Introduction
In previously untreated, medically fit patients with chronic lymphocytic leukaemia (CLL) and no 17p deletion, there is current research interest in improving survival outcomes and potentially sparing some patients from the standard 6 cycles of fludarabine, cyclophosphamide and rituximab (FCR). The phase II ICLL-07 (NCT02666898) trial, conducted by the French Innovative Leukemia Organization (FILO), aimed to explore the efficacy of obinutuzumab and ibrutinib treatment induction for 9 months, followed by a minimal residual disease (MRD)-driven strategy.
Methods
Following assessment at Month 9, patients in complete response (CR) with bone marrow (BM) MRD <0·01% continued only ibrutinib 420 mg po daily for 6 additional months (I arm). Otherwise, patients received 4x4-weekly cycles of fludarabine/cyclophosphamide (FC) and obinutuzumab 1000 mg iv, alongside continuing ibrutinib for 6 additional months (FCGA+I arm). Beyond Month 16, response was clinically assessed every 3 months and MRD in PB until Month 40 and every 6 months during 36 months. MRD assessment was by 8-colour flow cytometry (limit of detection 10-6). The primary objective was to demonstrate a 30% or higher rate of CR with BM MRD <0·01% at Month 16, by intent-to-treat (ITT) analysis. Progression-free survival (PFS) and overall survival (OS) were secondary endpoints.
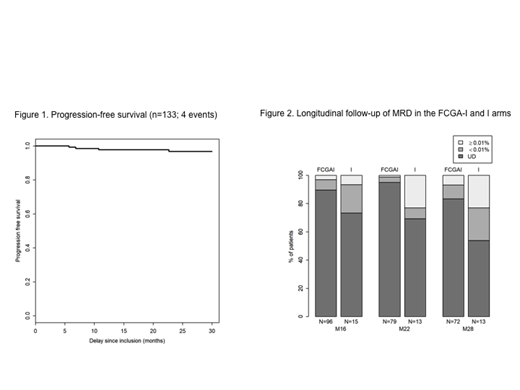
ResultsBetween 10/2015 and 05/2017, 135 patients were enrolled. At Month 9, only 8% of patients reached CR with BM MRD <0·01%, and thus, in accordance with the MRD-driven strategy, were included in the I arm and continued only ibrutinib for 6 additional months. Most patients were included in the FCGA+I arm and received 4 cycles of FC and obinutuzumab, alongside continuing ibrutinib for 6 additional months. At Month 16, the ITT rate of CR with BM MRD <0·01% was 62% (84/135; 90% confidence interval [CI] 55−69). Of note, the primary objective was exceeded, and this high ITT rate was achieved with no more than 4 cycles of FC and obinutuzumab. The CR rate was 73% by investigator assessment versus 75% by an independent review committee. The PB and BM MRD <0·01% rate was 79%. The most common haematological adverse event (AE) was thrombocytopenia in 45 (34%) of 133 patients at grade 1−2 in Months 1−9 and in 43 (33%) of 130 patients at grade 1−2 in Months 9−15. The most common non-haematological AE were infusion-related reaction in 83 (62%) patients at grade 1−2 in Months 1−9 and gastrointestinal disorders in 62 (48%) patients at grade 1−2 in Months 9−15. A total of 49 serious AE occurred, most frequently infections (10), cardiac events (8) and haematological events (8). No treatment-related deaths occurred. After a median follow-up of 26.3 months, the 2-year PFS rate was 98% (95% CI 95−100) (Figure 1) and the 2-year OS rate was 97.5% (95% CI 96−100). The longitudinal follow-up of PB MRD in the entire cohort showed durability of a deep response, with a PB MRD <0.01% rate of 96% (n=92 evaluable patients) at Month 22 and 91% (n=85 evaluable patients) at Month 28. According to the treatment arm, in the FCGA+I arm, the PB MRD <0.01% rate was 99% at Month 22 and 93% at Month 28; by contrast, in the I arm, 77% of patients had PB MRD <0.01% at each of Months 22 and 28. The strategy achieved deep and durable molecular remission with a high level of undetectable (UD) PB MRD that was maintained over time, as shown in Figure 2. At Month 28, the rate of UD PB MRD was 83% in the FCGA+I arm versus 54% in the I arm. According to the immunoglobulin heavy gene variable (IGHV) mutational status, the PB MRD ≥0.01% rate at Month 28 was 4% for the mutated group versus 23% for the unmutated group (p=0.075, Fisher test).
Conclusion
These findings from the ICLL-07 trial demonstrated that, in previously untreated, medically fit patients with CLL and no 17p deletion, treatment induction with obinutuzumab and ibrutinib followed by an MRD-driven strategy yielded a high rate of CR with BM and PB MRD <0.01%, together with prolonged PFS and OS. With longer follow-up, including assessing the evolution of PB MRD, the response is maintained. This strategy could be an option in the first-line setting, although randomised trial evidence is needed.
Salles:Roche, Janssen, Gilead, Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Educational events; Amgen: Honoraria, Other: Educational events. Leblond:Gilead: Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Honoraria, Speakers Bureau; Astra Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Cartron:Roche, Celgene: Consultancy; Sanofi, Gilead, Janssen, Roche, Celgene: Honoraria. Cymbalista:Sunesis: Research Funding; Roche: Research Funding; Janssen: Honoraria; Abbvie: Honoraria; AstraZeneca: Honoraria; Gilead: Honoraria. Le Garff-Tavernier:Alexion: Consultancy, Honoraria. Letestu:Alexion: Membership on an entity's Board of Directors or advisory committees, Other: speaker fee, expert contracts; Roche: Membership on an entity's Board of Directors or advisory committees, Other: speaker fee, expert contracts; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: speaker fee, expert contracts; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: speaker fee, expert contracts. Feugier:janssen: Honoraria, Research Funding, Speakers Bureau; gilead: Honoraria, Research Funding, Speakers Bureau; abbvie: Honoraria, Research Funding, Speakers Bureau; roche: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal