Abstract
Myeloproliferative neoplasms (MPNs) arise in the hematopoietic stem cell (HSC) compartment as a result of the acquisition of somatic mutations in a single HSC that provides a selective advantage to mutant HSC over normal HSC and promotes myeloid differentiation to engender a myeloproliferative phenotype. This population of somatically mutated HSC, which initiates and sustains MPNs, is termed MPN stem cells. In >95% of cases, mutations that drive the development of an MPN phenotype occur in a mutually exclusive manner in 1 of 3 genes: JAK2, CALR, or MPL. The thrombopoietin receptor, MPL, is the key cytokine receptor in MPN development, and these mutations all activate MPL-JAK-STAT signaling in MPN stem cells. Despite common biological features, MPNs display diverse disease phenotypes as a result of both constitutional and acquired factors that influence MPN stem cells, and likely also as a result of heterogeneity in the HSC in which MPN-initiating mutations arise. As the MPN clone expands, it exerts cell-extrinsic effects on components of the bone marrow niche that can favor the survival and expansion of MPN stem cells over normal HSC, further sustaining and driving malignant hematopoiesis. Although developed as targeted therapies for MPNs, current JAK2 inhibitors do not preferentially target MPN stem cells, and as a result, rarely induce molecular remissions in MPN patients. As the understanding of the molecular mechanisms underlying the clonal dominance of MPN stem cells advances, this will help facilitate the development of therapies that preferentially target MPN stem cells over normal HSC.
Introduction
Myeloproliferative neoplasm (MPN) stem cells are defined as a clonal population of rare cells within the bone marrow that harbor an MPN-initiating somatic mutation, are capable of indefinite self-renewal, and that, through a combination of cell-intrinsic and cell-extrinsic effects undergo clonal expansion. In this review, we focus on BCR-ABL–negative MPNs and highlight 5 main features of MPN stem cells: (1) MPN disease-initiating somatic mutations, (2) the effects of MPN somatic mutations on hematopoietic stem cell (HSC) function, (3) the role of MPN stem cells in the heterogeneity of disease phenotype in MPNs, (4) the MPN stem cell niche, and (5) therapeutic targeting of MPN stem cells.
MPN disease-initiating somatic mutations
The molecular basis of MPNs has been defined in almost all cases. In >95% of cases of MPNs, the mutations that drive the development of an MPN phenotype are accounted for by somatic mutations in 3 genes: JAK2, CALR, or MPL, and notably these mutations occur in a mutually exclusive manner.1 Mutations in JAK2 and MPL occur as gain-of-function point mutations (ie, JAK2V617F and MPLW515L/K, respectively), whereas the mutations in CALR occur as +1 base-pair frameshifts in the last coding exon of CALR, which result in the generation of a novel C-terminus.1,2 Recent work indicates that CALR mutations confer a neomorphic function on mutant CALR that results in activation of MPL signaling.3-6 Additional germ line and somatic mutations in JAK2 or MPL were recently identified in ∼19% of the so-called “triple-negative” MPN cases.7 Previously, negative regulators of the JAK-STAT signaling pathway, including LNK,8 c-CBL,9,10 and SOCS,11 were also shown to be somatically inactivated at low frequency in MPNs, highlighting the primacy of the JAK-STAT signaling pathway in MPN pathogenesis.
Evidence for an MPN disease-initiating role for JAK2, CALR, and MPL somatic mutations has been provided by retroviral bone marrow transplant assays, where ectopic expression of each mutation alone is sufficient to engender MPNs in mice5,6,12 ; however, it is important to note that the MPN is polyclonal in all these models. Recent work using a transgenic mouse model where human JAK2V617F is expressed from the endogenous human JAK2 promoter demonstrated that although MPN can be initiated by transplanting a single JAK2V617F-expressing long-term (LT)-HSC into a lethally irradiated wild-type recipient mouse, MPNs developed in only a minority of recipients in whom long-term reconstitution occurred,13 supporting the concept that JAK2V617F is not always disease initiating when modeled at the level of a single LT-HSC. The question of whether JAK2V617F is disease initiating in MPNs was also recently raised in the context of human hematopoiesis following a series of studies in which JAK2V617F mutations were detected in the peripheral blood of normal individuals who do not have any apparent hematological disease.14-16 This phenomenon, where clonally restricted somatic mutations in genes associated with hematological malignancies (including JAK2V617F) are found in normal individuals, has been termed clonal hematopoiesis of indeterminate potential (CHIP) and is strongly associated with increasing age.17 JAK2V617F is among the most common CHIP-associated mutations and in most cases the JAK2 mutations are isolated events that occur in the absence of other hematological malignancy-associated mutations, suggesting that JAK2V617F alone is sufficient to engender clonal hematopoiesis. However, intriguingly, the prevalence of JAK2V617F-positive MPNs is significantly lower than that of JAK2V617F-positive CHIP, suggesting that in many cases JAK2V617F alone may be sufficient to engender clonal hematopoiesis but insufficient to induce MPNs. This discrepancy may be explained in part by the observation from the Copenhagen General Population Study that higher JAK2V617F allele burden is associated with development of clinical MPNs, and that a minimum threshold JAK2V617F allele burden appears to be required for the development of overt disease.18 However, because the presence of concomitant somatic mutations was not assessed in the Copenhagen study, definitive conclusions regarding the sufficiency of JAK2V617F alone to cause MPNs cannot be made.
It is also important to note that other somatic genetic alterations, for example, TET2 loss-of-function mutations, can precede the acquisition of JAK2V617F as reported in the original description of TET2 mutations in myeloid malignancies19 and later validated in subsequent studies.20,21 Indeed, TET2 is also a common CHIP gene, as are other epigenetic genes, such as DNMT3A.14-16 Somatically mutated, clonally expanded HSC in which additional somatic mutations subsequently occur to drive the development of a disease phenotype have been termed “preleukemic” HSC, and their role in the development of myeloid malignancies has now been clearly established, validating the original description of TET2 mutations preceding JAK2V617F acquisition in MPNs.19 It has also been shown that TET2 mutations can follow JAK2V617F, and the order in which these mutations are acquired can impact both the age of onset and clinical features of MPNs.21
The acquisition of TET2 mutations in established MPNs has also been associated with leukemic transformation, as have mutations in other epigenetic genes such as ASXL1, in splicing genes such as SRSF2, and in additional genes including IDH1/2, TP53, NRAS, and RUNX1.22,23 Interestingly, acute myeloid leukemia (AML) that arises out of JAK2V617F-mutant MPNs retains the JAK2V617F allele only ∼50% of the time,24,25 suggesting that the acquisition of additional somatic mutations in MPN stem cells that harbor a phenotypic driver mutation (ie, JAK2, CALR, or MPL) is not the only route to AML. Leukemic transformation that emerges from a pre-MPN clone (lacking an MPN phenotypic driver mutation) may be promoted by inflammatory cytokines secreted by the MPN clone, particularly in the context of MF (see “The MPN stem cell niche”).
What is the impact of MPN disease-initiating somatic mutations on the cell of origin in MPNs?
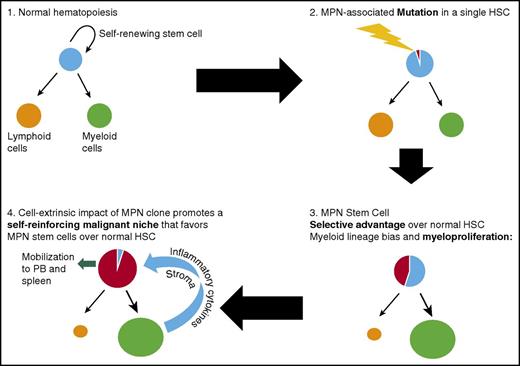
A number of lines of evidence support the concept that the original target cell that acquires an MPN-initiating mutation is an LT-HSC that resides at the apex of the hematopoietic hierarchy (Figure 1). In MPN patients, the JAK2V617F mutation is detectable in immunophenotypically defined (CD34+ CD38−) LT HSC26 and in all mature cell lineages,27,28 indicating that the mutation is acquired by a multipotent cell. By genotyping hematopoietic colonies from MPN patients, CALR mutations were also demonstrated to be present in the earliest phylogenetic node.1 Additional evidence from genetic mouse models further support the idea that Jak2V617F disease-initiating cells reside exclusively in the immunophenotypically defined LT-HSC compartment, and that Jak2V617F multipotent progenitors lack disease-propagating potential.29,30 Following the acquisition of an MPN-initiating mutation by a single HSC, the development of an overt MPN phenotype requires clonal expansion, conferred through a selective advantage of MPN stem cells over their normal counterparts (Figure 2). In contrast to acute leukemia, where myeloid progenitor cells can acquire aberrant self-renewal,31,32 disease initiation and propagation in MPNs can only be sustained by cells residing within the immunophenotypically defined LT-HSC compartment.30,33
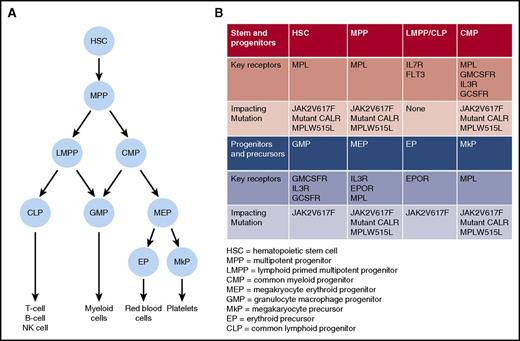
Key somatic mutations and growth factor receptors important for MPN development. (A) Simplified “roadmap” of hematopoietic development. (B) Distribution of key growth factor receptors in different stem, progenitor, and precursor cell populations. For each population, potential impact of JAK2V617F, CALR, or MPL mutation is indicated.
Key somatic mutations and growth factor receptors important for MPN development. (A) Simplified “roadmap” of hematopoietic development. (B) Distribution of key growth factor receptors in different stem, progenitor, and precursor cell populations. For each population, potential impact of JAK2V617F, CALR, or MPL mutation is indicated.
Key steps during MPN development from normal hematopoiesis following acquisition of an MPN-initiating mutation in a single HSC. The mutant HSC acquires a selective advantage over normal HSC and also promotes myeloid differentiation, eventually leading to a myeloproliferative phenotype. The expanded, abnormal myeloid clone disrupts the bone marrow microenvironment, promoting a self-reinforcing malignant niche that favors MPN stem cells over normal HSC and leads to eventual mobilization of MPN HSC into the peripheral blood (PB).
Key steps during MPN development from normal hematopoiesis following acquisition of an MPN-initiating mutation in a single HSC. The mutant HSC acquires a selective advantage over normal HSC and also promotes myeloid differentiation, eventually leading to a myeloproliferative phenotype. The expanded, abnormal myeloid clone disrupts the bone marrow microenvironment, promoting a self-reinforcing malignant niche that favors MPN stem cells over normal HSC and leads to eventual mobilization of MPN HSC into the peripheral blood (PB).
The impact of MPN phenotypic driver mutations on the expansion of the HSC pool can be inferred from the somatic mutant allele burden in granulocytes at diagnosis. In this regard, it is informative to compare the JAK2V617F mutant allele burden in chronic phase MPNs (ie, polycythemia vera [PV] and essential thrombocythemia [ET]) with that of more advanced phase disease MPNs (ie, myelofibrosis [MF]). In PV, the JAK2V617F-mutant allele burden is often low at diagnosis,34 and in ET, JAK2V617F heterozygous clones can remain stable over years,35 with recent evidence suggesting that homozygous JAK2V617F clones do not necessarily expand.36 Concordant with this observation, in a study of PV, ET, and MF patients, expansion of the CD34+ CD38− HSC compartment was not present in PV and ET patients but was present in MF patients.37 A separate study showed that JAK2V617F allele burden was higher in the CD34+ cell compartment of patients with MF as compared with PV and ET.38 Interestingly, this expansion of JAK2-mutant cells in the CD34+ compartment of patients with MF was found to be independent of JAK2V617F homozygosity, suggesting that clonal expansion in the CD34+ compartment in MF is driven by other somatic genetic alterations enriched in MF as compared with PV or ET (for example, epigenetic loss-of-function mutations). In contrast, the mutant CALR allele fraction measured in granulocytes is typically 40% to 50% at the time of ET diagnosis, suggesting that heterozygous mutant CALR HSC become clonally dominant quickly.39 Consistent with this observation, the age of onset of MPNs is younger in CALR-mutant patients as compared with JAK2-mutant patients.39,40
The impact of MPN-initiating mutations on HSC function has been studied in vivo using both genetic mouse models and patient-derived xenograft models. JAK2V617F in particular has been the subject of intensive study, and Jak2V617F genetic mouse models have been reviewed in detail elsewhere.12,41 Although these models have demonstrated that JAK2V617F alone is sufficient to engender MPNs and have shown that oncogenic JAK2V617F signaling does not confer self-renewal capacity upon non–self-renewing hematopoietic cells, the impact of JAK2V617F on HSC self-renewal (as assayed by competitive repopulation) has been highly variable, depending on the genetic targeting approach taken to generate the particular mouse model. Recently, using a transgenic JAK2V617F mouse model, the level of JAK2V617F expression in single LT-HSC was shown to influence reconstitution capacity, with high JAK2V617F-expressing single LT-HSC demonstrating an exhaustion phenotype in a serial transplantation assay, as compared with low JAK2V617F-expressing single LT-HSC, which retained their long-term repopulation capacity.13 Both Tet2 and Ezh2 loss have been shown to enhance the repopulating activity of Jak2V617F disease-propagating MPN stem cells in genetic mouse models42-46 ; in the case of Ezh2 loss, the enhanced repopulation was mediated through reactivation of a fetal lin28b program.45
MPN studies in xenografts have found that JAK2V617F-mutant CD34+ cells from patients with PV and ET engraft relatively poorly,47 whereas CD34+ cells from either the peripheral blood or the spleens of patients with MF demonstrate sustained engraftment,48,49 and in the case of splenic CD34+ cells, serial transplantation into secondary recipients was achieved.49 The fact that JAK2V617F severe combined immunodeficiency (SCID) repopulating cells (SRC) do not gain a proliferative advantage over wild-type SRC over time in CD122-depleted NOD/SCID mice50 suggests that JAK2V617F is not a strong driver of clonal expansion at the level of the HSC. However, 1 important caveat in the interpretation of these results is that incompatibilities between human cytokine receptors (expressed on transplanted CD34+ cells) and murine cytokines (produced by the recipient murine bone marrow) may significantly impact JAK2V617F SRC activity in these studies. Comutation of TET2 with JAK2V617F has been shown to increase the NOD-SCID repopulating capacity of CD34+ cells, when compared with CD34+ cells harboring JAK2V617F alone.19 In an effort to overcome issues with species incompatibility in xenografts, a humanized bone marrow ossicle transplantation model was recently employed to study MPN stem cells.33 In this study, the CD34+ compartment was fractionated into CD34+CD38+ and CD34+CD38lo/− subfractions, and engraftment was observed exclusively in ossicles transplanted with CD34+CD38lo/− cells, confirming that the MPN-initiating population is contained solely in the immunophenotypically defined LT-HSC fraction.33
Finally, the impact of cytokine receptor signaling in MPN stem cells has been assessed using various mouse models. In the HSC compartment, the thrombopoietin receptor, Mpl, is a key growth factor receptor that is required for normal HSC function,51 and importantly, all MPN-initiating mutations activate MPL signaling (Figure 1). Using genetic mouse models, a critical role for Mpl in Jak2V617F-driven MPN initiation has been demonstrated.52 A similar requirement for Mpl in mutant CALR-driven MPNs initiation was recently demonstrated using a retroviral bone marrow transplant model.6 Conversely, IL3Rb signaling has been shown to be expendable for MPN initiation,53 and in the case of erythropoietin signaling, restricting Jak2V617F expression to erythropoietin receptor (EpoR) expressing erythroid precursor cells has been found to result in a markedly attenuated MPN phenotype, supporting the absence of a role for the EpoR signaling in Jak2V617F-driven MPN initiation.30 Although these data firmly establish MPL as the crucial growth factor receptor for MPN development, we still have an incomplete understanding of the molecular events downstream of activated MPL signaling that allow MPN stem cells to gain a clonal advantage in the bone marrow.
How do MPN stem cells contribute to heterogeneity of disease phenotype in MPNs?
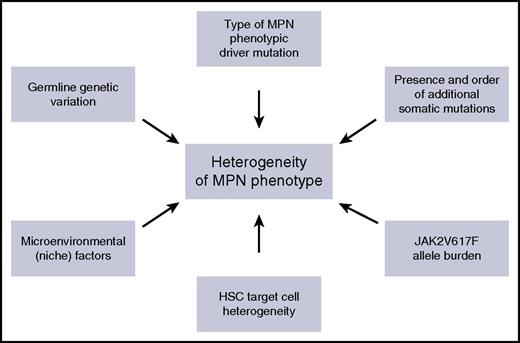
Although MPNs have many clinical and biological features in common, including activation of JAK-STAT signaling, they also exhibit a range of distinct clinical phenotypes. Understanding the factors that influence the impact of an MPN disease-initiating mutation on the HSC that acquires it, and on its clonal progeny, is of fundamental importance in understanding heterogeneity of disease phenotype in MPNs, and these factors are summarized in Figure 3. First, it is clear that MPN phenotype is partly determined by the specific phenotypic driver mutation that is acquired. For example, CALR and MPL mutations are almost always associated with an ET or MF phenotype, but not a PV phenotype. The association of distinct MPN phenotypes with CALR or MPL mutations partly relates to the mechanism by which these mutations induce MPNs, namely through activating signaling through MPL,3-6,54 which is selectively expressed on HSC and cells in the megakaryocyte differentiation pathway (Figure 1). Activation of this pathway consequently promotes megakaryopoiesis and a thrombocytosis phenotype. Specific signaling events downstream of a driver mutation can also influence MPN phenotype, through selectively promoting specific lineage differentiation, for example, JAK2 exon 12 mutations, which activate JAK2 signaling are associated with an isolated erythrocytosis phenotype both in MPN patients55 and in mouse models.56 Whether this lineage specificity occurs as a result of instructive signaling in multipotent progenitors, for example, by differentially enhancing erythropoietin signaling,57 or as a consequence of selective signaling in committed erythroid precursor cells, remains unclear.58
Summary of the factors that influence phenotypic heterogeneity in MPNs.
In contrast to the specific clinical phenotypes associated with CALR, MPL, and JAK2 exon 12 mutations, an intriguing aspect of MPN biology relates to the striking heterogeneity in clinical phenotype associated with the more common JAK2V617F mutation, which is associated with PV, ET, and MF phenotypes in humans and also in mouse models.12,41 An unresolved question is why a JAK2V617F mutation acquired by a single HSC can result in such a variety of clinical phenotypes. Furthermore, recent studies identifying the presence of CHIP in normal individuals14-16 indicate that many individuals who acquire a JAK2V617F mutation in an HSC will never develop MPN. A number of possible explanations have been proposed for JAK2V617F-associated clinical heterogeneity. First, evidence from MPN patients and mouse models indicates that phenotypic diversity in JAK2V617F-driven MPNs relates to both quantitative differences in JAK2V617F allele burden36,59 and qualitative differences in signaling downstream of JAK2V617F, including levels of STAT1 activation.60 Second, other modifier genes, through effects either on HSC or on the bone marrow microenvironment, could influence disease phenotype, for example, constitutional differences in MYB expression.61 It is interesting to note, however, that the same germ-line predisposition loci are associated with both JAK2V617F-positive CHIP and JAK2V617F-positive MPNs.62 Third, the presence of other somatic mutations, for example, in TET2, and the order in which the mutations are acquired in HSC, have been shown to influence the resulting disease phenotype.21 Fourth, cell-extrinsic environmental factors could influence eventual MPN phenotype. Finally, phenotypic heterogeneity could relate, at least in part, to the specific cell of origin of MPNs.
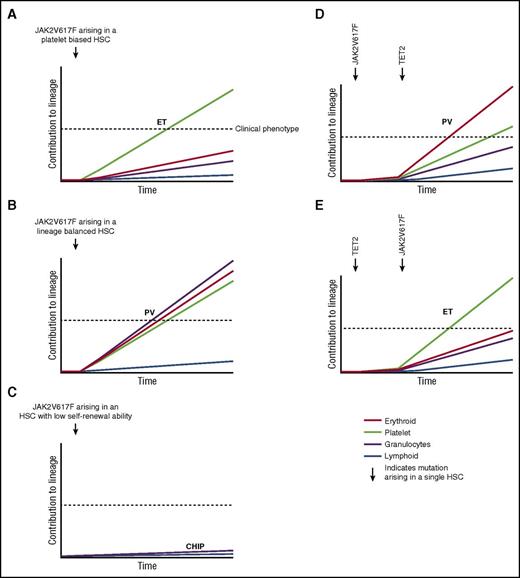
A number of lines of evidence suggest that HSC are highly heterogeneous in their self-renewal capacity and their intrinsic lineage bias.63 For example, platelet-biased HSC that reside at the apex of the hematopoietic hierarchy have been characterized.64 Furthermore, single-cell transplantation studies in mice have identified myeloid lineage-restricted, self-renewing progenitors,65 and a similar cell population has also been described in the context of native non–transplant hematopoiesis.66 Thus, characteristics of the specific individual HSC cell of origin that acquires a JAK2V617F mutation could also contribute to the phenotypic heterogeneity of JAKV617F-positive MPNs and may provide an explanation, at least in part, for the development of JAKV617F-positive CHIP without progression to MPNs. With respect to phenotypic heterogeneity, a JAK2V617F mutation arising in a platelet-biased HSC might result in an ET phenotype (Figure 4A), whereas the same mutation arising in a myeloid lineage-balanced HSC might promote a PV phenotype (Figure 4B). A recent study using single-cell transplantation approaches to analyze a JAK2V617F transgenic mouse model provides some evidence in support of this hypothesis.13 Single-stem/progenitor cells from genetically identical donor mice were found to be capable of generating MPNs in vivo with markedly variable phenotypes. There is also evidence to indicate that some HSC possess more limited self-renewal capability than others,63 and it may be that a JAK2V617F mutation arising in such a cell could result in CHIP (Figure 4C), with a clinical phenotype emerging only upon acquisition of a second mutation, for example in TET2, that enhances the self-renewal of the antecedent JAK2V617F-mutant clone42 (Figure 4D). Acquisition of a JAK2V617F mutation in an HSC already harboring a TET2 mutation could influence the clinical phenotype,21,67 in addition to the age of MPN onset21 (Figure 4E). Given the increased prevalence of CHIP in older individuals, it is interesting to note that aging has significant effects on HSC heterogeneity, including an increase in myeloid-biased differentiation, a decreased output of differentiated cells, and a reduced capacity for self-renewal in long-term secondary transplantation assays.68,69
Disease heterogeneity following acquisition of the JAK2V617F mutation in a single HSC. X-axis represents time following acquisition of JAK2V617F indicated by the arrow. Y-axis represents relative contribution from this HSC clone to each lineage, indicated by color. (A) JAK2V617F occurs in a platelet-biased HSC resulting in ET. (B) JAK2V617F occurs in a lineage-balanced HSC resulting in PV with trilineage myeloproliferation. (C) JAK2V617F occurs in an HSC with limited self-renewal capability resulting in CHIP. (D) JAK2V617F precedes acquisition of a TET2 mutation resulting in a PV phenotype. (E) TET2 precedes acquisition of a JAK2V617F mutation resulting in an ET phenotype. (A-C) represent hypotheses to explain how HSC heterogeneity may influence MPN phenotype. (D-E) represent an interpretation of published data demonstrating that the order in which JAK2V617F and TET2 mutations are acquired influences MPN phenotype.
Disease heterogeneity following acquisition of the JAK2V617F mutation in a single HSC. X-axis represents time following acquisition of JAK2V617F indicated by the arrow. Y-axis represents relative contribution from this HSC clone to each lineage, indicated by color. (A) JAK2V617F occurs in a platelet-biased HSC resulting in ET. (B) JAK2V617F occurs in a lineage-balanced HSC resulting in PV with trilineage myeloproliferation. (C) JAK2V617F occurs in an HSC with limited self-renewal capability resulting in CHIP. (D) JAK2V617F precedes acquisition of a TET2 mutation resulting in a PV phenotype. (E) TET2 precedes acquisition of a JAK2V617F mutation resulting in an ET phenotype. (A-C) represent hypotheses to explain how HSC heterogeneity may influence MPN phenotype. (D-E) represent an interpretation of published data demonstrating that the order in which JAK2V617F and TET2 mutations are acquired influences MPN phenotype.
The MPN stem cell niche
The bone marrow niche provides the microenvironmental signals that are essential for HSC function,70 and progress in understanding the cellular composition of the HSC niche has facilitated investigation of how these different components are perturbed during the development of myeloid neoplasms.71 Indeed, a number of lines of evidence indicate that targeted disruption of the bone marrow niche alone is sufficient to induce an MPN phenotype in vivo.72-75
Although the initiating MPN mutation acquired by a single HSC is likely to occur in the context of an unperturbed niche, as the MPN progresses, the expanding malignant clone exerts cell-extrinsic effects on components of the bone marrow niche, and evidence from mouse models suggests that this niche disruption can favor the survival and expansion of MPN stem cells over normal HSC, resulting in a so-called malignant “self-reinforcing” niche.76 For example, JAK2V617F-positive HSC have been shown to induce bone marrow neural damage through aberrant production of interleukin-1b, which in turn leads to a reduction in mesenchymal stem cell numbers and acceleration of MPN progression,77 culminating in the development of a clinical trial to test the efficacy of β3-adrenergic agonists in MPNs. Indeed, more advanced MPNs are characterized by the development of bone marrow fibrosis that is promoted by proinflammatory cytokines, including transforming growth factor β and tumor necrosis factor α (TNFα), which are produced by abnormal populations of myelomonocytic cells and megakaryocytes.76,78 Both transforming growth factor β and TNFα are also known negative regulators of normal HSC,79,80 and in a retroviral JAK2V617F model, TNFα was demonstrated to facilitate the preferential expansion of JAK2V617F-mutant hematopoietic cells.81 The bone marrow niche is also perturbed in MPNs as a result of enhanced angiogenesis as evidenced by increased bone marrow microvessel density in MPN patients,82 possibly occurring as a consequence of increased vascular endothelial growth factor expression.83 The disrupted niche can also lead to mobilization of HSC into peripheral blood and spleen, resulting in splenic extramedullary hematopoiesis, which is a cardinal feature of more advanced MPNs.49 Furthermore, the microenvironmental disruption of the bone marrow in MPNs might also promote leukemic transformation as demonstrated by an MF-xenograft study in which a high frequency of AML of mouse origin was noted. In this study, murine AML development was confined to the MF-xenograft group (and was not seen in xenografts of normal bone marrow or cord blood cells), suggesting that paracrine signaling arising from the MF cells drove leukemogenesis in normal mouse cells in vivo.84 Recent work has highlighted lipocalin as an important paracrine factor in promoting DNA damage and disease evolution in MPNs.85,86
Finally, a number of pathways mediating bone marrow fibrosis, inflammatory signals, and niche dysregulation are potentially amenable to therapeutic targeting. New approaches to modeling niche disruption such as those using humanized bone marrow ossicles in xenograft assays will be helpful in further exploring the underlying mechanisms of niche disruption in MPNs and in testing novel therapeutic interventions.33
Therapeutic targeting of MPN stem cells
The finding that MPNs originate from and are propagated by rare self-renewing stem cells that closely resemble normal LT-HSC phenotypically represents both a challenge and a therapeutic opportunity. The opportunity lies in the fact that effectively targeting MPN disease-propagating stem cells is not only required but is also likely to be sufficient to eradicate the disease and achieve a cure. However, in myeloid malignancies, stem cell populations have been demonstrated to be selectively resistant to therapies that are able to induce cytogenetic remissions87 and even the most successful targeted therapies in chronic myelogenous leukemia (CML) fail to eradicate leukemia stem cells in the majority of patients.88 This selective therapeutic resistance of leukemia stem cell populations is, at least in part, related to their marked quiescence in comparison with progenitor populations.89,90 Nevertheless, as somatic mutations confer a competitive advantage to MPN stem cells, the pathways that mediate this selective advantage should, in principle, be amenable to therapeutic targeting.
The only current curative therapy for MPNs, and therefore the only treatment that is able to eradicate MPN stem cells, is allogeneic HSC transplant. However, even following allogeneic transplant, relapse is unfortunately not an infrequent occurrence,91 indicating that the MPN stem cell populations can be resistant even to high-dose conditioning chemotherapy and alloimmunity-mediated clearance. Furthermore, due to the toxicity associated with the transplantation procedure, this approach is currently restricted to a minority of younger patients with advanced MPNs.92 Recently, CRISPR/Cas9 gene editing has emerged as a promising new approach with the potential to treat human disease, and although an enormous amount of work will be required to demonstrate the safety and feasibility of gene-editing as a therapeutic approach in patients, the recent demonstration that the causative mutation for sickle cell disease could be repaired in HSC from patients with sickle cell disease93 is an important advance. However, whether such a strategy could be used to treat clonal disorders such as MPNs by repairing JAK2V617F or MPLW515L/K mutations in HSC is uncertain at this point. Clearly, the challenges of applying this approach to a clonal disorder are considerably greater, as disease eradication cannot be achieved unless all MPN stem cells are successfully repaired.
The clinical development of JAK2 inhibitor therapies heralded a new era of molecular targeted therapy for BCR-ABL–negative MPNs. Ruxolitinib, the first clinically approved JAK2 inhibitor, has considerable efficacy in patients with PV and MF, with improvements in blood counts, spleen size, and symptoms. Despite this success, JAK2 inhibitors have been disappointing in their ability to induce molecular remissions in MPN patients, indicating that JAK2 inhibitors do not preferentially target MPN cells over normal cells, as evidenced by a lack of significant reduction in the JAK2V617F allele burden in patients undergoing treatment. Recently, the 4-year data from the COMFORT-I study, a phase 3 trial in MF, was reported. Only 12% of patients showed a >50% decrease in JAK2V617F allele burden, and <2% of patients achieved complete molecular remission.94 A retrospective analysis of CALR-mutant patients treated with ruxolitinib on the COMFORT-II study (another phase 3 trial in MF) was recently reported.95 Although CALR-mutant patients demonstrated comparable clinical responses to JAK2-mutant patients, there was no significant change in the CALR-mutant allele burden after a median of 60 weeks of treatment with ruxolitinib (n = 18). Underlying the failure of JAK2 inhibitors to induce molecular remission in BCR-ABL–negative MPNs is the lack of efficacy of these agents to target MPN stem cells, which is supported by evidence from both mouse models29 and MF patient samples.96 Encouragingly, complete inhibition of JAK2 in a retroviral MPLW515L MPN mouse model using a genetic knockout approach resulted in a superior therapeutic response than treatment with a JAK2 inhibitor.97 A new generation of type II JAK2 inhibitors are able to induce more effective JAK2 inhibition,98 and if developed for clinical use, such agents may be more successful at targeting MPN stem cells. However, JAK2 signaling is also essential for normal HSC function, as evidenced by several studies that have shown that hematopoietic-specific conditional genetic deletion of Jak2 in adult mice results in severe cell-intrinsic defects in HSC function, impaired hematopoiesis, and reduced survival.99-101 Although total loss of JAK2 protein as occurred in the case of genetic knockout mouse models may be different than more potent JAK2 kinase inhibition, these murine studies have raised concerns regarding the potential for on-target hematological toxicity from more potent JAK2 inhibitors. Recent work focused on the crystal structure and biochemical properties of the pseudokinase domain of JAK2, which is the domain in which the V617F mutation is found, may help to overcome this issue by advancing the development of JAK2V617F-mutant specific inhibitors.102,103
Interferon has been used in the treatment of MPNs for decades,104 and more recently, it has been recognized that a minority of interferon-treated patients achieve molecular remissions with interferon treatment, with reemergence of polyclonal hematopoiesis in some.105,106 Interferon treatment is not a “molecularly targeted” therapy, and its ability to induce molecular responses applies across a range of different hematological malignancies,107 including patients with CML and MPN patients with JAK2V617F105,106 and CALR mutations.108 Although acknowledging that interferon treatment likely has multiple effects in MPN patients, potentially including immunological effects and an impact on the bone marrow microenvironment, 1 possible mechanism by which it achieves molecular remissions in MPNs may relate to its ability to stimulate normally quiescent stem cell populations into cycle. As this cycling is more marked in JAK2V617F-positive HSC, interferon leads to preferential depletion of MPN stem cells in Jak2V617F mouse models,109,110 a finding that has also been corroborated in primary MPN samples.111 Long-term molecular remissions after discontinuation of interferon therapy have been reported, suggesting that JAK2V617F-mutant HSC can be eradicated by interferon.112 However, molecular relapses after cessation of interferon have also been observed,113 supporting the concept that in some patients, MPN stem cells persist and are able to mediate relapse. It should also be remembered that conventional chemotherapy agents such as busulfan have also been shown to induce molecular remissions in a small number of patients with PV,114 suggesting that the potential of conventional treatments to eradicate MPN stem cells in rare patients may not be restricted to interferon.
A number of agents in clinical development are showing promise with regards to their ability to target MPN stem cells. For example, in vitro treatment of JAK2V617F-positive CD34+ cells with MDM2 inhibitors (alone or in combination with interferon) has been found to reduce the degree of donor-derived chimerism and the JAK2V617F allele burden following injection of the treated CD34+ cells into xenografts, suggesting that the treatment targets MPN stem cells.115 Preclinical genetic mouse models have also identified selective sensitivity of MPN progenitors to estrogen-induced apoptosis,116 prompting initiation of a clinical trial (ISRCTN65011803) to test the efficacy of tamoxifen in MPNs, with a primary endpoint focused on molecular response. As research studies continue to advance the understanding of the mechanisms by which MPN stem cells become clonally dominant, the potential to exploit the biological insights gained in order to selectively target MPN stem cells in patients will become more feasible.
Future directions
Although tremendous progress has been made in the field of MPNs, particularly with regard to our understanding of the genetic basis of MPNs and to the development of molecularly targeted therapies with JAK2 inhibitors, significant gaps remain in our understanding of the mechanisms by which MPN stem cells become clonally dominant in the bone marrow. Furthermore, recent studies on CHIP have highlighted additional deficits in our understanding of the factors that constrain and promote the transition from JAK2V617F-driven clonal hematopoiesis to JAK2V617F-induced MPNs. This incomplete understanding of MPN stem cell biology underlies, at least in the part, the failure of current therapies to effectively target MPN stem cells, an essential step toward achieving cure. Progress in this area is further impeded by the failure of most clinical trials to incorporate appropriate biological sample banking that would allow future analysis of disease stem cells. Incorporation into clinical studies of biological secondary endpoints that focus on analysis of the stem cell compartment, such as assessment of molecular response in HSC, will provide an invaluable resource for future studies. Ultimately, effective eradication of MPN stem cells may require a multitargeted approach, tackling both the cell-intrinsic and the cell-extrinsic mechanisms that support MPN stem cell propagation.
Acknowledgment
The authors thank Julie-Aurore Losman for critically reviewing the manuscript.
Authorship
Contribution: A.J.M. and A.M. coauthored this review article.
Conflict-of-interest disclosure: A.J.M. declares receiving research funding, speaker funding, and advisor fees from Novartis. A.M. declares no competing financial interests.
Correspondence: Ann Mullally, Brigham and Women's Hospital, Harvard Medical School, 1 Blackfan Circle, Karp Building, Room 5.217, Boston, MA 02115; e-mail: amullally@partners.org; and Adam J. Mead, MRC Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Headington, Oxford OX3 9DS, United Kingdom; e-mail: adam.mead@imm.ox.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal