Key Points
Dasatinib, combined with low-intensity chemotherapy, gave 36% 5-year overall survival in Ph+ ALL patients older than age 55 years.
Prospective monitoring of mutations may be useful to personalize therapy in Ph+ ALL patients not eligible for intensive therapies.
Abstract
Prognosis of Philadelphia-positive (Ph+) acute lymphoblastic leukemia (ALL) in the elderly has improved during the imatinib era. We investigated dasatinib, another potent tyrosine kinase inhibitor, in combination with low-intensity chemotherapy. Patients older than age 55 years were included in the European Working Group on Adult ALL (EWALL) study number 01 for Ph+ ALL (EWALL-PH-01 international study) and were treated with dasatinib 140 mg/day (100 mg/day over 70 years) with intrathecal chemotherapy, vincristine, and dexamethasone during induction. Patients in complete remission continued consolidation with dasatinib, sequentially with cytarabine, asparaginase, and methotrexate for 6 months. Maintenance therapy was dasatinib and vincristine/dexamethasone reinductions for 18 months followed by dasatinib until relapse or death. Seventy-one patients with a median age of 69 years were enrolled; 77% had a high comorbidity score. Complete remission rate was 96% and 65% of patients achieved a 3-log reduction in BCR-ABL1 transcript levels during consolidation. Only 7 patients underwent allogeneic hematopoietic stem cell transplantation. At 5 years, overall survival was 36% and up to 45% taking into account deaths unrelated to disease or treatment as competitors. Thirty-six patients relapsed, 24 were tested for mutation by Sanger sequencing, and 75% were T315I-positive. BCR-ABL1T315I was tested by allele-specific oligonucleotide reverse transcription–quantitative polymerase chain reaction in 43 patients and detection was associated with short-term relapses. Ten patients (23%) were positive before any therapy and 8 relapsed, all with this mutation. In conclusion, dasatinib combined with low-intensity chemotherapy was well-tolerated and gave long-term survival in 36% of elderly patients with Ph+ ALL. Monitoring of BCR-ABL1T315I from diagnosis identified patients with at high risk of early relapse and may help to personalize therapy.
Introduction
Philadelphia (Ph) chromosome is the most frequent recurrent cytogenetic abnormality in elderly acute lymphoblastic leukemia (ALL) patients. Its incidence increases with age, reaching approximately 50% in ALL patients aged 60 years and older.1 Until the recent era of tyrosine kinase inhibitors (TKIs), most studies devoted to elderly patients with ALL made no distinction between Ph-positive (Ph+) and Ph-negative cases. This was largely due to equally poor survival irrespective of Ph chromosome status and the absence of differential treatments based on the identification of Ph+ ALL.2 The Ph chromosome arises from the t(9;22)(q34;q11.2) reciprocal translocation fusing the c-ABL1 tyrosine kinase gene on chromosome 9 and the BCR gene on chromosome 22. The deregulated BCR-ABL1 tyrosine kinase activity plays a major role in a transformation process in conjunction with other genetic alterations, leading to a differentiation block giving rise to Ph+ (or BCR-ABL1+) ALL.3
Designed as a targeted therapy against BCR-ABL1 tyrosine kinase activity, imatinib rapidly became a gold standard therapy in chronic myeloid leukemia and was proposed in relapsed/refractory Ph+ ALL.4 The addition of imatinib to age-adapted chemotherapy significantly improved complete remission rates and disease-free survival as compared with historical controls in elderly Ph+ ALL patients.5 A randomized study from the German Multicenter Study Group for Adult ALL (GMALL) established the benefit of imatinib against a chemotherapy-based induction.6 To reduce toxicity, the Italian Group for Haematological Diseases in Adults (GIMEMA) proposed combining high-dose imatinib (800 mg/day) with prednisone, without chemotherapy.7 Data from the combination of high-dose imatinib with vincristine and dexamethasone (DIV regimen) suggested that a low-intensity chemotherapy approach was feasible and effective both in relapsed and in elderly Ph+ ALL patients.8
Dasatinib is an oral, multitargeted kinase inhibitor of the BCR-ABL1 and SRC family kinases originally designed as an SRC kinase inhibitor. Dasatinib can bind to both the active and inactive conformations of the ABL kinase. Because it has less stringent binding requirements than imatinib, dasatinib has activity against many imatinib-resistant kinase domain mutations of BCR-ABL1. One exception is the T315I mutation within the adenosine triphosphate–binding pocket of the ABL tyrosine kinase that confers a high degree of resistance.9 The impact of new TKIs on the prognosis of Ph+ ALL is being prospectively assessed. The hyper cyclophosphamide, vincristine, doxorubicin (Adriamycin), and dexamethasone (CVAD) regimen was combined with dasatinib in a phase II trial. Complete remission rate was 96% and 5-year overall survival (OS) was 46%.10
The European Working Group on Adult ALL (EWALL) presented an international consensus defining a low-intensity chemotherapy backbone for adult ALL patients aged 55 years and older, the “EWALL backbone,” to minimize toxicity.11 In the present study, we combined the EWALL backbone with dasatinib (Sprycel, Bristol-Myers Squibb), for Ph+ ALL patients aged 55 years and older.
Patients and methods
Study design and population
The European Working Group on Adult ALL (EWALL) study number 01 for Ph+ ALL (EWALL-PH-01 international study) is the first prospective phase 2 study conducted by the EWALL group on behalf of the European Leukemia Net. Patients aged 55 years or older were eligible if they had newly diagnosed Ph+ and/or BCR-ABL1+ ALL with or without documented central nervous system (CNS) involvement and if they had not been previously treated, except with corticosteroids or single-dose vincristine. Other inclusion criteria are detailed in the supplemental Appendix, available on the Blood Web site. Written informed consent was obtained from all patients. The study was approved in May 2007 by the institutional review board of Ile de France XI, France.
The study was conducted in accordance with the Declaration of Helsinki (EudraCT: 2006-005694-21) and is registered on the EU Clinical Trial Web site (https://www.clinicaltrialsregister.eu/ctr-search/trial/2006-005694-21/FR).
Study treatments
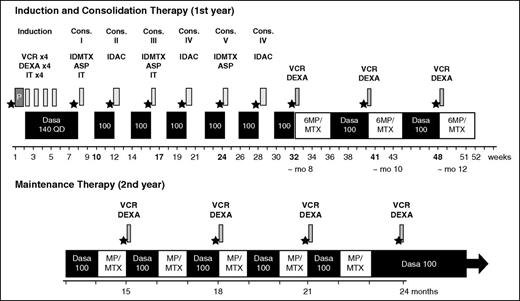
The planned protocol is summarized in Figure 1. After a prephase (dexamethasone 10 mg/day on days −7 to day −3 and 1 intrathecal therapy with methotrexate 15 mg), dasatinib was administered at 140 mg once a day during the induction period in combination with weekly vincristine 2 mg IV (1 mg for patients >70 years) and dexamethasone 40 mg for 2 days (20 mg for patients >70 years) for 4 weeks. Consolidation consisted of dasatinib 100 mg/day sequentially with methotrexate 1000 mg/m2 IV on day 1 and asparaginase 10 000 IU/m2 intramuscularly on day 2 for cycles 1, 3, and 5, and cytarabine 1000 mg/m2 IV every 12 hours day 1, day 3, and day 5 for cycles 2, 4, and 6, with 4-week cycles. Maintenance consisted of dasatinib 100 mg/day sequentially with 6-mercaptopurine (60 mg/m2 per day) and methotrexate (25 mg/m2 per week) orally, 1 every other month and dexamethasone/vincristine every 2 to 3 months up to 24 months. Postmaintenance therapy consisted of dasatinib alone (100 mg/day) until relapse or death. The protocol allowed both auto- or allogeneic hematopoietic stem cell transplantation (HSCT) with reduced-intensity conditioning or myeloablative conditioning. CNS prophylaxis included intrathecal methotrexate (15 mg), cytarabine (40 mg), and prednisone (40 mg) for a total of 6 injections (4 during induction and 2 during consolidations). Because of an excess rate of treatment discontinuation in the first 11 patients >70 years of age receiving dasatinib 140 mg/day, the protocol was amended after 15 months to reduce dasatinib and chemotherapy doses for patients aged 70 years and older (dasatinib 100 mg/day during induction, methotrexate 500 mg/m2, asparaginase 5000 IU/m2, and cytarabine 500 mg/m2 during consolidations). Comorbidities were assessed at inclusion by the Cumulative Illness Rating Scale for Geriatrics comorbidity index (CIRS-G).12
EWALL-PH-01 treatment strategy. ★, PCR analysis; ASP, asparaginase; IDAC, intermediate-dose cytarabine; Cons., consolidation; Dasa, dasatinib; DEXA, dexamethasone; IDMTX, intermediate-dose methotrexate; IT, intrathecal (triple IT, 15 mg MTX, 40 mg AraC, and 40 mg prednisone); 6MP, 6-mercaptopurine; MTX, methotrexate; mo, month; P, prephase with dexamethasone; QD, once a day; VCR, vincristine.
EWALL-PH-01 treatment strategy. ★, PCR analysis; ASP, asparaginase; IDAC, intermediate-dose cytarabine; Cons., consolidation; Dasa, dasatinib; DEXA, dexamethasone; IDMTX, intermediate-dose methotrexate; IT, intrathecal (triple IT, 15 mg MTX, 40 mg AraC, and 40 mg prednisone); 6MP, 6-mercaptopurine; MTX, methotrexate; mo, month; P, prephase with dexamethasone; QD, once a day; VCR, vincristine.
Molecular and cytogenetic assessments
Patients were monitored for BCR-ABL1 by reverse transcription–quantitative polymerase chain reaction (qRT-PCR) in national central laboratories in each country in accordance with current recommendations.13 Minimal residual disease (MRD) was assessed after induction (MRD1), during consolidation (MRD2), and throughout maintenance. Bone marrow (BM) results were considered preferentially when results from both peripheral blood and BM were available. In the absence of a BM sample, peripheral blood results were analyzed if available. During consolidation, the highest BCR-ABL1 value was considered to define MRD2.
Methods for BCR-ABL1T315I allele-specific oligonucleotide (ASO) qRT-PCR are described in the supplemental appendix. A sensitivity threshold of 0.05% was chosen that corresponded to the upper limit of the 95% confidence interval (CI) estimated from the distribution of nonspecific signals measured in 60 samples that were negative for BCR-ABL1T315I. Cytogenetic analyses were performed in each local laboratory at diagnosis and centrally reviewed.
Response definitions
Complete hematologic remission (CR) was defined as the presence of ≤5% blasts in the BM, with a granulocyte count ≥1.0 × 109/L, platelet count ≥100 × 109/L, and no extramedullary disease. Molecular response 5-log (MR5) was defined by a 5-log reduction in BCR-ABL1 transcript level (ratio positive ≤0.001% or null with at least 100 000 copies of ABL1 in the sample analyzed for BCR-ABL1). Major molecular response (MMR) was defined by a BCR-ABL1/ABL1 ratio ≤0.1%. Relapse was defined by recurrence of >5% lymphoblasts in the BM aspirate or by the presence of extramedullary disease after achieving CR. Induction death was defined as death occurring after the start of therapy without meeting the definition of CR or resistant disease. Resistant disease included patients who survived the induction treatment period but had persistent leukemia. Relapse-free survival (RFS) was calculated from the first day of therapy to the relapse date. Event-free survival (EFS) was defined as the time between inclusion and death under induction, hematologic relapse, or death in first CR, with other patients being censored at the time of last contact. OS was calculated from the date of initiation of therapy until death or last contact. Toxicity was evaluated according to the National Cancer Institute Common Toxicity Criteria, version 2.0.55.
Statistical methods
The primary end point was RFS at 12 months. Patients in remission were censored at the time of last follow-up. A sample size of at least 50 patients was calculated by Fleming’s single-stage design to ensure 80% power for proving lack of efficacy if the true CR rate was below 60%. Considering a rate below 40% would imply lack of efficacy, a sample size of at least 48 patients had to be recruited to detect a remission rate >60% with 80% power and a level of α = 2.5% (one-sided test). Allowing for potential censored data, a total of 55 patients were planned. This number was subsequently increased to 71 patients by amendment to compensate for early treatment discontinuation seen in patients aged >70 years. Secondary end points were cumulative incidence of relapse, cumulative incidence of nonrelapse-related mortality, and OS. The Kaplan-Meier method was used to estimate the EFS, RFS, and OS probabilities. Hazard ratios were given with the 95% CI. Analysis followed the intent-to-treat principle. Baseline characteristics were compared by nonparametric tests, either Fisher's exact test for qualitative variables or the Kruskal-Wallis test for quantitative variables. The censored end points (time to death, RFS) were estimated by the nonparametric Kaplan-Meier method with a 95% CI. Cumulative incidences were estimated using Aalen estimator.14 Prognostic factors of survival and progression-free survival were investigated using Cox proportional hazard models. Statistical analysis was performed with XLSTAT software (version 2014.2.03, Addinsoft) and R Project for Statistical Computing software (R, version 2.15.2). M.D. and P.R. analyzed the data and all authors had access to primary clinical trial data.
Results
Patient characteristics
Seventy-one patients were included between August 2007 and July 2010 in Belgium, Germany, Italy, and France. One patient had CNS involvement at diagnosis. Median age was 69 years (range, 59-83) and the sex ratio (male/female) was 0.73. Median follow-up on study was 32 months (range, 2-88 months) and median follow-up for surviving patients was 66 months (range, 21-88 months) at the time of analysis in January 2015. The median CIRS-G score value was 6 (range, 0-16). Overall, 77% of the patients had a CIRS-G score >4. Karyotype analysis was performed in all 71 patients and was successful in 64 (91%), identifying the t(9;22) chromosomal translocation in 61 of the 64 cases and additional cytogenetic aberrations (ACAs) in 48. BCR-ABL1 fluorescence in situ hybridization analysis demonstrating BCR-ABL1 fusion-specific signals was performed in 26 cases, including the 3 cases with a normal karyotype and 5 cases with karyotype failure. Minor breakpoint transcripts (e1a2 and e1a3) encoding for the p190BCR-ABL1 protein were identified in 54 patients (76%). Major breakpoint transcripts (e13a2 and e14a2) encoding the p210BCR-ABL1 protein were identified in 17 patients (24%) (Table 1).
Patient characteristics
| . | Patients (n = 71) . |
|---|---|
| Baseline | |
| Male/female, n | 30/41 |
| Median age, y (range) | 69 (59-83) |
| ECOG PS 0/1/2/3, n | 21/44/5/1 |
| Median CIRS (range) | 6 (0-16) |
| CIRS-G >4, n (%)* | 54 (77%) |
| CNS disease, n | 1 |
| Median WBC, 109/L (range) | 12.4 (0.8-348.3) |
| WBC ≥30 × 109/L, n (%) | 27 (38) |
| Karyotype | Successful (n = 64) |
| Ph on karyotype analysis | 61 (95%) |
| Presence of ACAs, n (%)† | 48 (79%) |
| Supernumerary Ph, n (%) | 19 (31%) |
| Hyperdiploidy 47-50, n (%) | 17 (28%) |
| Deletion 9p, n (%) | 15 (25%) |
| Monosomy 7 or deletion 7p, n (%) | 12 (20%) |
| Molecular biology, n | |
| Bcr subtype (m/M/both) | 54/17/0 |
| . | Patients (n = 71) . |
|---|---|
| Baseline | |
| Male/female, n | 30/41 |
| Median age, y (range) | 69 (59-83) |
| ECOG PS 0/1/2/3, n | 21/44/5/1 |
| Median CIRS (range) | 6 (0-16) |
| CIRS-G >4, n (%)* | 54 (77%) |
| CNS disease, n | 1 |
| Median WBC, 109/L (range) | 12.4 (0.8-348.3) |
| WBC ≥30 × 109/L, n (%) | 27 (38) |
| Karyotype | Successful (n = 64) |
| Ph on karyotype analysis | 61 (95%) |
| Presence of ACAs, n (%)† | 48 (79%) |
| Supernumerary Ph, n (%) | 19 (31%) |
| Hyperdiploidy 47-50, n (%) | 17 (28%) |
| Deletion 9p, n (%) | 15 (25%) |
| Monosomy 7 or deletion 7p, n (%) | 12 (20%) |
| Molecular biology, n | |
| Bcr subtype (m/M/both) | 54/17/0 |
ACAs, additional cytogenetic abnormalities; Bcr, BCR-ABL breakpoint; PS, performance status; CIRS, cumulative illness rating scale; m, minor breakpoint; M, major breakpoint; WBC, white blood cell count.
n = 70.
Only ACAs present in at least 20% of Ph+ cases are listed; ACAs were often combined.
Response to therapy and outcome
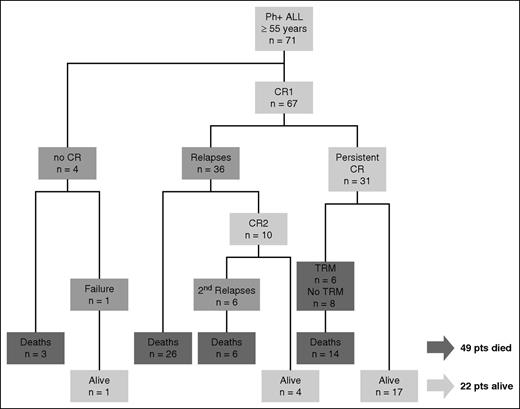
Sixty-seven of the 71 patients (96%) achieved CR. Four patients failed to obtain CR: 1 was refractory and obtained CR after salvage therapy and 3 died during induction. Among the 67 patients in CR after induction, 36 relapsed (54%). Ten patients obtained a second CR (28%), 6 of whom relapsed again. Forty-nine patients died (69% of the total population), including 32 deaths in relapse or refractory disease and 14 deaths in CR (nonrelapse-related mortality, 29%). Treatment-related mortality was reported in 6 patients (12%). Eight deaths were unrelated to disease or therapy (including 3 late deaths, 2 from adenocarcinoma after 49 and 66 months in study and 1 from meningioma after 68 months). Twenty-two patients (31% of the total population) were alive at the time of analysis. No patient received an autograft. Seven patients (median age, 60.3 years) underwent allogeneic HSCT, 5 with reduced-intensity conditioning and 2 with myeloablative conditioning. Three patients relapsed after transplant at 4.6, 6.3, and 6.5 months and died; 4 patients were alive in CR at 53, 56, 59, and 65 months. Figure 2 summarizes treatment outcomes.
Consort diagram. CR1, complete remission 1; CR2, complete remission 2; TRM, treatment-related mortality; pts, patients.
Consort diagram. CR1, complete remission 1; CR2, complete remission 2; TRM, treatment-related mortality; pts, patients.
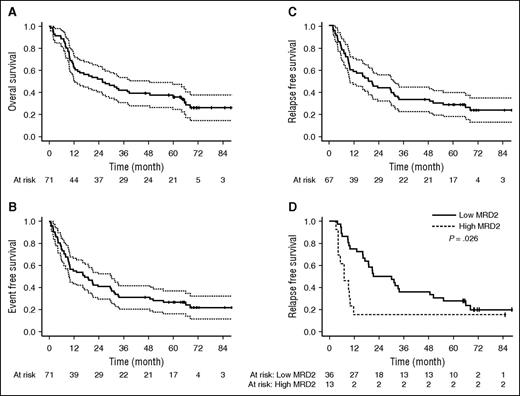
Among the 67 patients achieving first CR, 55 (82%) were evaluable for MRD. Characteristics of these 55 patients did not differ from those of the 12 CR patients not tested. At MRD1, 33 patients reached MMR (60%) and 11 patients reached MR5 (20%). At MRD2, 36 patients reached MMR (65%) and 13 patients reached MR5 (24%). RFS at 12 months was 58% (95% CI, 45-69). At 3 years, RFS, EFS, and OS were 33% (95% CI, 22-44), 31% (95% CI, 21-42), and 41% (95% CI, 29-52), respectively. At 5 years, RFS, EFS, and OS were 28% (95% CI, 18-39), 27% (95% CI, 17-37), and 36% (95% CI, 25-47), respectively. Median RFS, EFS, and OS were 19.1, 18.9, and 25.8 months, respectively (Figure 3). Including deaths unrelated to disease or treatment as competitors, OS was 45% (95% CI, 43-67) at 5 years (supplemental Figure 1). Taking allogeneic HSCT as a censoring event did not affect RFS, EFS, or OS (supplemental Figure 1).
Survival analysis. Overall survival (A, n = 71), event-free survival (B, n = 71), relapse-free survival (C, n = 67), and relapse-free survival according to MRD2 at the level of 0.1% (D, n = 49).
Survival analysis. Overall survival (A, n = 71), event-free survival (B, n = 71), relapse-free survival (C, n = 67), and relapse-free survival according to MRD2 at the level of 0.1% (D, n = 49).
Safety
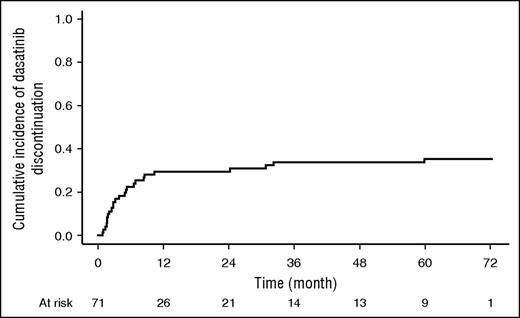
Median time on dasatinib therapy was 7.8 months (range, 0.6-72.4). Thirty-eight patients discontinued treatment because of relapse or death. Other reasons for dasatinib discontinuation were adverse events or investigator decision in 14 patients and allogeneic HSCT in 7 patients. Eight of 22 patients alive were still on dasatinib at the time of analysis (36%). The other patients were being treated with imatinib or nilotinib based on investigator decision. The cumulative incidence of dasatinib discontinuation was 35% (95% CI, 24-46) considering discontinuations at the time of death or relapse as competing risks (Figure 4).
Cumulative incidence of dasatinib discontinuation. Deaths or relapses were considered as competing risks for dasatinib discontinuation.
Cumulative incidence of dasatinib discontinuation. Deaths or relapses were considered as competing risks for dasatinib discontinuation.
During induction, the mean duration of neutropenia <0.5 × 109/L was 8.9 days (range, 0-41 days). Granulocyte colony-stimulating factor was administered to 73% of the patients as per protocol; 23 patients (32%) did not experience neutropenia. Mean duration of broad spectrum antibiotics was 12 days. Thirteen patients presented bacteremia or septicemia (4 with gram-negative and 9 with gram-positive strands). Two patients developed nonbacterial lung infections (1 pneumonia with Pneumocystis jiroveci and 1 with Aspergillosis). The mean duration of platelets <20 × 109/L was 3 days (range, 0-41 days). Consolidations with methotrexate and asparaginase were associated with very few episodes of neutropenia (2 during consolidation 1, 2 during consolidation 3, and none during consolidation 5). Consolidation with intermediate-dose cytarabine was associated with a mean duration of neutropenia <0.5 × 109/L of 4.2 days, 5.9 days, and 6.3 days during consolidation 2, 4, and 6, respectively, and a mean duration of platelets <20 × 109/L of 3.2 days, 4.5 days, and 4.9 days, respectively. During maintenance, 11 patients presented short episodes of neutropenia <0.5 × 109/L.
Pleural effusion occurred in 7 patients (10%), including 3 during induction, 2 during consolidation, 1 during maintenance, and 1 during postmaintenance therapy with dasatinib. The median age of patients with pleural effusion was the same as that of patients without. No concomitant lymphocytosis was seen. Median latency was 2.3 months, with only 1 late case (after 60.7 months). Three patients were receiving dasatinib at 140 mg/day and 4 at 100 mg/day at the onset of pleural effusion. Median duration of pleural effusion was 13 days (range, 4-41 days). As has previously been reported elsewhere,15 4 cases were associated with interstitial pneumonia and 1 case with pericardial effusion. In the late-occurring case, there was a strong suspicion of pulmonary hypertension based on echocardiography (supplemental Table 1). Of note, the RFS of patients who experienced pleural effusion was not different from those without (supplemental Figure 3).
Other serious adverse events included tumor lysis syndrome during induction (n = 2), renal failure (n = 6), subdural hemorrhage (n = 3, including 1 patient with concomitant acetyl salicylic acid therapy), digestive hemorrhage (n = 3), transaminase elevations (n = 5), pulmonary embolism (n = 3), atrial fibrillation (n = 4), and cardiac failure (n = 2).
Relapses and mutations
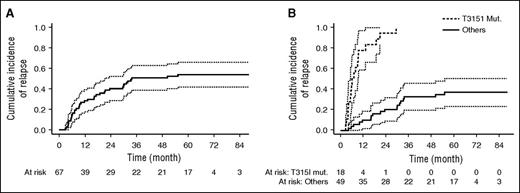
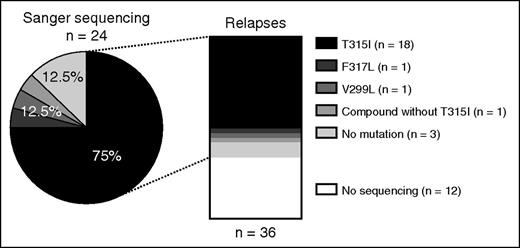
Thirty-six relapses were observed, including 14 during consolidation, 19 during maintenance, and 3 after allogeneic HSCT. Only 1 CNS relapse at 18 months was recorded. Cumulative incidence of relapse was 54% (95% CI, 42-66) at 5 years (Figure 5A). Standard mutation analysis with Sanger sequencing was available at relapse for 24 patients: 18 relapses were associated with the T315I mutation (including 1 compound mutation T315I+M244V+E255K), 1 relapse was associated with the F317L mutation, 1 with the V299L, 1 with a compound mutation without T315I (F137I+F359I+F359C), and mutations were not detectable in 3 patients. Sanger sequencing was not performed in 12 patients at relapse but ASO PCR for the detection of BCR-ABL1T315I was negative in all 7 patients tested (Figure 6).
Relapse analysis. Cumulative incidence of relapse in all 67 patients in CR (A) and in patients with BCR-ABL1T315I >0.05% compared with other patients (B) (P < .001).
Relapse analysis. Cumulative incidence of relapse in all 67 patients in CR (A) and in patients with BCR-ABL1T315I >0.05% compared with other patients (B) (P < .001).
Distribution of BCR-ABL1 tyrosine kinase domain mutations in 36 patients in first relapse.
Distribution of BCR-ABL1 tyrosine kinase domain mutations in 36 patients in first relapse.
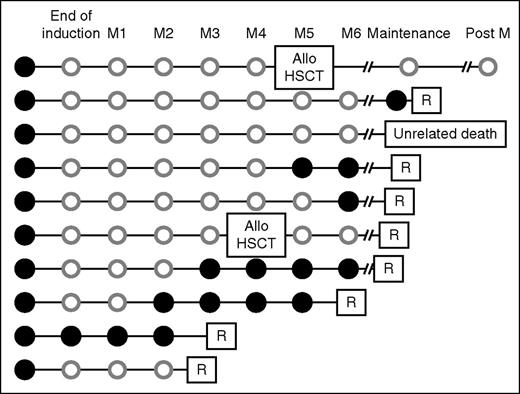
ASO PCR for the detection of BCR-ABL1T315I was retrospectively performed in 43 patients on prospectively collected RNA samples. No significant differences were seen between tested and nontested patients in terms of baseline characteristics. Of these 43 patients, 10 (23%) were BCR-ABL1T315I-positive by ASO PCR at the sensitivity level of 0.05% before any therapy (Figure 7). No mutation was detectable by Sanger sequencing on these samples. Eight patients relapsed, all with the T315I mutation, including 1 relapse 4 months after allogeneic HSCT. During follow-up, BCR-ABL1T315I was detected 1 to 3 months before hematologic relapse. Two patients did not relapse, 1 patient was transplanted and was still in CR 54 months after allogeneic HSCT, and the other died in CR at 9.6 months from lung adenocarcinoma. We compared the cumulative incidence of relapse (death was considered as a competing risk) between T315I mutated (BCR-ABL1T315I) and other patients at diagnosis. Quicker relapses were observed in cases of a detectable BCR-ABL1T315I (median, 7 months) compared with other patients (median not reached) (Fine and Gray regression coefficient, 14.80 [95%CI, 7.03-31.20], P < .001 (Figure 5B).
Follow-up of 10 patients positive for BCR-ABL1T315I by ASO PCR at inclusion. A systematic screening for presence of the T315I mutation at diagnosis was performed by ASO qRT-PCR in 43 patients and the T315I mutation was detected in 10 of them (23%). The kinetic of detection of the T315I mutation before relapse is indicated. Solid black circle, BCR-ABL1T315I detected by ASO PCR; open gray circle, BCR-ABL1T315I not detected by ASO PCR during follow-up. Allo, allogeneic; Post M, postmaintenance; R, relapse associated with BCR-ABL1T315I detected by ASO PCR and Sanger sequencing.
Follow-up of 10 patients positive for BCR-ABL1T315I by ASO PCR at inclusion. A systematic screening for presence of the T315I mutation at diagnosis was performed by ASO qRT-PCR in 43 patients and the T315I mutation was detected in 10 of them (23%). The kinetic of detection of the T315I mutation before relapse is indicated. Solid black circle, BCR-ABL1T315I detected by ASO PCR; open gray circle, BCR-ABL1T315I not detected by ASO PCR during follow-up. Allo, allogeneic; Post M, postmaintenance; R, relapse associated with BCR-ABL1T315I detected by ASO PCR and Sanger sequencing.
Prognostic factors
Influence of age, CIRS-G index (continuous variables), sex, Eastern Cooperative Oncology Group (ECOG) performance status, leukocytes <30 ×109/L, karyotype, BCR breakpoint, BCR-ABLT315I, and molecular response status at MRD1 and MRD2 on OS and RFS were assessed (supplemental Table 2). In univariate analysis age, sex, ECOG performance status, leukocyte count, and presence of the T315I mutation had a significant effect on OS. For RFS, only leukocytes count and achievement of MMR at MRD2 reached significance (Figure 3D). Multivariate analysis was conducted using variable with at least 69 not missing data for OS and 65 for RFS (age, CIRS-G, Sex, ECOG performance status, leukocyte count). Only sex, ECOG performance status, and leukocyte count remained significantly associated with OS, and the strength of association of leukocyte count with RFS increased in multivariate analysis.
Discussion
This study reports the long-term outcome of elderly Ph+ ALL patients treated with dasatinib, a second-generation TKI in combination with low-intensity chemotherapy. CR was achieved in 96% of the patients, RFS at 12 months was 58%, and 5-year OS 36%.
Relatively few studies have specifically investigated the use of imatinib in combination with chemotherapy in Ph+ ALL in the elderly. The GIMEMA proposed a study in Ph+ ALL patients aged >60 years evaluating the use of steroids combined with high-dose imatinib (800 mg/day), without additional chemotherapy. CR was obtained in all 29 patients with low toxicity; however, remission duration was limited in patients not eligible for allogeneic HSCT.7 The GMALL group performed a randomized study assessing induction therapy with either imatinib or multiagent, age-adapted chemotherapy followed by imatinib administered with consolidation chemotherapy in both arms. The overall CR rate was 96% in patients assigned to imatinib and 50% in patients allocated to chemotherapy. OS was not significantly different between the 2 cohorts.6 A low-intensity schedule (vincristine and dexamethasone) in combination with high-dose imatinib (800 mg/day) was proposed in the Group for Research on Adult Acute Lymphoblastic Leukemia (AFR07 study) for patients with relapsing or refractory Ph+ ALL (DIV regimen).8 A pilot study of this combination showed promising results with a CR rate of 90% in newly diagnosed patients older than 55 years, suggesting that high-dose chemotherapy may not be needed in this population.8 A similar induction regimen was used in the Acute Lymphoblastic Leukemia-Old-Philadelphia Positive-07 study from the Programa para el Tratamiento de Hemopatías Malignas group (53 patients aged >55 years), followed by maintenance without consolidation. High remission rates were observed (87%) and median survival was 37.3 months.16
The question as to whether dasatinib improves the prognosis of Ph+ ALL patients has been investigated in few studies. The GIMEMA group tested an induction regimen combining dasatinib at the initial dose of 70 mg twice daily with intrathecal chemotherapy and steroids for 84 days.17 All 53 patients (median age, 54 years) achieved CR, and disease-free survival was 51% at 20 months despite intensive consolidation therapies in 60% of the patients. A second study tested the combination of intermittent dasatinib (100-140 mg/day) with the hyper-CVAD regimen in 72 patients (median age, 55 years). Response rate was 96% and long-term survival was 46%, with a median follow-up of 67 months for surviving patients.18 The median age of the patients included in the current EWALL-PH-01 study (69 years) is the oldest reported to date in a large cohort. This elderly group of patients had a very low rate of allogeneic HSCT (10%). In this setting, the combination of dasatinib with low-intensity chemotherapy gave a 5-year OS of 36%, suggesting that a subgroup of patients may experience long-term survival without intensive therapies. Only 1 CNS relapse was observed in accordance with the protective effect of intrathecal CNS-directed prophylaxis, possibly enhanced by dasatinib because of its ability to achieve active concentrations in the CSF.19
The achievement of MMR during consolidation (MRD2) was a predictor for RFS in this study, as in other reports.20 Sixty-five percent of the patients reached MMR at MRD2, a proportion similar to that observed in the Group for Research on Adult Acute Lymphoblastic Leukemia study for young patients using imatinib and intensive consolidation.21 The deep molecular response rate was also comparable, suggesting that deep molecular responses can also be achieved with a potent TKI such as dasatinib without the need for intensive consolidation regimens.
The ECOG status was a strong prognostic factor in both univariate and multivariate analysis for OS and RFS. Overall, 77% of the patients included in the EWALL-PH-01 study had a CIRS-G score >4, highlighting the growing importance of the comorbidities in this population. Comorbidities may influence the choice of TKIs regarding the cardiovascular risk and may also trigger the risk of deaths unrelated to the disease. Eight of our patients died of causes independent of ALL or therapy. Five-year OS was 45%, taking into account deaths unrelated to disease or treatment as competitors. Safety was acceptable in this population, especially after the reduction of dasatinib daily dose from 140 to 100 mg/day for patients aged 70 years or more. With the use of a more potent TKI, the rate of induction deaths was low (4%). Treatment-related mortality compares favorably with studies using more intensive chemotherapy. As an example, the rate of the deaths in CR was 21.6% in the Group for Research on Adult Acute Lymphoblastic Leukemia study for young adults compared with 19.7% in our study.21 Pleural effusions are age- and dose-dependent in patients treated with dasatinib. Recent long-term follow-up studies reported an incidence of 28% to 35% at 5 years in chronic myeloid leukemia patients treated with dasatinib compared with only 10% in our aged ALL population.22 This low rate of pleural effusions in our study is likely related to the concomitant administration steroids and immunosuppressive chemotherapy.
Relapses were associated with clones harboring mutations known to confer a high degree of resistance to dasatinib, as previously described.23,24 Sanger sequencing was performed in 24 of 36 relapses and the T315I mutation was present in 18 of 24 relapsing patients (75%). Other mutations that may be selected by dasatinib were identified, such as the V299L mutation and the F317L mutation. With a specific ASO PCR for the detection of BCR-ABL1T315I, we were able to search for the T315I mutation at diagnosis in 43 patients. Ten patients (23%) were found to be positive. Eight relapsed, 1 patient died in CR during maintenance, and 1 died during allogeneic HSCT. Serial mutation analysis indicated that T315I became undetectable during remission and reappeared 1 to 6 months before relapse. DeBoer et al also reported relapsing patients with mutations (n = 13), including 5 patients with mutations at diagnosis.25
Whether the use of more powerful BCR-ABL1 inhibitors such as ponatinib may improve OS in Ph+ ALL remains to be determined. The cardiovascular toxicity of ponatinib may counteract the potential benefit of this drug in patients with a high comorbidity index, such as that of the EWALL-PH-01 study.26 A tailored choice based on patient status and adverse disease-related risk factors, such as the monitoring of mutations, could be 1 way to balance risk and efficacy in future trials. Other options may integrate immunoconjugates27 or BiTE antibodies such as blinatumomab28 into tyrosine kinase–based regimens.
In conclusion, despite a relatively small number of patients studied, we report high efficacy of dasatinib combined with low-intensity chemotherapy (EWALL backbone) in elderly Ph+ ALL patients with a 36% long-term survival without intensive chemotherapy or allogeneic HSCT. We report the first evidence supporting a prospective monitoring of the T315I mutation in patients not eligible for intensive therapies to personalize therapy before hematologic relapse.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families for participating in the trial, A. Beulaygue and A. Chabanne for data monitoring in France, J. Hermann for data monitoring in Germany, For Drug Consulting (FDC) for pharmacovigilance monitoring, and M. Hesham for his longstanding support and friendship.
This study was sponsored by the Versailles Clinical Research Office (Délégation à la Recherche Clinique et à l’Innovation, Versailles, France) and a research grant from Bristol-Myers Squibb, which also provided dasatinib. Sarah MacKenzie (medical writer), supported by funding from Centre Hospitalier de Versailles, provided editorial assistance.
Authorship
Contribution: P.R., O.G.O., H.D., D.H., N.G., A.D., J.R., and R.B. designed the study; L.M. provided administrative support; P.R., C.G.P., F.H., T.L., P.C., C.S., M.A., M.H., S.G., C. Bonmati, V.R., P.A., C. Berthou, E.J., J.F., L.S., A. Banos, O.R., B.L., X.T., N.I., A.D., J.R., H.D., and O.G.O. treated patients and collected data; P.R. and O.G.O. analyzed data and wrote the manuscript; A. Bornand provided geriatric expertise; J.-M.C., S.H., and H.P. performed the minimal residual disease studies; M.M.C. performed ASO polymerase chain reaction analysis; M.L.-P. performed cytogenetic review; and M.D. and P.R. performed the statistical analysis.
Conflict-of-interest disclosure: P.R. received a research grant from Bristol-Myers Squibb. The remaining authors declare no competing interests.
A complete list of the members of the European Working Group on Adult ALL (EWALL) group appears in “Appendix: study group members.”
Correspondence: Philippe Rousselot, Hemato-Oncology Unit, Hôpital André Mignot, Centre Hospitalier de Versailles, 177 rue de Versailles, 78150 Le Chesnay, France; e-mail: phrousselot@ch-versailles.fr.
Appendix: study group members
The members of the European Working Group on Adult ALL (EWALL) group are: Ulrich Jaeger, Alexander Hauswirth, Michel Doubek, Cyril Salek, Sabina Chiaretti, Giovanni Martinelli, Robin Foa, Nicola Goekbuget, Dieter Hoelzer, Heike Pfeifer,Anita Rijneveld, Jan Cornelissen, Adele Fielding, David Marks, Renato Bassan, Helene Hallbook, Sebastian Giebel, Jan Holowiecki, Jan Walewski, Josep Maria Ribera, Elena Parovichnikova,Viktor Savchenko, Boris Afanasiev, Martin Mistrik, Belinda Simoes, Oliver Ottmann, Mathilde Hunault, Philippe Rousselot, Ifrah Norbert, and Hervé Dombret.
References
Author notes
P.R. and M.M.C. equally contributed to this work.