Introduction: Omacetaxine is a semisynthetic formulation of HHT currently approved for CML patients (pts) in chronic (CP) or accelerated (AP) phase failing ≥2 tyrosine kinase inhibitors (TKIs). Preclinical studies showed synergy of HHT with imatinib. We hypothesized that combining HHT with imatinib might improve outcomes particularly for pts with advanced-phase disease.
Objective: To evaluate safety and efficacy of HHT combined with imatinib in the treatment of CML pts in CP, AP, or blast phase (BP)
Methods: This was an open-label, single-center study of pts with CML in CP (refractory or resistant to imatinib), or advanced phase (AP or BP; untreated or refractory to imatinib). Pts received HHT 2.5 mg/m2 by continuous 24-h intravenous (IV) infusion daily x5 days every 4 weeks with imatinib 400 mg orally daily for CP pts, and 600 mg daily for AP or BP pts. Hematologic efficacy, tolerance, and adverse events (AEs) were assessed prior to and during each cycle. Cytogenetic and molecular evaluations were done every 3 mo. Pts who did not achieve a meaningful hematologic or cytogenetic response (CyR) by the end of the 4th cycle were taken off study. Pts who achieved at least a complete hematologic response (CHR) could receive subsequent maintenance cycles with HHT 2.5 mg/m2 by continuous 24-hour infusions daily for 2 days every 4 weeks and imatinib at the dose received on the last induction cycle. The study was intended to follow a Simon 2-stage design to evaluate 18 pts in each stratum (CP, AP, and BP pts) for efficacy.
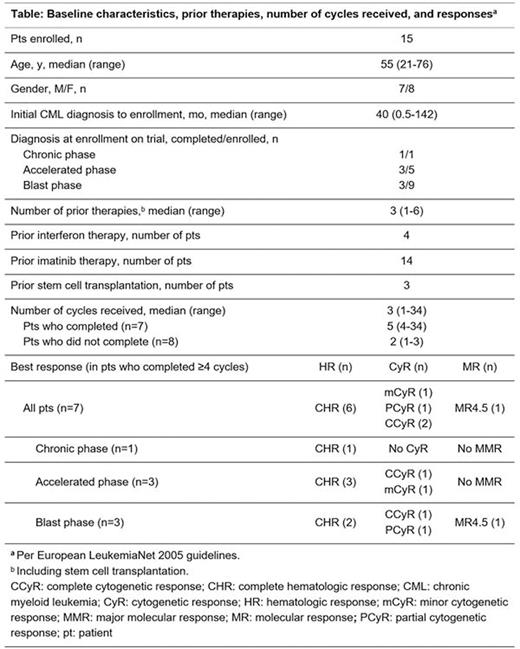
Results: We enrolled 15 pts between 10/2005 and 03/2009. Baseline characteristics, prior therapies, number of cycles received, and best responses are shown in the table. The median age was 55 y (range 21-76); 8 pts were male; 1 pt was in CP, 5 in AP, and 9 in BP. Seven pts completed the protocol-defined endpoint of ≥4 cycles of treatment for evaluation of response, including 3 in BP, 3 in AP, and 1 in CP (this pt had only achieved CHR without CyR after 7 mo of dasatinib and had previously failed imatinib and nilotinib). These 7 pts received a median of 4 cycles (range 4 to 34) and were on the study for a median of 5 mo (range 3 to 36). Of these 7 pts, at 3 mo, 2 achieved complete CyR (CCyR),1 partial CyR (PCyR), and 1 minor CyR (mCyR; per European LeukemiaNet 2005 guidelines). Three-month response by stage was: AP pts, 2 achieved CHR, 1 with CCyR and the other with mCyR; BP pts, 2 achieved CHR, 1 with PCyR and the other with CCyR at 3 mo and molecular response 4.5 at 4 mo; the CP pt achieved no CyR at 4 mo and was taken off study. Of the 8 pts who did not complete at least 4 cycles, 6 discontinued early for treatment failure, 1 discontinued per physician's recommendation, and 1 died on study from progressive disease and infection. These 8 pts received a median of 2 cycles (6 received at least 2 cycles). Overall response rate was 4/15 (27%) at 1 mo and 6/15 (40%) at 4 mo. Three of the 7 pts who completed the study had kinase domain mutations F359V, F317L, and T315I, respectively; 5 of the other 8 pts had mutations: T315I in 3 pts, F317L in 1 pt, and Q252H in 1 pt. Median survival for all enrolled pts was 4.6 mo. All the 15 enrolled pts reported at least 1 AE. Treatment-emergent AEs were rare and included vomiting (3%), anemia (2.4%), nausea (2.2%), neutropenia (2.2%), and thrombocytopenia (2.2%). Two pts discontinued due to unrelated AEs. One episode each of non-hematologic serious AEs (SAEs) occurred in 9/15 (60%) pts. SAEs occurred in 11/15 (73%) pts who experienced any AE; 10/27 (37%) of these SAEs were considered possibly, probably, or of unknown relationship to study drug; 3 SAEs were grade 2. Treatment-related SAEs occurred in 6/27 (22%) and were all associated with myelosuppression. Thirteen of the 15 enrolled pts died: 6 from BP, 3 from unknown causes, 1 from progressive disease and infection, 1 from intracranial hemorrhage (ICH), 1 from sepsis and ICH, and 1 in a car accident.
Conclusion: The combination of continuous IV infusion of HHT and imatinib was well tolerated; with most frequent treatment-related AEs being nausea, vomiting, and myelosuppression, and demonstrated clinical benefit in some pts. Given these preliminary observations, this approach merits further investigation in a carefully selected group of pts; combinations with newer TKIs may make the combination more effective.
Kantarjian:ARIAD: Research Funding; Amgen: Research Funding; Bristol-Myers Squibb: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. O'Brien:Janssen: Consultancy, Honoraria; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal