Abstract
Background: Genomic variations in AML are associated with disease outcome. Frameshift insertions of Nucleophosmin gene (NPM1) are common in AML with significantly higher prevalence in adults compared to childhood AML. NPM1 frameshift mutations are the result of 4 base pair (bp) insertions in exon 12 of the gene that encodes the Nuclear Export Signal (NES) domain, causing a premature truncation, leading to cytoplasmic mis-localization of NPM1 protein. Several genomic subsets of NPM1 insertions have been identified, with the Type A variant (insertion of TCTG) being the most common. Non-Type A NPM1 mutations occur in 25% of adult AML, but occur in >50% of pediatric AML, suggesting possible biologic and functional significance of NPM1genotype in AML. We studied the effect of different NPM1 insertion genotypes on disease characteristics and outcome among children and adults with de novo AML treated on COG and SWOG AML trials.
Methods: Genomic profile from 2301 patients treated on recent COG AML trials (CCG 2961, COG 03p1 and COG 0531, N=1915) and SWOG young adult AML trial S0106 (N=396) were studied. In this cohort of 2301 patients, initial NPM1 mutations were detected by fragment length analysis in 266 patients (12%) with prevalence of 9% in the COG Pediatric studies and 27% in the SWOG studies. There was a clear age-associated prevalence of NPM1 mutations with a prevalence of <1% in children younger than 2 years of age, 29% in those 10-18 years and 36% in those 45-60 years (p=0.004). Of the 266 cases with NPM1 mutations, diagnostic DNA was available from 206 patients for NPM1 insertion genotyping. Specific genotype of NPM1 insertion was determined and correlated with disease characteristics and outcome. Chi-square or Exact test was used to test proportion of patients. Mann-Whitney test was used to compare medians between the two groups. Kaplan-Meier methods were used to compare survival estimates of OS, EFS and RFS.
Results: Sequencing of exon 12 demonstrated multiple 4 bp insertion subtypes with Type A variant (TCTG) in 128 patients (62%) and non-Type A variants in 78 patients (38%). Those with non-Type A variants included type B in 15.5%, type D in 8.3%, type J in 2.9% and others in 11.2%. In our study, comparison was performed between type A vs. non-type A.
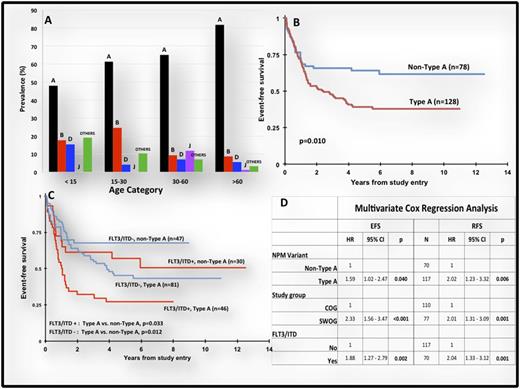
Comparison of disease characteristics between those with Type A vs. non-Type A NPM1 mutations demonstrated that Type A variants were more prevalent in older patients (p=0.018) with a clear age-based predominance of non-Type A variants in the younger patients (Figure A). FLT3/ITD prevalence was similar between Type A vs. non-Type A variants (36% vs. 39%, p=0.695). Evaluation of clinical outcome based on NPM1 genotypes showed no difference in CR rate (93% Type A vs. 91% non-Type A, p=0.625). However, those with Type A insertions had a 5 year event-free survival (EFS) of 39% vs. 64% for those with non-Type A variants (p=0.01), with a corresponding 5 year relapse-free survival of 41% vs. 70%, respectively (p=0.002, Figure B). As therapies between pediatric and adult trials differed, we performed a separate analysis for the COG and SWOG studies. In COG trials, 5 year EFS for those with and without Type A variant was 53% vs. 71% (p=0.058). Similar outcome evaluation for the SWOG study showed EFS of 25% vs. 50% (p=0.035) for those with and without Type A variants. Additionally, given significant overlap between FLT3/ITD and NPM1 mutations, we studied the impact of NPM1 mutation genotype in FLT3/ITD positive vs. negative cohorts. In FLT3/ITD positive patients, those with and without type A NPM1 variants had EFS of 27% vs. 57% (P=0.033) and in FLT3/ITD negative cohort, those with and without type A NPM1 variants had EFS of 45% vs. 68% (p=0.012), Figure C. Multivariate Cox regression analysis that included age (SWOG vs. COG) and FLT3/ITD status demonstrated that NPM1genotype was an independent predictor of EFS (HR 1.6, p=0.04) and RFS (HR 2.02, p=0.006) regardless of age or FLT3/ITD status, Figure D.
Conclusion: This COG/SWOG collaborative study of over 2300 pediatric and adult patients demonstrates clear functional biology behind age-based variation in NPM1 genotype where those with Type A variants have a significantly inferior outcome compared to non-Type A variants. Upon validation, specific genotype of NPM1 mutations might contribute to more accurate risk-based therapy allocation in AML.
Selim:CTI International Biopharma: Other: Supporting the research. Othus:Celgene: Consultancy; Glycomimetics: Consultancy. Radich:ARIAD: Consultancy; Novartis: Consultancy, Other: laboratory contract; TwinStrand: Consultancy; Pfizer: Consultancy; Bristol-MyersSquibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal