Abstract
INTRODUCTION: Ponatinib is a pan-tyrosine kinase inhibitor (TKI) with proven efficacy in multi-refractory CML patients (pts) who have failed other TKIs and approved in all CML stages after failure to other TKIs and for pts with T315I mutation. Despite excellent response rates, resistance or intolerance may develop in some cases. Treatment options in these pts are limited and the outcomes have not been described.
METHODS: We conducted a retrospective review of the outcomes of pts with refractory chronic (CP) and accelerated (AP) phase CML who discontinued ponatinib in the salvage setting. Pts were assessed for the cause of ponatinib discontinuation, therapies (Rxs) received after discontinuation, response to such Rx, and survival post-ponatinib discontinuation.
RESULTS: Among 55 pts treated (32 treated in CP, 23 in AP), 36 (65 %) have discontinued ponatinib at the time of this analysis. Nineteen pts were either lost to follow up (F/U) or died on Rx and excluded from this analysis. Of the 36 pts analyzed, 19 were in CP and 17 in AP at initiation of ponatinib. Pts had received a median of 4 Rxs (2-6) prior to ponatinib. Median age at discontinuation was 67 yrs (22-94). Median duration on ponatinib Rx was 17 mo (1-61).
Of 19 CP pts, 5 discontinued due to toxicity (pancreatitis = 2, stroke = 1, headache = 1, thrombocytopenia = 1), 13 for lack of response, 1 per pt choice. At discontinuation, 14 were still in CP [complete cytogenetic response (CCyR) = 3, no/minor CyR (NR/mCyR) = 10, major molecular response (MMR) = 1]; 3 had progressed to AP, and 2 to blast phase (BP). Subsequent therapy for those still in CP included stem cell transplant (SCT) = 4, supportive care only (NT) = 3, dasatinib = 2, omacetaxine = 2, and bosutinib + decitabine (DAC), nilotinib, and imatinib (1 each); the 3 pts that progressed to AP received dasatinib + DAC, low-dose Ara-C (LDAC), and NT = 1 each, respectively; and BP pts received Hyper - CVAD + dasatinib followed by SCT = 1, ponatinib + LDAC = 1. Of the 3 CP pts in CCyR, 1 died from sepsis 1 mo after discontinuing ponatinib due to thrombocytopenia; 1 pt received SCT and died in MMR 11 mo post SCT; 1 developed 7q- /Ph- on ponatinib and received SCT (MMR at 47 mo F/U). The CP pt in MMR discontinued ponatinib after a stroke and lost MMR 38 mo later (still in CCyR off Rx). Two CP pts improved to CCyR after SCT (1 died in 8 mos; 1 in MMR at 25 mo). Thirteen pts (CP 9, AP 3, BP 2; all non-SCT Rx) did not improve their responses post ponatinib (remained in NR/mCyR). The Median survival (OS) post ponatinib discontinuation of the 19 CP pts was 26 mo [Fig 1]. Twelve pts have died: 5 disease related (CP 2, BP 2, AP 1), 4 cause unknown (CP 3, AP 1), 3 from sepsis (CP 2, AP 1).
Of 17 AP pts who discontinued, 15 stopped due to poor response and 2 for toxicity (stroke = 1, nausea = 1). At discontinuation, 14 were still in AP [NR/mCyR = 13, partial CyR (PCyR) = 1], 3 had progressed to BP. Subsequent therapy included NT = 5, dasatinib = 4, dasatinib + DAC = 2, SCT = 2, hydroxyurea = 1 for those still in AP, and SCT = 1, BIDFA = 1, mitoxantrone + etoposide + ponatinib = 1 for those in BP. The pt with PCyR at discontinuation died 3 mos after discontinuation of heart failure. Three pts (AP = 2, BP = 1) received SCT: 1 BP pt achieved MMR but died 12 mos post SCT of unknown cause; 1 AP pt maintains MMR 63 mos after SCT; the other AP pt did not respond and relapsed in AP, then received Rx with dasatinib + DAC and died of progressive disease 5 mos later. The remaining 14 pts (AP 12, BP 2; all non-SCT Rx) did not improve their responses post ponatinib (remained in NR/mCyR). The OS post ponatinib discontinuation (17 AP) was 9 mo [Fig 1]. Twelve pts have died: sepsis 3 (AP 3); progression 4 (AP 3, BP1), unidentified 5 (BP 1, AP 4); other 1 (BP 1).
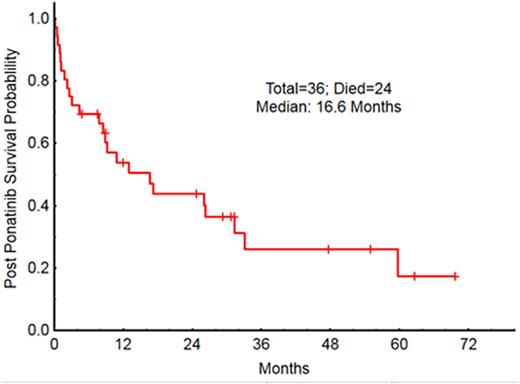
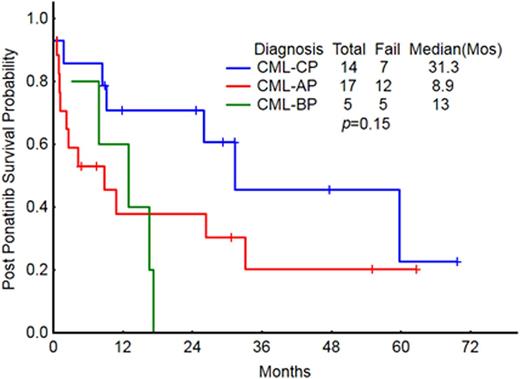
The OS for all 36 pts was 16 mos [Fig 2]; OS by stage at discontinuation was 31mo in CP, 9 mo in AP, 13 mo in BP [Fig 3]. The 12-mo survival probabilities for individual post-discontinuation Rx cohorts were: TKI 74%, SCT 56%, supportive 30%, other 50%. The OS for pts who stopped ponatinib because of toxicity vs resistance was 60 mo and 11 mo respectively.
CONCLUSIONS: Long term outcomes of pts with ponatinib failure are poor with estimated 1-year OS and EFS rates of 54% and 40% respectively. Lack of response to ponatinib, after failing other TKIs, predicts a considerable risk for subsequent Rx failure. New Rx options are required for this small subset of patients.
OS post ponatinib by stage at start of ponatinib
OS after failure for pts treated in CP or AP
OS post ponatinib by stage at the time of ponatinib dc
Kantarjian:Amgen: Research Funding; ARIAD: Research Funding; Bristol-Myers Squibb: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding. Daver:Otsuka: Consultancy, Honoraria; Sunesis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Karyopharm: Honoraria, Research Funding; Kiromic: Research Funding; BMS: Research Funding; Ariad: Research Funding. Kadia:BMS: Research Funding; Novartis: Honoraria. Ravandi:BMS: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding. Jain:Pfizer: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Infinity: Research Funding; BMS: Research Funding; Servier: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Genentech: Research Funding; Abbvie: Research Funding; Novartis: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; Novimmune: Consultancy, Honoraria; Seattle Genetics: Research Funding; Celgene: Research Funding. Burger:Gilead: Research Funding; Portola: Consultancy; Roche: Other: Travel, Accommodations, Expenses; Janssen: Consultancy, Other: Travel, Accommodations, Expenses; Pharmacyclics, LLC, an AbbVie Company: Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal