Abstract
Introduction: Imatinib mesylate is standard therapy for patients (pts) with chronic myeloid leukemia (CML). In February 2016 a generic formulation was introduced in the US and many patients have migrated to this formulation at the request of their insurance provider. The use of generic drugs can reduce the financial burden on patients. However, since the approval of generic drugs does not generally require comparative studies, physicians and pts are frequently concerned about whether switching from innovator to generic drugs may affect efficacy and/or safety. To address this concern, we analyzed the outcome of pts switched from innovator to generic imatinib for changes in efficacy or adverse event profile.
Methods: We reviewed the records of pts diagnosed with CML in chronic phase who were treated with innovator imatinib either in frontline or after interferon from year 2000 to 2017 and were subsequently switched to generic imatinib. After switch we increased the frequency of blood counts and chemistry to every 1-2 months, performed PCR after 3 months and reviewed changes or new adverse events with pts.
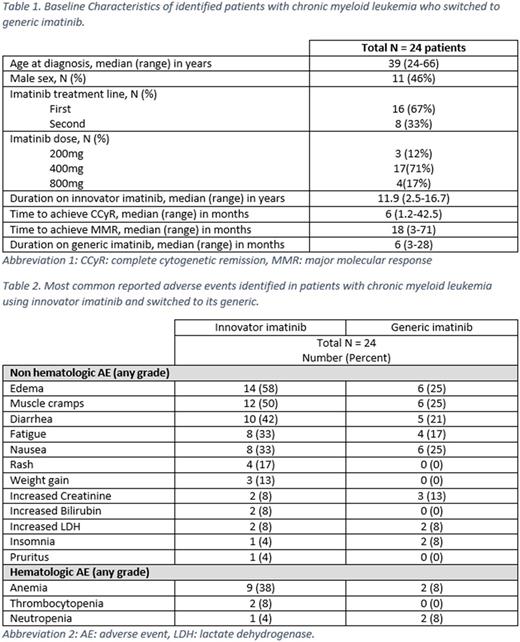
Results: A total of 24 patients have switched to generic imatinib. Baseline characteristics are summarized in the table 1 below. Most (71%) were receiving imatinib at a dose of 400 mg; in no instance was the dose changed at the time of switching. Before the switch, all patients were in complete cytogenetic remission (CCyR) and major molecular response (MMR), including 15 (63%) with MR4.5. The median duration of innovator imatinib use was 11.9 (2.5-16.7) years. Pts have received generic imatinib for a median of 6 (3-28) months. All pts have maintained the same level of cytogenetic and molecular response they had before the switch. Of all patients, 21 (88%) experienced at least one adverse event while on innovator imatinib, mostly edema in 14 (58%) patients, muscle cramps in 12 (50%), diarrhea in 10 (42%), and nausea and fatigue in 8 (33%) each. Anemia was seen in 9 patients (38%), neutropenia in 1 (4%), thrombocytopenia in 2 (8%), increased creatinine in 2 (8%), and increased bilirubin in 2 (8%) pts. None of the adverse events were grade 3-4. After switching to generic imatinib, 11 (46%) pts reported new or worsening adverse events including 6 (25%) pts with grade 1 edema, 6 (25%) muscle cramps, 6 (25%) nausea, 5 (21%) diarrhea, and 4 (17%) fatigue. New grade 1 anemia occurred in 2 (8%) pts, and a grade 1 transient increase in creatinine levels in 3 (13%) pts (from 1.12 to 1.43, 1.1 to 1.32 and 0.63 to 1.37, respectively). One pt had the dose of generic imatinib reduced from 400 mg to 300 mg due to fatigue and diarrhea after 2 month of switching. One patient skipped 4 doses because of worsening periorbital edema, then resumed without problems. Four patients discontinued treatment with generic imatinib: one had elective discontinuation because of sustained MR4.5, two pts changed back to innovator imatinib because of diarrhea, and 1 pt switched to dasatinib because of grade 1 creatinine elevation; after the switch creatinine level went down from 1.43 to 1.1. All patients are alive and none has progressed.
Conclusion: A change to generic imatinib for pts who have been receiving innovator imatinib appears to maintain the response. Although few new adverse events are reported, the change appears to be generally safe. More patients and longer follow-up is required to confirm these observations.
Kantarjian: ARIAD: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Bristol-Meyers Squibb: Research Funding; Amgen: Research Funding; Delta-Fly Pharma: Research Funding. Burger: Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Novartis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding. Jabbour: Bristol-Myers Squibb: Consultancy. Verstovsek: Gilead: Research Funding; CTI BioPharma Corp: Research Funding; Genentech: Research Funding; Celgene: Research Funding; Pfizer: Research Funding; Genentech: Research Funding; Galena BioPharma: Research Funding; Bristol Myers Squibb: Research Funding; Celgene: Research Funding; NS Pharma: Research Funding; Promedior: Research Funding; Roche: Research Funding; Incyte: Research Funding; Seattle Genetics: Research Funding; Pfizer: Research Funding; Astrazeneca: Research Funding; NS Pharma: Research Funding; Blueprint Medicines Corp: Research Funding; Blueprint Medicines Corp: Research Funding; CTI BioPharma Corp: Research Funding; Bristol Myers Squibb: Research Funding; Promedior: Research Funding; Lilly Oncology: Research Funding; Gilead: Research Funding; Astrazeneca: Research Funding; Roche: Research Funding; Seattle Genetics: Research Funding; Lilly Oncology: Research Funding; Galena BioPharma: Research Funding; Incyte: Research Funding. Cortes: Pfizer: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Sun Pharma: Research Funding; ARIAD: Consultancy, Research Funding; Teva: Research Funding; BMS: Consultancy, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal