Key Points
MPL P106L induces thrombocytosis due to an incomplete trafficking defect that allows very low cell-surface levels.
The P106L mutation uncouples MPL signaling from its THPO clearance functions.
Abstract
The mechanisms behind the hereditary thrombocytosis induced by the thrombopoietin (THPO) receptor MPL P106L mutant remain unknown. A complete trafficking defect to the cell surface has been reported, suggesting either weak constitutive activity or nonconventional THPO-dependent mechanisms. Here, we report that the thrombocytosis phenotype induced by MPL P106L belongs to the paradoxical group, where low MPL levels on platelets and mature megakaryocytes (MKs) lead to high serum THPO levels, whereas weak but not absent MPL cell-surface localization in earlier MK progenitors allows response to THPO by signaling and amplification of the platelet lineage. MK progenitors from patients showed no spontaneous growth and responded to THPO, and MKs expressed MPL on their cell surface at low levels, whereas their platelets did not respond to THPO. Transduction of MPL P106L in CD34+ cells showed that this receptor was more efficiently localized at the cell surface on immature than on mature MKs, explaining a proliferative response to THPO of immature cells and a defect in THPO clearance in mature cells. In a retroviral mouse model performed in Mpl−/− mice, MPL P106L could induce a thrombocytosis phenotype with high circulating THPO levels. Furthermore, we could select THPO-dependent cell lines with more cell-surface MPL P106L localization that was detected by flow cytometry and [125I]-THPO binding. Altogether, these results demonstrate that MPL P106L is a receptor with an incomplete defect in trafficking, which induces a low but not absent localization of the receptor on cell surface and a response to THPO in immature MK cells.
Introduction
The thrombopoietin (THPO)/MPL/JAK2 axis is central for the regulation of platelet production.1,2 Hereditary thrombocytosis (HT) and essential thrombocythemia (ET) are 2 forms of chronic thrombocytosis associated with genetic alterations targeting this axis.3,4 Both HT and ET can be the consequence of germ line or sporadic activating mutations in JAK2 and MPL.5-9 HT can be also related to an excess of plasma THPO levels,3,4 which can be the result of increased synthesis or defect in clearance.10,11 The latter is regulated through THPO binding to MPL on megakaryocytes (MK) and platelets.12,13 In addition, senescent platelets induce THPO synthesis in hepatocytes by binding to the Ashwell-Morell receptor.14 The MK/platelet mass through plasma membrane MPL remains the main determinant of THPO clearance. This process requires higher MPL expression at the cell-surface plasma membrane than required for THPO-induced signaling. Thus, a low level of membrane MPL can paradoxically lead to thrombocytosis via increased available THPO to early MK progenitors, as demonstrated in transgenic/ knock-in models with low MPL platelet expression.11,15-17
Several MPL mutations are associated with a profound thrombocytopenia or inversely with thrombocytosis associated with high plasma THPO.18-21 Congenital amegakaryocytic thrombocytopenia is due to loss of function mutations of MPL, which leads either to a complete absence of MPL plasma membrane expression, such as MPL R102P,22 or to an absence of THPO binding, such as MPL F104S.22,23 Two HT forms are associated with MPL mutations (MPL K39N and P106L) and high THPO.20,21 MPL K39N (MPL Baltimore), which induces a thrombocytosis with a high penetrance, is expressed at low levels on the cell surface due to a posttranslational processing defect.20 In contrast, it was recently reported that MPL P106L was absent from the cell surface due to a complete trafficking defect and accumulated in the endoplasmic reticulum (ER). However, it was suggested to induce constitutively cell survival and to respond to THPO when MPL P106L is located in the ER membrane.24 This model proposed for MPL P106L could mimic the mechanism of MPL activation by the calreticulin (CALR) mutants in myeloproliferative neoplasms that binds the extracellular domain of MPL in the lumen of the ER.25-27 However, CALR is an intrinsically synthesized ER protein, unlike THPO, which must be endocytosed and remains in the cytosol, but thus cannot bind to the extracellular domains of MPL, which is localized in the ER lumen.25
Here, we investigated the trafficking and function of MPL P106L in order to understand the mechanisms behind its thrombocytosis effects.
Materials and methods
More information regarding retroviral vector construction and production, flow cytometry analysis, cell proliferation, western blot analysis, confocal microscopy, binding studies, internalization, senescence-associated (SA)-β-galactosidase staining, cell cycle, degradation analysis, and statistics can be found in the supplemental Materials and methods, available on the Blood Web site.
Patients
Peripheral blood was obtained from patients after informed consent. Platelets and plasma were isolated. CD34+ cells were isolated using an immunomagnetic cell sorting system (Automacs; Miltenyi Biotec) and cultured in plasma clot.28
Plasma THPO levels were measured according to the manufacturer’s instructions (Human Thrombopoietin Quantikine ELISA Kit; R&D Systems).
Cell culture and transduction
Ba/F3 cells and UT-7 cells were grown and transduced as previously described.5,29 CD34+ cells isolated from adult bone marrow (hip surgery) were grown in serum-free medium, as previously described,28 in the presence of THPO (10 ng/mL) and stem cell factor (SCF; 25 ng/mL), and transduced on the fourth day of culture.
Mouse model
All procedures were approved by the local Gustave Roussy ethics committee. Bone marrow reconstitution was performed as described,30 using Mpl−/− recipients and donors.31 Lin− cytokine prestimulated cells were transduced with MPL wild-type (WT) and MPL P106L and injected into Mpl−/− knockout lethally irradiated mice (8.5 Gy) in a ratio of 1 donor per recipient. Plasma THPO levels were measured according to the manufacturer’s instructions (Mouse Thrombopoietin Quantikine ELISA Kit; R&D Systems).
Results
Families
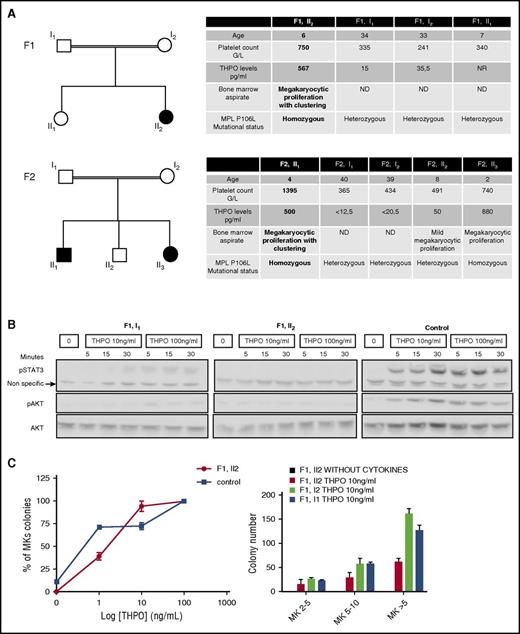
We studied 2 families, 1 from Kuwait (F1) and the other from Saudi Arabia (F2). In the 2 families, the probands (F1, II2 and F2, II1), aged 4 to 6 years, always presented with marked thrombocytosis and paradoxically high-serum THPO levels associated with megakaryocytic hyperplasia and clustering in the bone marrow aspirate. They were asymptomatic (no bleeding or thrombosis). THPO and MPL sequencing detected a homozygous germ line MPL P106L mutation. A sibling (F2, II3) of the second family proband was also homozygous for the mutation with the same biological characteristics. All other members of the 2 families were heterozygous for the mutation with absence of thrombocytosis and normal THPO levels (Figure 1A). In the F1 family, we studied the signaling response to THPO of platelets from the father (F1, I1) and proband (F1, II2). Although the father presented an intermediate response, the proband had no activation of STAT3 and AKT compared with the control (Figure 1B). However, the response to THPO of the proband colony-forming unit–megakaryocyte (CFU-MK) was nearly normal, although the absolute number of circulating CFU-MK–derived colonies was lower than in the parents, and no differences in the size of the colonies were observed. Of note, no spontaneous megakaryocytic growth was detected. Altogether this suggests that, in contrast to platelets, CFU-MK may respond normally to THPO (Figure 1C).
Patient thrombocytosis paradoxically associated with a loss of function receptor. (A) Family trees and biological characteristics: in 2 families, probands are homozygous for the MPL P106L mutation and exhibit thrombocytosis with high levels of THPO and bone marrow megakaryocytic proliferation. (B) Platelet signaling study: STAT3 and AKT activation over a time course were examined by western blot after starvation and subsequent stimulation with THPO at 10 ng/mL or 100 ng/mL and compared with a healthy control. (C) Megakaryocytic proliferation: the MK progenitor compartment response to THPO was studied by plasma clot culture in relative percentage compared with the control or in absolute values. MK colonies were split in 2-5, 5-10, and >10 MKs per colony. The error bars represent mean ± standard deviation of triplicates from a representative experiment.
Patient thrombocytosis paradoxically associated with a loss of function receptor. (A) Family trees and biological characteristics: in 2 families, probands are homozygous for the MPL P106L mutation and exhibit thrombocytosis with high levels of THPO and bone marrow megakaryocytic proliferation. (B) Platelet signaling study: STAT3 and AKT activation over a time course were examined by western blot after starvation and subsequent stimulation with THPO at 10 ng/mL or 100 ng/mL and compared with a healthy control. (C) Megakaryocytic proliferation: the MK progenitor compartment response to THPO was studied by plasma clot culture in relative percentage compared with the control or in absolute values. MK colonies were split in 2-5, 5-10, and >10 MKs per colony. The error bars represent mean ± standard deviation of triplicates from a representative experiment.
Finally, we tested if MPL P106L was expressed on the surface of MKs from patients. We could detect cell-surface expression of MPL on MKs from patient F1, II2 but at a slightly lower level than from her heterozygous parents (supplemental Figure 1). The differences observed here might be in reality more important, as we used an indirect fluorescent labeling, which is not entirely quantitative.32
Lower but not absent cell-surface MPL P106L levels in primary MKs
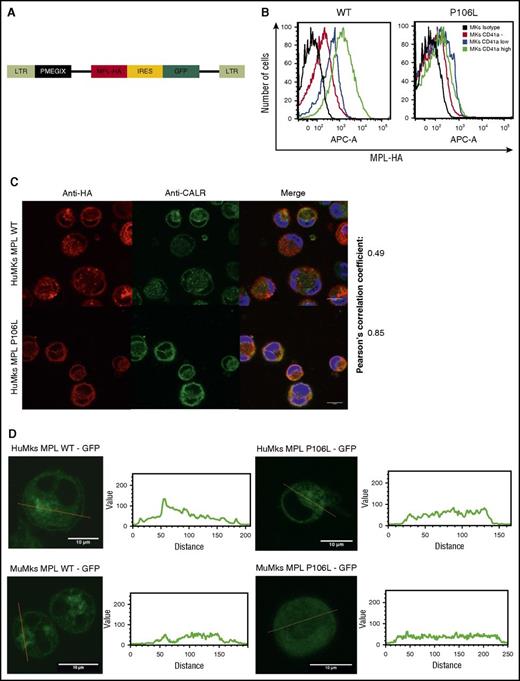
Following the results in patient cells, we transduced MPL P106L or MPL WT into CD34+ cells cultured for 4 days with THPO and SCF (Figure 2A). On the eighth day, we analyzed receptor expression by flow cytometry in the different stages of MK differentiation with anti-CD41a and antihemagglutinin (HA) labeling, confirming lower, but not absent, MPL P106L expression at plasma membrane, when compared with MPL WT. The difference between MPL WT and MPL P106L cell-surface localization clearly increases in mature MKs CD41a high (geometric mean difference 1173) compared with CD41a-negative cells (geometric mean difference 89), suggesting an uncoupling between MPL-dependent proliferation in response to THPO and THPO clearance via MPL (Figure 2B).
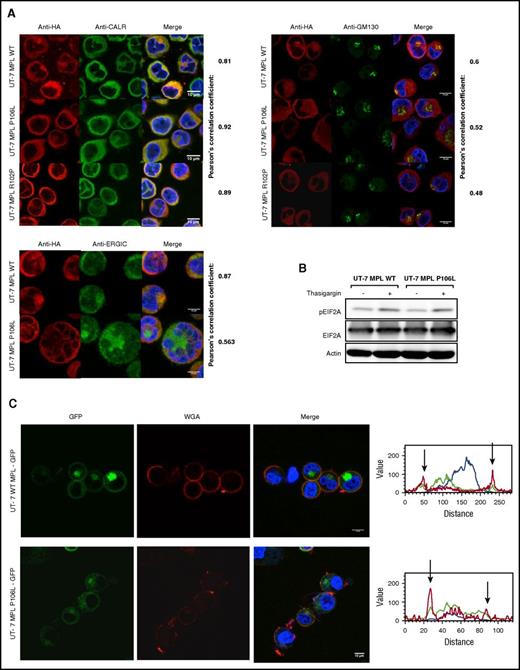
MPL P106L cellular localization in transduced human MKs. (A) Retroviral construction. Cells were transduced with the retroviral vector pMEGIX-IRES-GFP containing either the human MPL WT or the mutated P106L tagged with HA. (B) Receptor expression on transduced human MKs: human MKs cultured 4 days with THPO were labeled with anti-HA and anti-CD41a antibodies and analyzed by flow cytometry 96 hours after transduction. MPL P106L is less expressed at the cell-surface membrane than the WT receptor, especially in mature MKs. A range from 3 × 103 to 4 × 104 cells were analyzed. (C) Human MPL localization: human CD34+ cells were transduced with the human MPL WT or P106L on the fourth day of culture with THPO and SCF. Four days later, cells were stained with either an anti-HA antibody to analyze MPL or an anti-CALR antibody to analyze the ER compartment. (D) Murine MPL-GFP localization: human CD34+ or murine lin− cells were transduced with the pREX-IRES-CD4-muMPL-HA fused to GFP and analyzed by confocal microscopy at days 8 and 5, respectively.
MPL P106L cellular localization in transduced human MKs. (A) Retroviral construction. Cells were transduced with the retroviral vector pMEGIX-IRES-GFP containing either the human MPL WT or the mutated P106L tagged with HA. (B) Receptor expression on transduced human MKs: human MKs cultured 4 days with THPO were labeled with anti-HA and anti-CD41a antibodies and analyzed by flow cytometry 96 hours after transduction. MPL P106L is less expressed at the cell-surface membrane than the WT receptor, especially in mature MKs. A range from 3 × 103 to 4 × 104 cells were analyzed. (C) Human MPL localization: human CD34+ cells were transduced with the human MPL WT or P106L on the fourth day of culture with THPO and SCF. Four days later, cells were stained with either an anti-HA antibody to analyze MPL or an anti-CALR antibody to analyze the ER compartment. (D) Murine MPL-GFP localization: human CD34+ or murine lin− cells were transduced with the pREX-IRES-CD4-muMPL-HA fused to GFP and analyzed by confocal microscopy at days 8 and 5, respectively.
In parallel experiments, to focus on the intracellular pool of receptors, we examined MPL WT and P106L localization by confocal microscopy using an ER marker (anti-CALR antibody). Both colocalized with CALR, attesting their ER localization, but only MPL WT could be observed on the cell surface. The low amount of MPL P106L on the cell surface could not be detected by this technique, due to lack of sensitivity (Figure 2C). We also used a different approach, transducing human and murine MKs with a retroviral construct containing either the murine Mpl WT receptor or the murine Mpl P106L fused to GFP and looked at its cellular localization by confocal microscopy. Mpl P106L was not detected on the surface and was detected in a diffuse pattern in the cytoplasm, whereas the WT receptor was detected on the cell surface and the Golgi area (Figure 2D). These results demonstrate that MPL P106L has a low cell-surface expression due to partial retention in the ER.
Cell biology of MPL P106L and R102P in cell lines
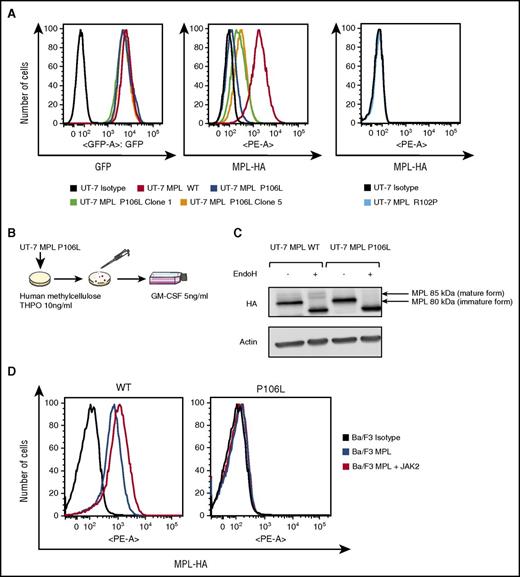
Our results indicate that MPL P106L exhibits a defect in traffic that is not complete, as we could detect cell-surface levels of MPL P106L in primary transduced MKs and in patient MKs. We aimed to investigate this in detail, particularly because in cell lines the study of Stockklausner et al24 indicated that MPL P106L is completely blocked in the ER. We transduced either MPL P106L, MPL R102P, or MPL WT into the human UT-7 cell line, which responds to granulocyte-macrophage colony-stimulating factor (GM-CSF), but does not express MPL, nor responds to THPO.33 All 3 cell lines respond to GM-CSF in a similar manner.
We first assessed the cell-surface receptor expression after transduction in UT-7 cells. For the same GFP expression level using flow cytometry analysis, cell-surface MPL P106L was much weaker, but to our surprise detectable at the cell-surface membrane (Figure 3A), the sensitivity of flow cytometry being between 500 and 700 receptors/cell.34 Thus, in UT-7 cells, which are megakaryocytic leukemia cells, we can detect low levels of MPL P106L at the cell surface. We next selected UT-7 MPL P106L clones, which respond to THPO. To this end, UT-7 MPL P106L cells were cloned in methylcellulose in the presence of 10 ng/mL THPO, and we expanded the different clones in THPO (Figure 3B). The clones responding to THPO expressed a higher level of MPL P106L at the cell surface, but always lower than WT (Figure 3A). By western blot, MPL P106L was present mainly as an immature endoglycosidase-H–sensitive form compared with MPL WT, raising the possibility that the receptor carrying the P106L mutation was essentially blocked in the ER (Figure 3C). We also performed similar experiments in Ba/F3 cells, which are IL3-dependent Pro B cells.35 Interestingly, in contrast to UT-7 cells, we could not detect cell-surface MPL P106L in Ba/F3 cells, even under conditions where we enhanced the levels of JAK2, which acts a chaperone to stabilize MPL (Figure 3D).36,37 JAK2 overexpression normally protects MPL WT from proteasomal- and lysosomal-mediated degradation,36,37 and its lack of effect in Ba/F3 cells suggests that the MPL P106L defect is not due to enhanced degradation (supplemental Figure 2A-B). However, these negative results in Ba/F3 cells confirm those of Stockklausner et al24 in other cell types and indicate that apart from in MKs and megakaryocytic cell lines, the traffic of MPL P106L to the cell surface appears to be completely defective.
Retrovirally transduced MPL P106L is localized at the cell-surface membrane, but at much lower levels than MPL WT. (A) Receptor expression in transfected UT-7 cell lines: cells cultured with GM-CSF were incubated with an anti-HA antibody coupled with phycoerythrin (PE) and analyzed by flow cytometry. For similar GFP expression, MPL P106L is minimally expressed at the cell-surface membrane, unlike the clones, which present more surface receptors, although considerably less than the cell line expressing MPL WT. As already known, MPL R102P is not detectable at the cell-surface membrane. (B) Clones selection: UT-7 MPL P106L cells were grown under THPO 10 ng/mL in methylcellulose assay. Colonies responding to THPO were selected and plated in supplemented minimum essential medium alpha supplemented with GM-CSF. (C) Analysis of the mature and immature forms of MPL: UT-7 cells cultured with GM-CSF were incubated with endoglycosidase H before western blot with an anti-HA antibody. The mature form of MPL (85 kDa), which is resistant to endoglycosidase H digestion and capable of reaching the plasma membrane, is not present in UT-7 cells transduced with MPL P106L. (D) Receptor expression on Ba/F3 cells cotransfected with JAK2: Ba/F3 cells cultured with supernatant from WEHI-3B cells were cotransduced with MPL and JAK2 and analyzed by flow cytometry after anti-HA labeling. There was no increase in MPL P106L cell-surface expression, in contrast to MPL WT. At least 3 × 104 cells were analyzed in all flow cytograms.
Retrovirally transduced MPL P106L is localized at the cell-surface membrane, but at much lower levels than MPL WT. (A) Receptor expression in transfected UT-7 cell lines: cells cultured with GM-CSF were incubated with an anti-HA antibody coupled with phycoerythrin (PE) and analyzed by flow cytometry. For similar GFP expression, MPL P106L is minimally expressed at the cell-surface membrane, unlike the clones, which present more surface receptors, although considerably less than the cell line expressing MPL WT. As already known, MPL R102P is not detectable at the cell-surface membrane. (B) Clones selection: UT-7 MPL P106L cells were grown under THPO 10 ng/mL in methylcellulose assay. Colonies responding to THPO were selected and plated in supplemented minimum essential medium alpha supplemented with GM-CSF. (C) Analysis of the mature and immature forms of MPL: UT-7 cells cultured with GM-CSF were incubated with endoglycosidase H before western blot with an anti-HA antibody. The mature form of MPL (85 kDa), which is resistant to endoglycosidase H digestion and capable of reaching the plasma membrane, is not present in UT-7 cells transduced with MPL P106L. (D) Receptor expression on Ba/F3 cells cotransfected with JAK2: Ba/F3 cells cultured with supernatant from WEHI-3B cells were cotransduced with MPL and JAK2 and analyzed by flow cytometry after anti-HA labeling. There was no increase in MPL P106L cell-surface expression, in contrast to MPL WT. At least 3 × 104 cells were analyzed in all flow cytograms.
We further employed confocal microscopy to assess the precise localization of MPL P106L, R102P, and WT in UT-7 cells. Costaining of MPL was performed with ER (CALR), ER-Golgi intermediate compartment, and Golgi markers. MPL WT was more markedly costained with the Golgi (43% of cells) than MPL P106L (5%) and MPL R102P (0.4%), suggesting that MPL P106L is partially blocked in the ER and that MPL R102P is completely blocked in the ER (Figure 4A). This blockage in the ER did not induce an ER stress as examined by the level of pEIF2A that could explain the phenotype (Figure 4B).
MPL P106L exhibits cellular trafficking defects without inducing ER stress. (A) Human MPL intracellular localization of UT-7 MPL WT and UT-7 MPL P106L: cells were stained with antibodies directed against the ER (anti-CALR), the Golgi apparatus (anti-GM130), the ER-Golgi intermediate compartment (anti-ERGIC), and MPL (anti-HA), and immunofluorescence was analyzed by confocal microscopy. (B) ER stress analysis: UT-7 MPL WT and P106L cells were treated with 1 μM thapsigargin for 30 minutes and analyzed by western blotting. (C) Localization of the murine MPL receptor fused to GFP: cells were transduced with the retroviral vector pREX-IRES-CD4-muMPLHA fused to GFP and directly analyzed without fixation by confocal microscopy. Wheat germ agglutinin served as membrane marker.
MPL P106L exhibits cellular trafficking defects without inducing ER stress. (A) Human MPL intracellular localization of UT-7 MPL WT and UT-7 MPL P106L: cells were stained with antibodies directed against the ER (anti-CALR), the Golgi apparatus (anti-GM130), the ER-Golgi intermediate compartment (anti-ERGIC), and MPL (anti-HA), and immunofluorescence was analyzed by confocal microscopy. (B) ER stress analysis: UT-7 MPL WT and P106L cells were treated with 1 μM thapsigargin for 30 minutes and analyzed by western blotting. (C) Localization of the murine MPL receptor fused to GFP: cells were transduced with the retroviral vector pREX-IRES-CD4-muMPLHA fused to GFP and directly analyzed without fixation by confocal microscopy. Wheat germ agglutinin served as membrane marker.
In addition, when UT-7 cells were transduced with the murine Mpl WT and P106L fused to GFP, accumulation of Mpl P106L was more diffuse around the nuclear membrane than Mpl WT, which was more focused. Mpl P106L was not detected by confocal microscopy on the plasma membrane (Figure 4C). The main difference with the previous experiments using the human MPL P106L was the detection of a part of the receptor population in the Golgi (supplemental Figure 3A-B), indicating that a larger portion of murine Mpl P106L escapes the ER blockage compared with its human homolog.
Low receptor activity for MPL P106L and no activity for MPL R102P in cell lines
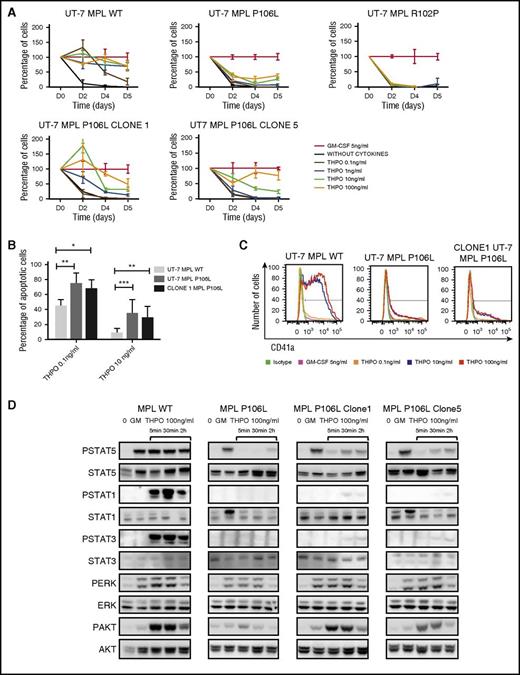
We next evaluated receptor activity by studying proliferation in a dose- and time-response manner to THPO of UT-7 MPL WT, UT-7 MPL P106L, and UT-7 MPL R102P cultures. UT-7 MPL P106L only responded to high doses of THPO (10 and 100 ng/mL) with low proliferation. No spontaneous survival was observed. UT-7 MPL R102P had no response, as expected (Figure 5A).
MPL P106L exhibits lower activity than MPL WT. (A) UT-7 cells proliferation in response to THPO: UT-7 MPL WT, UT-7 MPL P106L, UT-7 MPL P106L clone 1 and 5, and UT-7 MPL R102P cells were seeded at the same concentration (1 × 105/mL) after cytokine deprivation and stimulated with various concentrations of THPO compared with the control (GM-CSF). Cell proliferation is expressed in relative percentage compared with the control representing 100%. Viable cells were counted at different time points using KOVA slide. (B) UT-7 cell apoptosis in culture with THPO: cells were cultured for 3 days with various concentrations of THPO, and the percentage of apoptotic cells (Annexin V–positive) was analyzed by flow cytometry using Annexin V assay (105 cells analyzed per condition). (C) UT-7 cells phenotype in response to THPO: cells were cultured with various concentrations of THPO, and CD41a expression was analyzed by flow cytometry after 3 days. At least 3 × 104 cells were analyzed. (D) UT-7 cells signaling in response to THPO: cells were deprived for 5 hours and then stimulated with various concentrations of THPO compared with GM-CSF and analyzed by western blotting at different time points. STAT1, STAT3, STAT5, AKT, and ERK1/2 phosphorylation was examined. Cell-surface MPL expression for UT-7 MPL WT, MPL P106L, MPL P106L clone 1 and 5 correspond to Figure 3A. Intracellular MPL expression corresponds to Figure 3C. *P < .05; **P < .01; ***P < .001.
MPL P106L exhibits lower activity than MPL WT. (A) UT-7 cells proliferation in response to THPO: UT-7 MPL WT, UT-7 MPL P106L, UT-7 MPL P106L clone 1 and 5, and UT-7 MPL R102P cells were seeded at the same concentration (1 × 105/mL) after cytokine deprivation and stimulated with various concentrations of THPO compared with the control (GM-CSF). Cell proliferation is expressed in relative percentage compared with the control representing 100%. Viable cells were counted at different time points using KOVA slide. (B) UT-7 cell apoptosis in culture with THPO: cells were cultured for 3 days with various concentrations of THPO, and the percentage of apoptotic cells (Annexin V–positive) was analyzed by flow cytometry using Annexin V assay (105 cells analyzed per condition). (C) UT-7 cells phenotype in response to THPO: cells were cultured with various concentrations of THPO, and CD41a expression was analyzed by flow cytometry after 3 days. At least 3 × 104 cells were analyzed. (D) UT-7 cells signaling in response to THPO: cells were deprived for 5 hours and then stimulated with various concentrations of THPO compared with GM-CSF and analyzed by western blotting at different time points. STAT1, STAT3, STAT5, AKT, and ERK1/2 phosphorylation was examined. Cell-surface MPL expression for UT-7 MPL WT, MPL P106L, MPL P106L clone 1 and 5 correspond to Figure 3A. Intracellular MPL expression corresponds to Figure 3C. *P < .05; **P < .01; ***P < .001.
The low level of UT-7 MPL P106L cell proliferation could reflect the lower cell-surface MPL expression, but also a decreased affinity of the receptor to THPO or a senescent/antiproliferative process, as previously reported in some UT-7 MPL WT and Ba/F3 MPL cells expressing high JAK2 levels.33 The latter was ruled out by an absence of senescence-associated–β-galactosidase staining and of differences in cell-cycle analysis on UT-7 MPL P106L (supplemental Figure 4A-B). Moreover, proliferation of UT-7 MPL P106L cells was restored by GM-CSF addition (supplemental Figure 4C).
To evaluate why UT-7 MPL P106L did not proliferate under low doses of THPO, we measured apoptosis. As expected, we found a major increase in Annexin V binding in UT-7 MPL P106L in comparison with UT-7 MPL WT. The increased apoptosis of UT-7 MPL P106L under THPO stimulation was only partially rescued by higher THPO doses (Figure 5B). Moreover, THPO stimulation had no impact on CD41 expression of UT-7 MPL P106L, unlike UT-7 MPL WT cells (Figure 5C).
To test for low constitutive or hyperactivity of MPL P106L, we studied signaling after cytokine deprivation and restimulation by different concentrations of THPO at different time points. No spontaneous signaling was observed for UT-7 MPL P106L cells. P-ERK and P-AKT were observable after THPO stimulation, but no phosphorylated forms of STAT5 or STAT3 were detected (Figure 5D). To eliminate the possibility of any receptor cytokine hypersensitivity or autonomous activity, we performed in UT-7 cell background, respectively, the following: (i) a proliferation assay with no cytokines or increasing doses of GM-CSF from 0.005 ng/mL and (ii) colony assays. Both approaches confirmed the absence of basal activity of the receptor (supplemental Figure 5A-B). The same pattern of proliferation was observed in Ba/F3 cells transduced with MPL P106L (supplemental Figure 5C). Altogether, these results show that MPL P106L exhibits low activity in comparison with MPL WT, and MPL R102P exhibits no activity at all, suggesting that the number of receptor expressed at the cell surface determines the phenotype.
To confirm this hypothesis, we characterized in detail 2 UT-7 MPL P106L clones, which express more cell-surface receptors. Cell proliferation with THPO stimulation was greater in the 2 clones than in the original UT-7 MPL P106L, but lower than in UT-7 MPL WT, thus correlating with cell-surface levels (Figure 5A). THPO signaling was studied in these 2 clones and showed, in contrast to the UT-7 MPL P106L cell line that had not been selected in THPO, that we could detect the induction of phosphorylation of STAT5 and STAT3 and an increased phosphorylation of ERK and AKT (Figure 5D).
MPL P106L is a functional receptor
As the thrombocytosis of homozygous MPL P106L patients is associated with high circulating THPO levels reflecting a decreased THPO clearance, we investigated if this could solely be due to defects in receptor cell-surface localization or also in its internalization. Therefore, we studied MPL internalization over time following THPO stimulation in 1 clone of UT-7 MPL P106L and could show a decrease in receptor internalization compared with the WT receptor (Figure 6A). This decrease in internalization further increases the defect of MPL P106L in THPO clearance.
Lower MPL P106L activity is related to the cell-surface receptor number. (A) MPL receptor internalization after THPO binding: receptor cell-surface expression in UT-7 MPLWT and UT-7 MPL P106L clone 1 was measured by flow cytometry at different times after THPO binding. Analyzed were 2 × 105 cells per point, and results are means ± standard deviation of 3 independent experiments. (B) MPL binding to [125I]THPO (upper panel): the specific radioactivity bound to 5 × 105 UT-7 parental cells (empty circle), MPL WT (filled circle), P106L (empty square), and P106L clone 1 (filled square) UT-7 cells following 2-hours incubation at 15°C with radiolabeled ligand. A representative of 3 independent experiments is shown, together with a nonlinear fit to a 1-site model of specific saturation binding for UT-7 MPL WT and UT-7 MPL P106L clone 1. Binding to UT-7 parental and UT-7 MPL P106L cells was too low in this assay to unambiguously fit to model. Scatchard plot of [125I]THPO binding to UT-7 MPL WT and P106L cells (lower panel). (C) UT-7 MPL transduced cell proliferation in response to eltrombopag: UT-7 MPL WT, P106L, and THPO-selected clones were washed and then cultivated with THPO or eltrombopag at a concentration of 1 × 105 cells/mL and compared with the control GM-CSF, representing 100%. Viable cells were counted using KOVA slide. (D) UT-7 MPL WT and UT-7 MPL P106L signaling induced by THPO and eltrombopag. Cells were starved of cytokine for 5 hours and then stimulated with various concentrations of THPO or eltrombopag and compared with the control (GM-CSF). STAT1, STAT3, STAT5, AKT, and ERK1/2 activation was analyzed by western blotting using corresponding antibodies. Cell-surface MPL expression data for UT-7 MPL WT and MPL P106L clone 1 correspond to Figure 3A.
Lower MPL P106L activity is related to the cell-surface receptor number. (A) MPL receptor internalization after THPO binding: receptor cell-surface expression in UT-7 MPLWT and UT-7 MPL P106L clone 1 was measured by flow cytometry at different times after THPO binding. Analyzed were 2 × 105 cells per point, and results are means ± standard deviation of 3 independent experiments. (B) MPL binding to [125I]THPO (upper panel): the specific radioactivity bound to 5 × 105 UT-7 parental cells (empty circle), MPL WT (filled circle), P106L (empty square), and P106L clone 1 (filled square) UT-7 cells following 2-hours incubation at 15°C with radiolabeled ligand. A representative of 3 independent experiments is shown, together with a nonlinear fit to a 1-site model of specific saturation binding for UT-7 MPL WT and UT-7 MPL P106L clone 1. Binding to UT-7 parental and UT-7 MPL P106L cells was too low in this assay to unambiguously fit to model. Scatchard plot of [125I]THPO binding to UT-7 MPL WT and P106L cells (lower panel). (C) UT-7 MPL transduced cell proliferation in response to eltrombopag: UT-7 MPL WT, P106L, and THPO-selected clones were washed and then cultivated with THPO or eltrombopag at a concentration of 1 × 105 cells/mL and compared with the control GM-CSF, representing 100%. Viable cells were counted using KOVA slide. (D) UT-7 MPL WT and UT-7 MPL P106L signaling induced by THPO and eltrombopag. Cells were starved of cytokine for 5 hours and then stimulated with various concentrations of THPO or eltrombopag and compared with the control (GM-CSF). STAT1, STAT3, STAT5, AKT, and ERK1/2 activation was analyzed by western blotting using corresponding antibodies. Cell-surface MPL expression data for UT-7 MPL WT and MPL P106L clone 1 correspond to Figure 3A.
The P106L substitution is localized close to the high-affinity THPO binding site F10423,38 and could potentially perturb ligand binding and explain this internalization defect. We tested this hypothesis by performing a saturation binding assay with iodine-125 ([125I])-labeled human THPO on UT-7 MPL WT and P106L cells as well as in clone 1. [125I]-THPO binding was not detected in parental UT-7 cells, but was weakly detected in P106L cells and was significant on UT-7 MPL P106L selected cells, albeit at lower levels than in UT-7 MPL WT cells, reflecting an independent indication of the lower cell-surface expression of mutant receptor. Although binding to both UT-7 MPL P106L cell lines was significant, it was insufficient to accurately determine binding affinity in this assay (Figure 6B). However, where it was possible to fit the data to a 1-site model of saturation binding, we could compare the maximum number of binding sites, which indicated UT-7 MPL WT cells express ∼ 4 times the number of functional THPO receptors per cell as UT-7 MPL P106L clone 1.
In order to definitively exclude a defect in THPO binding to MPL P106L, we studied the response of UT-7 MPL P106L cells to Eltrombopag, a THPO mimetic that binds to residue H499 at the outset of the MPL transmembrane domain, far from the THPO binding site.39,40 In proliferation assays, UT-7 MPL P106L clone 1 responded to Eltrombopag, but even less than to THPO (Figure 6C). Signaling in response to Eltrombopag for UT-7 MPL P106L confirms that there was no rescue by this molecule, suggesting that the lower receptor activity is not due to a ligand affinity defect, but rather to the number of receptor expressed at the cell surface (Figure 6D).
Having established that MPL P106L is localized at low levels at the cell surface in MKs and in the UT-7 MK cell line, the 2 major questions are the following: (i) why does this P106L mutation induce a traffic defect, and (ii) by which pathway do low numbers of receptor chains escape blockage in the ER and arrive at the surface? The first question is also related to why MPL R102P is completely retained in the ER, suggesting that mutations in the region around R102-P106 might induce changes in receptor folding. For the second question, it has been shown in cell lines that MPL WT could exit the ER and enter the autophagy compartment before going to the cell surface in an immature N-glycosylated form.41 Here, using surface immunoprecipitation, we detected an immature form of MPL P106L on the cell surface (supplemental Figure 6B) that partially colocalizes with LC3 (supplemental Figure 6A). Thus, for a small fraction of MPL P106L, there is an escape mechanism from the ER mediated via the nonconventional autophagy pathway, leading to low cell-surface MPL P106L and likely explaining the internalization defect observed. These results in cell lines need to be extended to primary patient MKs.
Mouse model reproduces the pathology
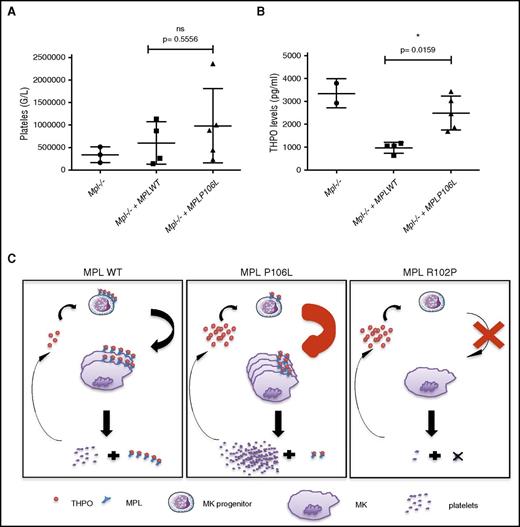
We asked whether expression of MPL P106L in vivo in mice can on its own induce a thrombocytosis with high THPO, as we observed in homozygous patients. We produced a retroviral murine model in which deficient C57/Bl6 Mpl−/− lin− cells were retrovirally transduced with either the human MPL WT or MPL P106L receptor and then injected into lethally irradiated Mpl−/− mice to avoid endogenous hematopoiesis recovery. We followed platelet count from 4 weeks after transduction and could observe that certain MPL P106L mice exhibited thrombocytosis, whereas MPL WT transduced mice did not. Although not statistically significant at P < .05 (due to variability and a low number of mice), when averaged for all reconstituted mice, the platelet levels in retrovirally MPL P106L transduced mice was higher than in retrovirally MPL WT transduced mice, the latter appearing to regain normal platelets values compared with Mpl−/− mice (Figure 7A). THPO levels were clearly not reduced to normal ranges in retrovirally MPL P106L transduced mice and stayed elevated, contrary to retrovirally MPL WT transduced mice, including in mice with thrombocytosis (Figure 7B). These results indicate that the disease induced by MPL P106L appears similar to the paradoxical thrombocytosis reported for engineered mice where platelet levels of MPL are low, impairing the clearance function,17 but the localization at the cell surface of early megakaryocytic progenitors allows stimulation and eventual production of a high number of platelets (Figure 7C).
Murine model partially reproduces the human pathology.Mpl knockout lin− cells were transduced with MPL WT or MPL P106L and then injected into lethally irradiated Mpl−/− mice. (A) Platelets and (B) THPO levels, respectively, were analyzed 1 month after transduction using impedance measure and enzyme-linked immunosorbent assay. At least 2 mice were used to constitute each group, apart from the control group, in 2 different experiments. (C) Schematic model of the mechanism induced by MPL P106L. *P < .05. Filled circles, filled boxes, and filled triangles defined the level for each mouse. ns, nonsignificant.
Murine model partially reproduces the human pathology.Mpl knockout lin− cells were transduced with MPL WT or MPL P106L and then injected into lethally irradiated Mpl−/− mice. (A) Platelets and (B) THPO levels, respectively, were analyzed 1 month after transduction using impedance measure and enzyme-linked immunosorbent assay. At least 2 mice were used to constitute each group, apart from the control group, in 2 different experiments. (C) Schematic model of the mechanism induced by MPL P106L. *P < .05. Filled circles, filled boxes, and filled triangles defined the level for each mouse. ns, nonsignificant.
Discussion
At present, there are 4 main classes of MPL mutations that are responsible for HT. Two of them (MPLS505N and MPLW515R) lead to a constitutive activation of the receptor and are also associated with sporadic ET.42 The 2 others (MPL K39N and MPL P106L) are germ line mutations associated with HT and can be considered rare polymorphisms.20,21 K39N is found in ∼8% of African American individuals.20,43 MPL P106L is present in ∼3% of the Arabic population.21,24 Recently, it was shown that MPL P106L exhibits a trafficking defect with a blockage in the ER with an absence of surface expression.24 Nonetheless, the mutated receptor was apparently capable of inducing a survival signal and was suggested to respond to extracellular THPO in the ER, but the topology of MPL would impose its extracellular domain to be located within the ER and not on the cytosolic face, as shown by Stockklausner et al.24 Although CALR mutants were shown to bind the extracellular MPL domain in the ER,25 there is no evidence that exogenously added THPO can be present in the ER lumen to bind to the extracellular domain of MPL.
Here, we show that the MPL P106L can traffic to the cell surface in megakaryocytic progenitors, especially in immature MKs, and in UT-7 megakaryocytic leukemia cells, but not in Ba/F3 cells. This difference in trafficking explains the pathology induced by this mutant and previous apparently contradictory results.24
On the other hand, we have clearly confirmed that MPL P106L exhibits a trafficking defect. It accumulates in the ER as an incompletely glycosylated immature form in a similar way as MPL R102P.24 This partial blockage leads to a marked defect in cell-surface plasma membrane localization. The receptor was undetectable or barely detectable at the cell surface in cell lines without cytokine selection. However, by selection in THPO, it was possible to derive clones that respond to exogenous THPO with a cell-membrane expression fourfold lower than MPL WT (Figure 6B). A low but detectable plasma membrane expression of MPL P106L was also found in the absence of any selection in MKs derived from CD34+ cells after MPL P106L retroviral transduction (Figure 2B). It is presently unknown why mutations of amino acids in close proximity, such as R102P, P106L, and F104S, induce different abnormalities in MPL trafficking. R102P induces a nearly complete block in the ER, P106L a partial block, and F104S a normal trafficking.23 It seems likely that the R102P and P106L, but not F104S, induce a folding defect in MPL, which leads to total or partial block in the ER.
One major difference between R102P and P106L could be that P106L remains able to traffic to the cell surface by an unconventional route.
Importantly, we did not find any evidence for gain-of-function constitutive activation of the MPL P106L receptor using several approaches, including by showing the absence of THPO-induced signaling in platelets from patients. It should be noted that a lower number of circulating CFU-MK was found in the proband compared with the parents, although a marked MK hyperplasia was found on bone marrow aspiration. Thus, we cannot exclude some defect in MK progenitor trafficking in this disorder.
In a mouse model, we only partially reproduced the disease by retrovirally transducing MPL P106L in Mpl−/− hematopoietic stem cells, with only rare mice developing thrombocytosis. In such a system, the level of MPL expression is difficult to control.17,44,45 However, in all mice, the THPO plasma level was higher than in those reconstituted with MPL WT, including in the mice with thrombocytosis (Figure 7B).
In homeostatic conditions, mice have a higher platelet count and a higher THPO plasma level than humans. It is very interesting to note that the mouse sequence is identical to the K39N mutation in human, and thus, the MPL K39N in human mimics the mouse physiology.20 This observation also highlights the importance of the glycosylation pattern of MPL and the MPL trafficking in the pathogenesis of thrombocytosis. This observation is true for MPL P106L and K93N, but also in the mechanism of MPN thrombocytosis. Indeed, both JAK2 V617F and CALR mutants induce a MPL trafficking defect with the presence of an incompletely glycosylated form at the cell surface.25,36,46-48 Although in the pathogenesis of MPN THPO is dispensable, it augments the phenotype as recently shown by targeting MPL in xenograft transplantations.49
Overall, our data on the pattern of MPL expression can explain the disease. The level of MPL P106L at the plasma membrane was higher in immature than in mature MKs, suggesting that there are enough receptors for proliferation and differentiation and not enough for THPO clearance. This pattern of MPL expression is reminiscent of the induction of a thrombocytosis in Mpl−/− mice rescued with a low Mpl expression17 and the transgenic mice described by Tiedt et al.11 In this last model, Mpl is expressed well during the early phase of megakaryopoiesis and is turned down in latter stages. This leads to a thrombocytosis with a normal or slightly reduced THPO level.11 A similar observation has been noted in mice, with a lack of MPL expression only in MKs and platelets.16 In conclusion, MPL P106L represents a loss-of-function receptor mutation for THPO clearance due to a partial trafficking defect, more manifest at late stages of MK differentiation. Because the THPO clearance defect is more severe than the signaling defect in earlier progenitors, the induced phenotype is thrombocytosis (Figure 7C). This underscores the crucial role of the THPO/MPL axis in the regulation of normal and pathological platelet production.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Roxana I. Albu for the MPL-GFP fusion. The authors are grateful to P. Rameau for cell sorting experiments and to S. Salomé-Desnoulez for confocal microscopy experiments. They acknowledge Céline Legrand for her help in mouse experiments and Kristell Lebozec for her contribution to retroviral production.
Support is acknowledged to S.N.C. from Ludwig Institute for Cancer Research, Fund for Scientific Research–Fund for Scientific Research, Salus Sanguinis Foundation, the Action de Recherche Concertée (ARC) project ARC10/15–027 of the University catholique de Louvain, the Fondation contre le Cancer, the Pôle d'attraction interuniversitaires Programs BCHM61B5, and Belgian Medical Genetics Initiative. J.P.D., R.-I.A., and V.G. were supported by Fund for Research Training in Industry and Agriculture, Télévie, and Belgian Federal Science Policy Office Interuniversity Attraction Poles postdoctoral fellowship. L.N.V. was supported by a Haas-Teichen fellowship and a postdoctoral fellowship at the de Duve Institute from the Maurange Fund, Belgium. This work was supported by grants from the Agence Nationale pour la Recherche (Thrombocytosis [W.V.]), the Ligue Nationale contre le Cancer (Equipe labellisée), and the Fondation Laurette Fugain (W.V.). F.F. was supported by the INSERM (Poste d’accueil) and the ARC Foundation, and M.K. was supported by the University Paris-Diderot.
Authorship
Contributions: W.V. and S.N.C. conceived and designed the study, interpreted the data, and wrote the paper; F.F. and K.M. designed and performed experiments, analyzed the data, prepared the figures, and wrote the paper; L.N.V. and S.B. designed, performed experiments, and analyzed the data; J.P.D., V.G., C.P., R.-I.A., and O.B. performed experiments; R.F., P.B., and G.L. collected the families and performed the THPO enzyme-linked immunosorbent assay assays; and I.P., H.R., and N.D. gave experimental/intellectual input and corrected the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William Vainchenker, UMR U1170, Gustave Roussy, 114 Rue Edouard Vaillant, 94805 Villejuif, France; e-mail: verpre@igr.fr; and Stefan N. Constantinescu, Signal Transduction and Molecular Hematology Unit, Ludwig Institute for Cancer Research Ltd, de Duve Institute, Université Catholique de Louvain, Avenue Hippocrate 74, UCL 75-4 Brussels B-1200, Belgium; e-mail: stefan.constantinescu@bru.licr.org.
References
Author notes
F.F. and K.M. contributed equally to this study.
S.N.C. and W.V. are joint senior authors.

![Figure 6. Lower MPL P106L activity is related to the cell-surface receptor number. (A) MPL receptor internalization after THPO binding: receptor cell-surface expression in UT-7 MPLWT and UT-7 MPL P106L clone 1 was measured by flow cytometry at different times after THPO binding. Analyzed were 2 × 105 cells per point, and results are means ± standard deviation of 3 independent experiments. (B) MPL binding to [125I]THPO (upper panel): the specific radioactivity bound to 5 × 105 UT-7 parental cells (empty circle), MPL WT (filled circle), P106L (empty square), and P106L clone 1 (filled square) UT-7 cells following 2-hours incubation at 15°C with radiolabeled ligand. A representative of 3 independent experiments is shown, together with a nonlinear fit to a 1-site model of specific saturation binding for UT-7 MPL WT and UT-7 MPL P106L clone 1. Binding to UT-7 parental and UT-7 MPL P106L cells was too low in this assay to unambiguously fit to model. Scatchard plot of [125I]THPO binding to UT-7 MPL WT and P106L cells (lower panel). (C) UT-7 MPL transduced cell proliferation in response to eltrombopag: UT-7 MPL WT, P106L, and THPO-selected clones were washed and then cultivated with THPO or eltrombopag at a concentration of 1 × 105 cells/mL and compared with the control GM-CSF, representing 100%. Viable cells were counted using KOVA slide. (D) UT-7 MPL WT and UT-7 MPL P106L signaling induced by THPO and eltrombopag. Cells were starved of cytokine for 5 hours and then stimulated with various concentrations of THPO or eltrombopag and compared with the control (GM-CSF). STAT1, STAT3, STAT5, AKT, and ERK1/2 activation was analyzed by western blotting using corresponding antibodies. Cell-surface MPL expression data for UT-7 MPL WT and MPL P106L clone 1 correspond to Figure 3A.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/26/10.1182_blood-2016-06-722058/5/m_blood722058f6.jpeg?Expires=1767784929&Signature=MF~MGwitQOVZb8jjU34XOAvUEZY3Pn7eJVvJYwOY1o2aRcTQ3xF4z60THchBvMirJwfRN16yvFuI8kzTDKocHjKu-4kSz8YK0jyjAWaVmuegas6Ekc4nv~Jsi2OZDTmS7X0-cPXeCA1qHWuidunvyFp6lsxmWYljSpLSOr2rZPJyRd3y0VcSkUUDZ4HPbBwkYlbgqjRYauZcGupiZRST7MIUZ0nB9NlMyQ45nRk1UGJvf60-TN2HO8wrB8VXw1MsEq-gR-yT3Kvw7ZJjTmNbLxNP~I-M41GRGHitFhtf4U0ZDlczgUUMb~F~CuAugTjT3mCNXvBnSC5crwwQjOFNhQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)