Background: FLT3-mutated (mu) AML pts have poor outcomes. IDH1mutated (IDH1m) and IDH2m co-occur with FLT3 mutations in 15-27% and 8-30% of AML, respectively (Boddu P, et al. Leukemia 2017; 31:2526-2529). FLT3-ITD is a strong risk factor for relapse and since multiple FLT3 inhibitors (FLT3i's) are available, it is important to further determine how the efficacy of these targeted is impacted by concurrent mutation/s.
Methods: We reviewed FLT3-mu AML pts with concurrent IDHm's between Jan 2011-Dec 2018 who had received at least one first or second generation FLT3-inhibitor (FLT3i)-based therapy in frontline and/or relapsed/refractory (R/R) setting. We are not discussing IDHi based therapies in double mutant pts this abstract. Mutation testing was performed using a NGS-based analysis for the detection of somatic mutations in the coding sequences of 28 or 81 genes on DNA extracted from BMA, as previously described (33). We analyzed the characteristics of these pts, responses to therapy, and outcomes.
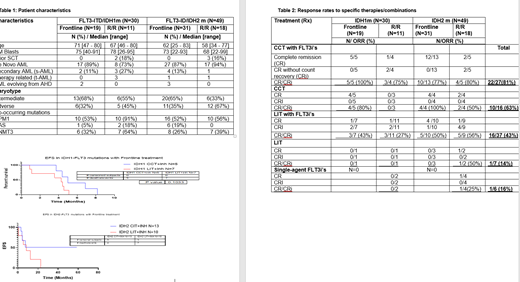
Results: Eighty two FLT3, IDH 'double mutated' AML pts were identified. One pt had FLT3-TKD mu and IDH1m, and 2 pts had FLT3-TKD mu and IDH2m and were excluded. We focus here on the 79 pts with concurrent FLT3-ITD mu and IDH1m or IDH2m. 30 pts had concurrent FLT3/IDH1m: 19 (63%) in frontline and 11 (37%) in the R/R setting. 49 had concurrent FLT3/IDH2m: 31 (63%) in frontline and 18 (37%) in the R/R setting. The median follow-up was 8.3 months [1.2 - 115] and 10.5 months [0.3 - 57.8] months for pts with concurrent FLT3/IDH1m and FLT3/IDH2m, respectively. The median number of prior therapies was 2 [1 - 8] in both R/R settings. The most frequent mutations were IR132H (77%) and IR140Q (98%) in pts with FLT3/IDH1m and FLT3/IDH2m, respectively. Patient characteristics are shown in Table 1. As expected, the incidence of adverse cytogenetics in FLT3/IDH mutated pts was low (7/79; 9%). The most common co-occurring mutations in FLT3/IDH1 pts were NPM1 (66%) and DNMT3A (43%). Similarly in FLT3/IDH2: NPM1 (53%) and DNMT3A (31%).
FLT3i's were given as a single agent or in combination with cytotoxic chemotherapy (CCT) or low intensity therapy (LIT) (hypomethylating agents and low-dose cytarabine) in the frontline or R/R setting (Table 2). CR/CRi rate was highest with CCR with FLT3i, with 83% and 78% of frontline and R/R FLT3/IDH pts achieving CR/CRi with this approach. Among pts who could not receive CCT based therapies, LIT with FLT3i showed encouraging activity with CR/CRi rates of 47% and 40% in frontline and R/R FLT3/IDH pts, respectively. LIT alone was not very effective with CR/CRi rates of 0 and 33% in frontline and R/R FLT3/IDH pts, although numbers are small. Similarly, single agent FLT3 showed modest activity with CR/CRi in 16% of the R/R FLT3/IDH pts.
As expected FLT3/IDH2 pts had improved overall survival (OS) and event free survival (EFS) compared with FLT3/IDH1 pts. The median OS was 11.2 and 2.88 in frontline and R/R FLT3/IDH1 pts, respectively. The median OS was 20.6 and 10.3 in frontline and R/R FLT3/IDH2 pts, respectively. The median EFS was 4.98 and 4.27 in frontline FLT3/IDH1 pts who received CCT+FLT3i and LIT+FLT3i, respectively (p=0.10). The median EFS was 14.2 and 8.2 in frontline FLT3/IDH2 pts who received CCT+FLT3i and LIT+FLT3i, respectively (p=0.09).
Conclusion:FLT3/IDH2 pts have better EFS and OS than FLT3/IDH1 pts in frontline and R/R setting with FLT3i in combination with CCT or LIT. CCT in combination with FLT3i appears to be optimal therapy for FLT3/IDH co-mutated pts with CR/CRi rates of 83% in frontline, and 78% in R/R setting. In pts unable to tolerate CCT a combination of LIT with FLT3i is a reasonable approach yielding CR/CRi rates of 40-45% in frontline and R/R FLT3/IDH pts. LIT alone and FLT3-inhibitor alone demonstrated lower activity with CR/CRi rates of 14-16% in double mutant pts.
Kadia:Pfizer: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Bioline RX: Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Research Funding. Konopleva:Astra Zeneca: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Ascentage: Research Funding; Kisoji: Consultancy, Honoraria; Genentech: Honoraria, Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Eli Lilly: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria; Calithera: Research Funding; Agios: Research Funding. Ravandi:Macrogenix: Consultancy, Research Funding; Xencor: Consultancy, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Menarini Ricerche: Research Funding; Cyclacel LTD: Research Funding; Selvita: Research Funding. Cortes:Novartis: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; BiolineRx: Consultancy; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Biopath Holdings: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Sun Pharma: Research Funding. Jabbour:Pfizer: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Cyclacel LTD: Research Funding. Garcia-Manero:Onconova: Research Funding; H3 Biomedicine: Research Funding; Merck: Research Funding; Amphivena: Consultancy, Research Funding; Helsinn: Research Funding; Novartis: Research Funding; AbbVie: Research Funding; Celgene: Consultancy, Research Funding; Astex: Consultancy, Research Funding. Short:Takeda Oncology: Consultancy, Research Funding; AstraZeneca: Consultancy; Amgen: Honoraria. Sasaki:Pfizer: Consultancy; Otsuka: Honoraria. Pemmaraju:novartis: Consultancy, Research Funding; plexxikon: Research Funding; Daiichi-Sankyo: Research Funding; abbvie: Consultancy, Honoraria, Research Funding; sagerstrong: Research Funding; affymetrix: Research Funding; celgene: Consultancy, Honoraria; cellectis: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; samus: Research Funding; incyte: Consultancy, Research Funding; mustangbio: Consultancy, Research Funding. Borthakur:Argenx: Membership on an entity's Board of Directors or advisory committees; FTC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Tetralogic Pharmaceuticals: Research Funding; Janssen: Research Funding; NKarta: Consultancy; Bayer Healthcare AG: Research Funding; Novartis: Research Funding; Incyte: Research Funding; Agensys: Research Funding; Merck: Research Funding; GSK: Research Funding; Arvinas: Research Funding; Xbiotech USA: Research Funding; Cyclacel: Research Funding; BioTheryX: Membership on an entity's Board of Directors or advisory committees; Strategia Therapeutics: Research Funding; BMS: Research Funding; AstraZeneca: Research Funding; PTC Therapeutics: Consultancy; Cantargia AB: Research Funding; BioLine Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai: Research Funding; Oncoceutics: Research Funding; Eli Lilly and Co.: Research Funding; Oncoceutics, Inc.: Research Funding; Polaris: Research Funding; AbbVie: Research Funding. Kantarjian:Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Amgen: Honoraria, Research Funding; BMS: Research Funding; Cyclacel: Research Funding; Takeda: Honoraria; Immunogen: Research Funding; Ariad: Research Funding; Novartis: Research Funding; Agios: Honoraria, Research Funding; Jazz Pharma: Research Funding; Astex: Research Funding; Pfizer: Honoraria, Research Funding. DiNardo:celgene: Consultancy, Honoraria; jazz: Honoraria; syros: Honoraria; daiichi sankyo: Honoraria; agios: Consultancy, Honoraria; medimmune: Honoraria; notable labs: Membership on an entity's Board of Directors or advisory committees; abbvie: Consultancy, Honoraria. Daver:Celgene: Consultancy; Glycomimetics: Research Funding; NOHLA: Research Funding; BMS: Consultancy, Research Funding; Sunesis: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; Immunogen: Consultancy, Research Funding; Otsuka: Consultancy; Karyopharm: Consultancy, Research Funding; Jazz: Consultancy; Forty-Seven: Consultancy; Novartis: Consultancy, Research Funding; Agios: Consultancy; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Servier: Research Funding; Hanmi Pharm Co., Ltd.: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Astellas: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal