Abstract
Purpose: Progression to acute myeloid leukemia (AML) from myeloproliferative neoplasms (MPNs) is almost always fatal. To explore the best treatment strategies for this serious complication, we analyzed the survival outcome of patients with MPNs who progressed to AML based on treatment received at our institution.
Patients and Methods: A total of 273 patients who were diagnosed with AML secondary to MPNs between 1989 and 2016 were retrospectively analyzed. Progression-free survival (PFS) and overall survival (OS) were calculated.
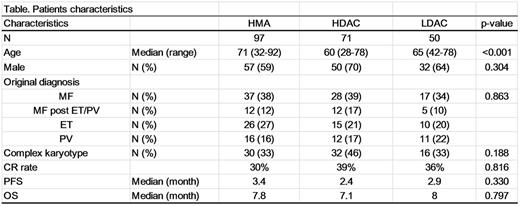
Results: The patients progressed to AML from essential thrombocythemia (ET, N=60), polycythemia vera (PV, N=49), primary myelofibrosis (PMF, N=105), post-ET/PV MF (N=37), and MPN-unclassifiable (N=22). The median age of the patients was 67 (range: 28-92). The median time to AML transformation from MPN diagnosis was 64.5 months (range: 0.9-477.7 months). Complex karyotype was seen in 99 patients at the time of transformation (38%). With a median follow up of 16.5 months (range: 2.7-115.7 months), 247 patients had died (88% from AML, 6% from treatment-related mortality, 6% with other cause including infection and GVHD). Ninety-nine patients received hypomethylating including agent (HMA), 71 a high-dose cytarabine regimen, 52 a low-dose cytarabine regimen, 28 other treatments, and 24 patients did not receive any treatment due deterioration of the patientfs condition or patientfs decision. The median PFS and OS after progression to AML were 2.6 months and 7.0 months, respectively. There was no significant improvement in PFS and OS by date of diagnosis (1989 to 2016). Also, complete response (CR) rate and survival outcome were not significantly different by type of treatment (Figure A) or prior MPN subtype. Overall, 46 patients (27 in CR, 14 not in CR, 4 unknown due to the transplant outside) proceeded to allogeneic transplant (allo-SCT), and the median overall survival after transplant was 15.3 months (6.1-29.4 months). Although it was not statistically significant, patients who achieved a CR before allo-SCT had longer OS than those who received allo-SCT without achieving a CR (35.1 vs 8.9 months; Figure B). Complex karyotype was associated with both shorter PFS (Hazard ratio: 2.2, 95%CI: 1.6-2.9) and OS (Hazard ratio: 2.0, 95%CI: 1.5-2.6).
Summary: Survival outcome for AML secondary to MPN remains very poor, and there has been no improvement in survival outcome since 1989. Further studies are urgently needed to test new treatment strategies and to detect residual disease post allo-SCT. Considering dismal outcome after transformation, studies are also needed to explore strategies to prevent this fatal complication.
DiNardo:Daiichi Sankyo: Research Funding; Abbvie: Research Funding; Agios: Research Funding; Celgene: Research Funding; Novartis: Research Funding. Konopleva:Reata Pharmaceuticals: Equity Ownership; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Stemline: Consultancy, Research Funding; Eli Lilly: Research Funding; Cellectis: Research Funding; Calithera: Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal