatic mutations in cytopenic patients suspected of having MDS, we performed a prospective study examining diagnostic samples from consenting individuals. A total of 144 enrolled patients with at least 1 unexplained, clinically meaningful cytopenia (defined as a hemoglobin level <11 g/dL, a neutrophil count of <1800 × 109/L, or a platelet count <100 000 × 109/L) had diagnostic blood and bone marrow samples sent to Genoptix Medical Laboratory (supplemental Table 1). Routine diagnostic procedures were performed (morphologic analysis, metaphase karyotyping, FISH, and flow cytometry) and patients were assigned to 1 of 3 groups based on expert hematopathology review (Figure 1): 24 patients were found to meet the diagnostic criteria for MDS and were assigned to group A, 21 patients had evidence of dysplasia that did not meet diagnostic criteria for MDS and were assigned to group B, and 99 patients were found to have no evidence of dysplasia or an MDS defining karyotype abnormality and were assigned to group C. The 120 patients in groups B and C can be described as having ICUS, suggesting that among cytopenic patients suspected of having MDS, the occurrence of ICUS is many times higher than the diagnosis of MDS. To corroborate this finding, we examined similar cases sent to Genoptix during 2014. Among several thousand diagnostic samples from cytopenic patients suspected of having MDS, 30% had an alternative diagnosis, 62% did not meet any diagnostic criteria, and only 8% were diagnosed with MDS.
Prospective trial schema and diagnostic groups. (A) Schema showing how patients were screened and selected for entry. Patients were placed into 1 of 3 groups based on their bone marrow findings. (B) Proportions of patients with and without mutations in each diagnostic group are shown after DNA sequencing 22 genes associated with myeloid neoplasms.
Prospective trial schema and diagnostic groups. (A) Schema showing how patients were screened and selected for entry. Patients were placed into 1 of 3 groups based on their bone marrow findings. (B) Proportions of patients with and without mutations in each diagnostic group are shown after DNA sequencing 22 genes associated with myeloid neoplasms.
DNA isolated from the bone marrow of trial participants underwent next generation sequencing of 22 MDS-associated genes (supplemental Table 2). One or more somatic mutations were identified in 71% of patients with MDS (group A), 62% of patients with only rare dysplastic morphology (group B), and 20% of patients with no evidence of dysplasia (group C) (P < .0001). Combining groups B and C, 33 of 120 of these ICUS patients (28%) carried at least 1 somatic mutation indicative of clonal hematopoiesis (Figure 1). Another 9 ICUS patients without a detected somatic mutation harbored a clonal chromosomal abnormality. Therefore, we identified 42 patients with CCUS compared with 24 with a diagnosis of MDS in this prospectively defined cohort.
Characteristics of somatic mutations in cytopenic patients
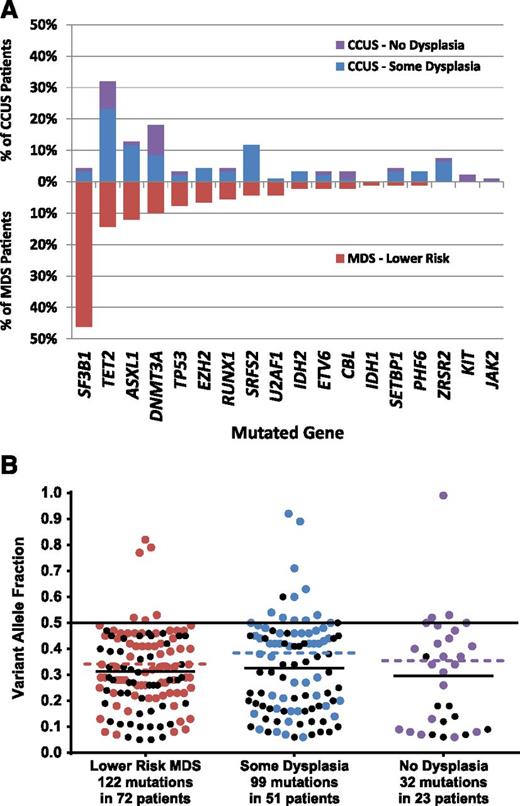
Group A patients with clear evidence of MDS were more likely than the group B or C patients to have 2 or more somatic mutations, and had a higher mean number of mutations per patient with at least 1 mutation (2.5 vs 1.5 and 1.3 in groups B and C, respectively; P = .004). The genes mutated in each group were largely, but not completely, overlapping (Figure 2). SF3B1 mutations and TET2 mutations were the most common in group A and B patients, consistent with prior studies of somatic mutations in MDS. In contrast, group C patients with no evidence of dysplasia most frequently had mutations of TP53 followed by TET2 and DNMT3A, but lacked mutations of SF3B1 and other splicing factors (supplemental Figure 1A).
Mutations and VAFs in prospective study groups. (A) The frequency of mutations in each gene listed on the x-axis is shown for prospective cohort patients with MDS (red bars in lower half) and CCUS, separated by whether dysplasia was seen in the bone marrow or not (blue and purple bars, respectively, in upper half). (B) Variant allele fractions by diagnostic group. The VAF for each mutation identified in the prospective MDS and CCUS groups is shown. Colored dots represent the highest VAF found in a patient whereas black dots represent mutations with VAFs less than the maximum for a given patient. The colored dotted lines represent the mean of the highest VAFs (colored dots) whereas solid black lines represent the mean of all mutations (colored and black dots).
Mutations and VAFs in prospective study groups. (A) The frequency of mutations in each gene listed on the x-axis is shown for prospective cohort patients with MDS (red bars in lower half) and CCUS, separated by whether dysplasia was seen in the bone marrow or not (blue and purple bars, respectively, in upper half). (B) Variant allele fractions by diagnostic group. The VAF for each mutation identified in the prospective MDS and CCUS groups is shown. Colored dots represent the highest VAF found in a patient whereas black dots represent mutations with VAFs less than the maximum for a given patient. The colored dotted lines represent the mean of the highest VAFs (colored dots) whereas solid black lines represent the mean of all mutations (colored and black dots).
The digital nature of our sequencing approach allowed us to estimate the variant allele frequency (VAF) for each of the detected mutations. When treating mutations in each patient as independent, the mean VAFs among mutation carriers were similar across diagnostic groups (33% for group A, 30% for group B, and 23% for group C; P = .20; Figure 2). However, in patients with 2 or more mutations, secondary mutations may be subclonal to more ancestral lesions. When only the mutation with the greatest VAF in each mutated patient was considered, the mean VAFs differed across groups (45% for group A, 32% for group B, and 25% for group C; P = .014).
Retrospective validation of prospective findings
To validate our findings from the prospective study, we examined a larger cohort of patients with unexplained cytopenias undergoing a diagnostic bone marrow examination. We identified 249 diagnostic samples sent to Genoptix over a 6-month period that were determined to have ICUS and had undergone sequencing with the 22-gene panel. Of these, 133 (53%) were deemed to have no evidence of dysplasia analogous to group C patients in the prospective study. A cohort of 91 samples from patients diagnosed with lower-risk MDS (ie, low or intermediate-1 risk by the IPSS) during the same 6-month period was identified as a comparator group. These MDS patients are analogous, but not identical, to the group A cohort in the prospective trial because group A included higher-risk MDS.
Somatic mutations were found in 79% of patients with lower-risk MDS, 44% in ICUS patients with some dysplasia, and only 17% of ICUS patients with no evidence of dysplasia (Figure 3; P < .0001). Overall, 38% of ICUS patients had evidence of clonal hematopoiesis indicative of CCUS when clonal chromosomal abnormalities were included. Among mutation carriers, the mean number of mutations per patient in the lower-risk MDS group did not differ from that of the CCUS group (1.7 vs 1.8 per patient, respectively, P = .7). The VAFs also did not vary between CCUS and MDS groups (Figure 3; P > .25). As with the prospective study, SF3B1 and TET2 were the most frequently mutated genes in the lower-risk MDS cohort. In the CCUS group, mutations of TET2 and DNMT3A were most common whereas SF3B1 mutations were relatively rare (supplemental Figure 1B).
Mutations and VAFs in retrospective study groups. (A) The frequency of mutations in each gene listed on the x-axis is shown for retrospective cohort patients with MDS (red bars in lower half) and CCUS, separated by whether dysplasia was seen in the bone marrow or not (blue and purple bars, respectively, in upper half). (B) Variant allele fractions by diagnostic group. The VAF for each mutation identified in the retrospective MDS and CCUS groups is shown. Colored dots represent the highest VAF found in a patient whereas black dots represent mutations with VAFs less than the maximum for a given patient. The colored dotted lines represent the mean of the highest VAFs (colored dots) whereas solid black lines represent the mean of all mutations (colored and black dots).
Mutations and VAFs in retrospective study groups. (A) The frequency of mutations in each gene listed on the x-axis is shown for retrospective cohort patients with MDS (red bars in lower half) and CCUS, separated by whether dysplasia was seen in the bone marrow or not (blue and purple bars, respectively, in upper half). (B) Variant allele fractions by diagnostic group. The VAF for each mutation identified in the retrospective MDS and CCUS groups is shown. Colored dots represent the highest VAF found in a patient whereas black dots represent mutations with VAFs less than the maximum for a given patient. The colored dotted lines represent the mean of the highest VAFs (colored dots) whereas solid black lines represent the mean of all mutations (colored and black dots).
Clinical associations with somatic mutations
Somatic mutations have been associated with clinical features in patients with MDS including age, sex, bone marrow findings, and cytopenias. To determine whether these relationships exist for nonclonal ICUS (ncICUS) and CCUS patients, we combined our prospective and retrospective cohorts and examined them for associations between clinical features and the presence of somatic mutations indicative of clonal hematopoiesis (Table 1). In both cohorts, the mean age did not materially differ between MDS and CCUS patients (74.3 vs 75.1 years, respectively; P = .87); however, ICUS patients without evidence of clonality had a significantly lower mean age than the MDS and CCUS groups (66.6 years, P < .001). The male-to-female ratio was significantly higher among the CCUS patients (1.65) in comparison with the MDS (ratio = 1) and ncICUS patients (0.68; P = .0003). CCUS patients defined by the presence of mutations vs karyotype abnormalities alone showed little difference in clinical measures (supplemental Figure 2; supplemental Table 3).
Clinical measures by mutation status for both cohorts
| . | MDS . | CCUS . | Nonclonal ICUS . | P . |
|---|---|---|---|---|
| Group size, N | 115 | 136 | 233 | |
| Male:female ratio | 1.0 | 1.65 | 0.68 | .0003 |
| Age, mean (SD), y | 74.3 (10.4) | 75.1 (12.5) | 66.6 (13.6) | <.0001* |
| Hgb, mean (SD), g/dL | 9.8 (1.8) | 10.7 (1.8) | 11.1 (2.0) | <.0001† |
| ANC, mean (SD), ×109/L | 3.2 (2.1) | 3.5 (2.3) | 3.4 (2.4) | .71 |
| Platelets, mean (SD), ×109/L | 184 (110) | 164 (104) | 179 (101) | .27 |
| . | MDS . | CCUS . | Nonclonal ICUS . | P . |
|---|---|---|---|---|
| Group size, N | 115 | 136 | 233 | |
| Male:female ratio | 1.0 | 1.65 | 0.68 | .0003 |
| Age, mean (SD), y | 74.3 (10.4) | 75.1 (12.5) | 66.6 (13.6) | <.0001* |
| Hgb, mean (SD), g/dL | 9.8 (1.8) | 10.7 (1.8) | 11.1 (2.0) | <.0001† |
| ANC, mean (SD), ×109/L | 3.2 (2.1) | 3.5 (2.3) | 3.4 (2.4) | .71 |
| Platelets, mean (SD), ×109/L | 184 (110) | 164 (104) | 179 (101) | .27 |
ANC, absolute neutrophil count; SD, standard deviation.
No difference between MDS and CCUS (P = .87).
No difference between CCUS and ICUS (P = .21).
Cytopenias were similar among the ncICUS and CCUS patients, and somewhat less severe than the MDS patients (Table 1), suggesting that blood counts were not strongly associated with the presence of mutations. There was a decreasing trend in anemia across the patient groups: MDS (hemoglobin [Hgb] = 9.8 g/dL), CCUS (Hgb = 10.7 g/dL), and ncICUS (Hgb = 11.1 g/dL; P < .0001). However, the difference in anemia between CCUS and ncICUS was not significant (P = .21). There was no difference in ANC across the 3 patient groups (P = .71). The mean platelet count was slightly lower in the CCUS group (164 × 109/L) compared with the MDS (184 × 109/L) and ncICUS groups (179 × 109/L; P = .27). This difference may reflect the relatively conserved platelet counts in patients with SF3B1 mutations for which the MDS group was enriched. Restricting the comparison with patients without SF3B1 mutations reversed this difference, whereby those with MDS now had a lower platelet count (137 × 109/L) than CCUS patients (158 × 109/L; P = .18). Exclusion of SF3B1 mutant patients did not alter the significant difference in hemoglobin level between the MDS (9.6 g/dL) and CCUS (10.6 g/dL) groups (P = .0003).
Discussion
This study determined the frequency and clinical associations of MDS-related somatic mutations in 2 cohorts of patients with unexplained cytopenias. In the prospective study, patients were assigned a diagnostic group prior to genetic sequencing. In the retrospective group, sequential cases of ICUS that had undergone genetic sequencing were compared with 91 cases of lower-risk MDS diagnosed in the same period. Both cohorts demonstrated that somatic mutations indicative of clonal hematopoiesis are common in patients with ICUS, particularly if they demonstrate some degree of dysplasia.
The frequency of ICUS in cytopenic patients suspected of having MDS could be estimated from our prospective cohort. In this group, ICUS was diagnosed 5 times as often as MDS, with the majority of ICUS patients showing no evidence of dysplasia in their bone marrow. There is little data on the incidence of ICUS, but a review of cases sent to Genoptix for evaluation of cytopenias is consistent with our findings. However, an accurate measure of the incidence of ICUS will require larger, carefully controlled prospective studies. Our retrospective study included a higher proportion of ICUS patients with some degree of dysplasia vs none. However, it is likely that ICUS patients without dysplasia were underrepresented in this group because they were less likely to have a genetic sequencing test ordered by the reviewing pathologist.
Although the proportion of patients with evidence of clonal hematopoiesis was lower in ICUS patients, the absolute number with CCUS was substantially larger than the number diagnosed with MDS. The actual fraction of patients with CCUS may actually be higher because some patients may have had mutations not detected in our panel. Our findings conservatively suggest that the incidence of CCUS is as great, or greater, than the incidence of MDS. This has implications for the diagnostic utility of somatic mutations in cytopenic patients. Outcomes for CCUS patients are not known. It is unlikely that they all will progress to frank MDS as some may never develop dysplasia or excess blasts and others might develop AML without an intervening diagnosis of MDS.30 Recognition of CCUS will allow these patients to be studied so that the incidence and the clinical significance of their somatic mutations can be determined.
Mutations of different genes may have distinct prognostic significance in CCUS as they do in MDS. Our data demonstrate that the spectrum of mutation in CCUS and MDS is fairly similar. Even mutated genes associated with poor outcomes in MDS, like TP53, ASXL1, RUNX1, and DNMT3A, were identified in patients with CCUS at comparable frequencies. The only mutated gene overrepresented in MDS patients was SF3B1. This is likely because of the strong association between SF3B1 mutations and the presence of ring sideroblasts, a readily identifiable feature associated with MDS.31,32 In fact, without consideration of ring sideroblasts, all 37 cases classified as having refractory anemia with ring sideroblasts (RARS) in the retrospective study would not have met the diagnostic criteria for MDS. Furthermore, pathologists who see ring sideroblasts even in the absence of significant dysplasia may be more inclined to order genetic sequencing in these patients. Knowledge of an SF3B1 mutation (or another mutation) could influence the quantification of ring sideroblasts and other forms of dysplasia in favor of meeting the diagnostic criteria for MDS. However, in this study, all diagnoses were made prior to the receipt of sequencing results and were not altered by this information.
The second major impediment to the use of somatic mutations as diagnostic biomarkers comes from the high rate of age-dependent somatic mutations in persons without a known hematologic disorder. These individuals are said to have clonal hematopoiesis of indeterminate potential (CHIP).21-23 The prevalence of CHIP increases greatly with age and approaches 10% among 70- to 80-year olds, the typical age range for MDS patients. Because the incidence of MDS in this age group is orders of magnitude lower than 10%, most persons with somatic mutations indicative of clonal hematopoiesis never develop MDS (or any other hematologic malignancy).33 Therefore, the presence of a somatic mutation in the blood of a patient with unexplained cytopenias may be an incidental finding unrelated to their blood counts and cannot currently be relied upon to establish a diagnosis of MDS absent traditional criteria.10,26 It is certainly possible that mutations found in our cohorts were unrelated to their blood counts. However, more than one-third of ICUS patients had CCUS as evidenced by somatic mutations or chromosomal abnormalities. Even in patients with no evidence of dysplasia, 27% had CCUS, which is over twofold greater than the rate of CHIP in a similarly aged “normal” population. Furthermore, CHIP is a significant risk factor for developing a hematologic malignancy, particularly when allele frequency of a somatic variant is >10%. In our studies, the majority of CCUS patients had mutations with VAFs >10% and their distribution of VAFs showed little difference when compared with lower-risk MDS patients. This alone would indicate that CCUS patients have a substantially increased risk, compared with an age-matched population, of developing a frank hematologic malignancy, albeit not necessarily MDS.21,30,34,35 Whether the presence of a clinically significant cytopenia further increases that risk remains unknown and the clinical utility of gene sequencing in ICUS patients has not been proven.
The lack of long-term follow-up is a limitation of our study. Additional research into the clinical significance of somatic mutations in ICUS patients is needed. A formal definition of CCUS would help standardize how these patients are identified and facilitate comparisons between studies. A simple approach would be to combine the proposed definition of CHIP with the cytopenia thresholds commonly used to define MDS (Table 2).26 Adding a requirement that unexplained cytopenias persist for 6 months would help exclude transient benign processes, although this more rigorous criteria was not used in our study. Similarly, it would be useful to know outcomes in ICUS patients without evidence of clonal hematopoiesis. A lack of abnormalities in commonly mutated MDS genes may have prognostic significance.8,11,36
Proposed criteria for CCUS
| Peripheral blood findings . | Bone marrow findings . | Genetic findings . |
|---|---|---|
| 1 or more of the following: | None of the following: | 1 or more of the following: |
| Hemoglobin, <11 g/dL | ≥10% dysplasia in the granulocytic, erythroid, or megakaryocytic lineage | An acquired chromosomal abnormality not diagnostic of a heme malignancy |
| ANC <1500/μL, 1.5 × 109/L | Myeloblasts comprise ≥5% of total cellularity | Presence of a somatic mutation with a VAF ≥2% in a heme malignancy–associated gene in the peripheral blood or bone marrow |
| Platelet count <100 000/μL, 100 × 109/L | An acquired chromosomal abnormality specific for MDS/AML | |
| Additional criteria: No other likely cause of cytopenias or evidence of another hematologic disorder. | ||
| Peripheral blood findings . | Bone marrow findings . | Genetic findings . |
|---|---|---|
| 1 or more of the following: | None of the following: | 1 or more of the following: |
| Hemoglobin, <11 g/dL | ≥10% dysplasia in the granulocytic, erythroid, or megakaryocytic lineage | An acquired chromosomal abnormality not diagnostic of a heme malignancy |
| ANC <1500/μL, 1.5 × 109/L | Myeloblasts comprise ≥5% of total cellularity | Presence of a somatic mutation with a VAF ≥2% in a heme malignancy–associated gene in the peripheral blood or bone marrow |
| Platelet count <100 000/μL, 100 × 109/L | An acquired chromosomal abnormality specific for MDS/AML | |
| Additional criteria: No other likely cause of cytopenias or evidence of another hematologic disorder. | ||
In conclusion, somatic mutations identified on a commercial myeloid gene sequencing panel indicate the presence of clonal hematopoiesis in a larger-than-expected fraction of patients with clinically meaningful cytopenias. This includes patients with no evidence of dysplasia. The pattern of mutation, implied clonal size, and number of mutations closely resembles lower-risk MDS. Although the natural history of patients with CCUS vs ncICUS is not known, our study suggests that clonality may underlie the cytopenias seen in a substantial fraction of these patients. Recognition of CCUS as a clinical entity would help identify it as a formally defined diagnostic group that would aid in future outcome studies in this population.
An earlier analysis of the prospective study data was presented as a poster at the 56th annual meeting of the American Society of Hematology (San Francisco, CA, December 6-9, 2014).
An earlier analysis of the retrospective study data was presented orally at the 56th annual meeting of the American Society of Hematology (San Francisco, CA, December 6-9, 2014).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors recognize the invaluable contributions of the CLARITY Trial Steering Committee: Drs Benjamin Ebert, Tim Graubert, Rami Komrokji, Alan List, Guillermo Garcia-Manero, Gail Roboz, and David Steensma, and the contribution of the participating sites and investigators listed in supplemental Table 4.
This work was supported by Genoptix Medical Laboratory and by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant K08DK091360 (R.B.).
Authorship
Contribution: B.K., J.M.H., and R.B. designed the study; C.E.H., J.A.-H., and C.V. conducted site recruitment, monitoring, and data retrieval for the prospective study; J.A.-H. was responsible for regulatory oversight and monitoring of the prospective study; E.B. was responsible for data entry and database construction and maintenance for the prospective study; J.M.H. was responsible for obtaining funding and guidance, site recruitment, and data monitoring and retrieval for the prospective study; B.K., Y.X., P.R., K.L., R.F., B.D., and A.Y. performed bone marrow morphologic, flow cytometric, and cytogenetic review; J.K. carried out DNA sequencing; M.J.M. and S.A.N. performed DNA sequencing analysis; J.S.W. performed the statistical analysis; and R.B., B.K., J.M.H., and J.S.W. wrote the manuscript which was reviewed and edited by all authors.
Conflict-of-interest disclosure: J.M.H., B.K., P.R., K.L., R.F., A.Y., Y.X., J.A.-H., E.B., C.V., C.E.H., M.J.M., S.A.N., J.K., and B.D. are employees of Genoptix. J.S.W. has served as a consultant for Genoptix. R.B. has served as a consultant for Genoptix and Celgene. R.B. has intellectual property licensed by Genoptix.
Correspondence: Rafael Bejar, Moores Cancer Center, University of California, San Diego, 3855 Health Sciences Dr, MC 0820, La Jolla, CA 92093-0820; e-mail: rabejar@ucsd.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal