Abstract
Hematological toxicity is the most common adverse event after chimeric antigen receptor (CAR) T-cell therapy. Cytopenias can be profound and long-lasting and can predispose for severe infectious complications. In a recent worldwide survey, we demonstrated that there remains considerable heterogeneity in regard to current practice patterns. Here, we sought to build consensus on the grading and management of immune effector cell–associated hematotoxicity (ICAHT) after CAR T-cell therapy. For this purpose, a joint effort between the European Society for Blood and Marrow Transplantation (EBMT) and the European Hematology Association (EHA) involved an international panel of 36 CAR T-cell experts who met in a series of virtual conferences, culminating in a 2-day meeting in Lille, France. On the basis of these deliberations, best practice recommendations were developed. For the grading of ICAHT, a classification system based on depth and duration of neutropenia was developed for early (day 0-30) and late (after day +30) cytopenia. Detailed recommendations on risk factors, available preinfusion scoring systems (eg, CAR-HEMATOTOX score), and diagnostic workup are provided. A further section focuses on identifying hemophagocytosis in the context of severe hematotoxicity. Finally, we review current evidence and provide consensus recommendations for the management of ICAHT, including growth factor support, anti-infectious prophylaxis, transfusions, autologous hematopoietic stem cell boost, and allogeneic hematopoietic cell transplantation. In conclusion, we propose ICAHT as a novel toxicity category after immune effector cell therapy, provide a framework for its grading, review literature on risk factors, and outline expert recommendations for the diagnostic workup and short- and long-term management.
Introduction and state of the art
The last decade has firmly established chimeric antigen receptor (CAR) T-cell therapy as a practice-changing immunotherapy platform for an increasing number of refractory B-cell malignancies.1-7 Although durable remissions can be achieved, this comes with the caveat of a unique spectrum of side effects ranging from cytokine release syndrome (CRS), to immune effector cell–associated neurotoxicity syndrome (ICANS), and immune effector cell–associated hemophagocytic lymphohistiocytosis-like syndrome (IEC-HS).8-11 Real-world evidence has underlined the growing importance of hematological toxicity as the most frequent Common Terminology Criteria for Adverse Events (CTCAE) grade ≥3 adverse event after CAR T-cell therapy.12-14 Similarly high rates of cytopenias have been reported for other T-cell–based immunotherapies such as bispecific antibodies.15-19 Notably, profound and often long-lasting cytopenias can add to the immunosuppression conferred by B-cell aplasia and consecutive hypogammaglobulinemia.20 Importantly, severe infections are a major driver of both morbidity and nonrelapse mortality following CAR T-cell therapies.21-23
Hematological side effects have been described after CAR T-cell therapy regardless of the target antigen (eg, CD19 vs CD22 vs B-cell maturation antigen [BCMA]) and across various disease entities (eg, large B-cell lymphoma [LBCL], B-cell precursor acute lymphoblastic leukemia [BCP-ALL], mantle cell lymphoma [MCL], multiple myeloma [MM], and follicular lymphoma [FL]).3-5,24-29 Several features underline the unique nature of CAR T-cell–related hematotoxicity. First, cytopenias can persist long after the resolution of clinical CRS, and have been reported as long as months to years after CAR T-cell infusion.30 Hematopoietic count recovery often follows a biphasic trajectory, with intermittent recovery followed by second, or multiple, dips.12,13 Second, patients can develop very severe bone marrow (BM) aplasia that is often refractory to therapeutic measures such as growth factor support.13,31,32 Finally, the underlying pathophysiology remains to be elucidated, although recent evidence points toward the importance of both baseline hematopoietic reserve and the systemic inflammatory state of the host.13 Moreover, the inflammatory stress conferred by severe CRS and the associated alterations in cytokine patterns can exert myelosuppressive effects.33-35
In a recent international survey led by the European Hematology Association (EHA) and European Society for Blood and Marrow Transplantation (EBMT), we identified a high degree of heterogeneity in regard to both the grading and management of cytopenias.36 Current grading systems, such as the CTCAE, describe cytopenias predominantly in quantitative terms by assigning severity grades based on the depth of cytopenia. However, they are not clinically actionable and fail to capture the distinct nature of post–CAR T-cell hematopoietic reconstitution, such as the biphasic and/or delayed course. Furthermore, the cumulative risk of secondary complications (eg, infections or bleeding) primarily increases with the respective duration of observed cytopenia.22,37 Classification systems that were developed for cytopenia following classic cytotoxic chemotherapies may not apply to patients receiving novel T-cell–based immunotherapies. To accommodate these unique features of hematological side effects in adult patients receiving such therapies, we herein introduce the concept of immune effector cell–associated hematotoxicity (ICAHT). Based on a novel framework for grading, we outline expert recommendations for its diagnostic workup and management.
Methodology
This workshop is based on the EBMT Practice Harmonization and Guidelines committee method.38 In September 2022, K.R. and M.S. proposed to set up a workshop to issue European recommendations regarding the grading and management of ICAHT, particularly after autologous CAR T-cell therapy. As a first step, an international survey on current practices at >50 global CAR T-cell therapy centers was sent out and results were analyzed.36 Experts from different countries and belonging to EBMT and EHA were subsequently invited to join the workshop. As a second step, several teleconferences took place to discuss and advance the first draft. Along with the results of the international survey, a comprehensive literature review was carried out by the workshop participants within each subgroup, which served as the basis for the discussions. The third step consisted of a 2-day face-to-face meeting, which took place in Lille, France in March 2023.
These recommendations are intended to be general in scope and applicable to all diseases and types of CAR T-cell therapies or other T-cell–based immunotherapies (eg, bispecific antibody constructs) adopted as standard clinical practice. They are intended to reflect current best practices in this new and rapidly evolving field and aim to help clinicians and other health care professionals in providing consistent, high-quality patient care. These recommendations were created because of the growing number of autologous CAR T-cell therapies currently available outside of clinical trials for the treatment of hematological malignancies. Given the lack of high-quality evidence from randomized trials in this area (expected evidence levels 3-5, Oxford Centre for Evidence Based Medicine), the decision was made not to grade these recommendations. They therefore represent the consensus point of view of the authors. When administering CAR T-cell therapies within clinical trials, physicians are advised to follow respective trial protocols.
Consensus recommendations
ICAHT grading
On the basis of the results of the international survey on behalf of EHA and EBMT, the expert panel defined early ICAHT as cytopenia occurring during the first 30 days after CAR T-cell infusion. Conversely, late ICAHT was classified as cytopenia observed beyond day +30. The expert panel resolved that the main clinical action points of post–CAR T-cell cytopenias concerned profound and/or prolonged neutropenia, and that isolated thrombocytopenia or anemia represent rare occurrences. Concomitantly, a grading system based on neutropenia was pursued. For early ICAHT (day 0-30), a grading system based on both depth and duration of neutropenia was defined due to the associated clinical sequelae (Table 1, top). Late ICAHT was graded based on the elapsed time from CAR T-cell infusion (eg, occurring after day +30) with the severity (grade 1-4) defined by the depth of neutropenia (Table 1, bottom). For anemia and thrombocytopenia, the expert panel refers to existing grading systems and recommends that institutional guidelines should be followed, as further outlined in “Management of cytopenias” and Table 3 (refer to “Transfusions”).
ICAHT grading
| Grading . | 1 . | 2 . | 3 . | 4 . |
|---|---|---|---|---|
| Early ICAHT (day 0-30) | ||||
| ANC ≤500/μL | <7 d | 7-13 d | ≥14 d | Never above 500/μL |
| ANC ≤100/μL | — | — | ≥7 d | ≥14 d |
| Late ICAHT (after day +30)∗ | ||||
| ANC | ≤1500/μL | ≤1000/μL | ≤500/μL | ≤100/μL |
| Grading . | 1 . | 2 . | 3 . | 4 . |
|---|---|---|---|---|
| Early ICAHT (day 0-30) | ||||
| ANC ≤500/μL | <7 d | 7-13 d | ≥14 d | Never above 500/μL |
| ANC ≤100/μL | — | — | ≥7 d | ≥14 d |
| Late ICAHT (after day +30)∗ | ||||
| ANC | ≤1500/μL | ≤1000/μL | ≤500/μL | ≤100/μL |
Measured ≥2 time points, or nontransient neutropenia.
Risk factors for developing post–CAR T-cell cytopenias
The overall incidence of hematological toxicity in the key registrational trials for CAR T-cell products endorsed by the European Medicines Agency are outlined in the supplemental Table 1, available on the Blood website. Furthermore, we performed an extensive literature review of prominent real-world studies with a specific focus on correlative studies and potential risk factors (supplemental Table 2). Overall, a plethora of factors contribute to the development of cytopenias after CAR T-cell therapy, some of which remain incompletely understood. Broadly, they relate to the underlying disease and its previous treatments, baseline risk factors (eg, hematopoietic reserve, BM infiltration, and systemic inflammation), as well as CAR T-cell product features and CRS-related inflammatory patterns (summarized in Table 2 and the supplemental Material).12,13,23,30,33,34,39-56
Risk factors associated with an increased risk of post–CAR T-cell cytopenias
| . | Risk factors . | Comments . | References . |
|---|---|---|---|
| Disease-related features | Underlying disease (BCP-ALL > B-NHL) | Evidence concerning the rate of cytopenias in patients with MM still emerging | 39 |
| Disease burden prior to CAR T-cell infusion (progressive disease, high LDH) | Especially BM disease burden | 14,102 | |
| Prior therapies | Number of prior therapy lines | Associated with baseline hematopoietic function | 39 |
| Prior hematopoietic stem cell transplantation | 104 | ||
| Bridging therapy | 41 | ||
| Baseline marrow status | BM infiltration | 42,40 | |
| Preexisting cytopenias | Particularly preexisting thrombocytopenia | 13,33 | |
| Clonal hematopoiesis of indeterminate potential (CHiP)? | Has been linked to increased inflammation, potential emerging risk factor | 46,47,85 | |
| Baseline inflammatory status | Increased serum CRP | 13 | |
| Increased serum ferritin | 13 | ||
| CAR T-cell product and postinfusion risk factors | Costimulatory molecule (CD28 > 41BB) | May also reflect differences in lymphodepletion dosing (cyclophosphamide dosing) | 39 |
| Type of construct (tandem > single target) | 39 | ||
| Severe CRS | 33,34 | ||
| Sustained increased inflammatory markers | 33 | ||
| Oligoclonal T-cell expansion | In select patients; the success of autologous stem cell boost argues against this as a general mechanism | 35 | |
| Active infection | Mainly viral or in case of concomitant sepsis | 105 | |
| CRS/MAS or IEC-HS | Cytopenia as overlapping symptomology | 10,11,106 |
| . | Risk factors . | Comments . | References . |
|---|---|---|---|
| Disease-related features | Underlying disease (BCP-ALL > B-NHL) | Evidence concerning the rate of cytopenias in patients with MM still emerging | 39 |
| Disease burden prior to CAR T-cell infusion (progressive disease, high LDH) | Especially BM disease burden | 14,102 | |
| Prior therapies | Number of prior therapy lines | Associated with baseline hematopoietic function | 39 |
| Prior hematopoietic stem cell transplantation | 104 | ||
| Bridging therapy | 41 | ||
| Baseline marrow status | BM infiltration | 42,40 | |
| Preexisting cytopenias | Particularly preexisting thrombocytopenia | 13,33 | |
| Clonal hematopoiesis of indeterminate potential (CHiP)? | Has been linked to increased inflammation, potential emerging risk factor | 46,47,85 | |
| Baseline inflammatory status | Increased serum CRP | 13 | |
| Increased serum ferritin | 13 | ||
| CAR T-cell product and postinfusion risk factors | Costimulatory molecule (CD28 > 41BB) | May also reflect differences in lymphodepletion dosing (cyclophosphamide dosing) | 39 |
| Type of construct (tandem > single target) | 39 | ||
| Severe CRS | 33,34 | ||
| Sustained increased inflammatory markers | 33 | ||
| Oligoclonal T-cell expansion | In select patients; the success of autologous stem cell boost argues against this as a general mechanism | 35 | |
| Active infection | Mainly viral or in case of concomitant sepsis | 105 | |
| CRS/MAS or IEC-HS | Cytopenia as overlapping symptomology | 10,11,106 |
BCP-ALL, B-cell precursor acute lymphoblastic leukemia; B-NHL, B-cell non-Hodgkin lymphoma; CRP, C-reactive protein; LDH, lactate dehydrogenase; MAS, macrophage activation syndrome.
What scoring systems to use
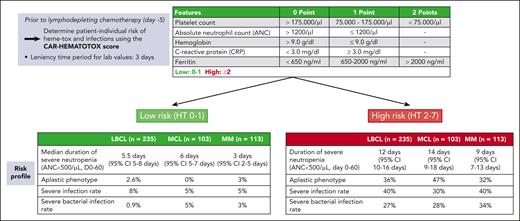
Based on several of the risk factors delineated earlier, the CAR-HEMATOTOX score was developed to identify patients at high risk for prolonged neutropenia, and especially the development of the aplastic phenotype of neutrophil recovery.13 An online calculator can be found on the website of the German Lymphoma Alliance (https://www.german-lymphoma-alliance.de/Scores.html). The score incorporates factors related to hematopoietic reserve (absolute neutrophil count [ANC], hemoglobin, and platelet count) and baseline inflammatory state (C-reactive protein and ferritin) and was validated for a primary end point of severe neutropenia (ANC < 500/μL) lasting ≥14 days during the first 60 days after CAR T-cell infusion. Importantly, the CAR-HEMATOTOX score is determined before lymphodepleting chemotherapy and thus enables early risk-stratification into a high vs low risk of developing severe hematotoxicity after CAR T-cell treatment (Figure 1). In subsequent studies, the score also identified patients at risk for severe infections and poor treatment outcomes across multiple disease entities (eg, LBCL, MCL, and MM).22,42-44,57 However, it is important to note that the score remains to be validated prospectively and for adult and pediatric patients with B-cell precursor acute lymphoblastic leukemia. Furthermore, the test characteristics (high sensitivity, but lower specificity) indicate a lower positive predictive value, meaning that not all patients deemed high risk will develop severe hematotoxicity. Conversely, the high negative predictive value suggests that the score is particularly helpful in ruling out patients at risk for severe hematotoxicity.
The CAR-HEMATOTOX score as a risk stratification tool. Data presented in the tables are based on multicenter retrospective analyses examining hematotoxicity and infectious complications in patients receiving CAR-T for relapsed/refractory LBCL,13,22 MCL,43 or MM.44 CI, confidence interval; HT, CAR-HEMATOTOX score; MCL, mantle cell lymphoma. MM, multiple myeloma.
The CAR-HEMATOTOX score as a risk stratification tool. Data presented in the tables are based on multicenter retrospective analyses examining hematotoxicity and infectious complications in patients receiving CAR-T for relapsed/refractory LBCL,13,22 MCL,43 or MM.44 CI, confidence interval; HT, CAR-HEMATOTOX score; MCL, mantle cell lymphoma. MM, multiple myeloma.
Assessment and diagnostic workup of ICAHT
In patients with a high-risk profile for developing ICAHT (Table 2; Figure 1), baseline BM studies (prior to apheresis or lymphodepletion) should be considered to risk-stratify patients for hematological toxicity and to identify underlying marrow infiltration as a pertinent risk factor. Cryopreservation of the BM aspirate and/or peripheral blood mononuclear cells is optional but may provide useful information in case the patient develops secondary BM failure (eg, presence of CHiP clone).
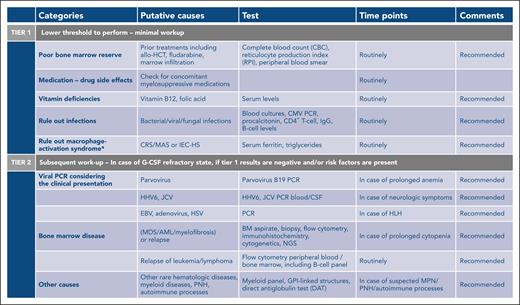
In case of cytopenia that persists beyond the expected reconstitution of lymphodepleting chemotherapy (typically after week 2-3 after CAR T-cell infusion), the first step in the workup lies in defining the differential diagnosis, which can include drug-induced cytopenia, vitamin deficiencies, infectious causes, sustained inflammatory stressors, relapse, and/or active BM disease. The expert panel recommends performing an incremental diagnostic workup, with an initial tier 1 assessment comprising standard diagnostic tests that should be performed in all cases of severe, or grade ≥3, ICAHT (Figure 2). In case the tier 1 results are inconclusive and cytopenias persist and/or are granulocyte colony-stimulating factor (G-CSF) refractory (absence of count recovery despite ≥5 days of G-CSF support), a subsequent tier 2 diagnostic workup can be pursued. Importantly, this includes extended viral studies, as well as BM aspiration and biopsy. The expert panel would reserve cytogenetics and next-generation sequencing to rule out an underlying myeloid malignancy to either cases of profound, long-lasting marrow aplasia (eg, no count recovery above an ANC of ≥500/μL by day +30, pancytopenia), or new-onset pancytopenia that is refractory to therapeutic measures late after CAR T-cell infusion.
Step-by-step diagnostic workup depending on ICAHT severity. ∗In case of elevated ferritin and clinical suspicion of MAS, refer to supplemental Table 3 and supplemental Figure 1. AML, acute myeloid leukemia; CMV, cytomegalovirus; CRS/MAS, cytokine release syndrome with macrophage activation syndrome; EBV, Epstein-Barr virus; GPI, glycosylphosphatidylinositol; HHV6, human herpesvirus 6; HSV, herpes simplex virus; IgG, immunoglobulin G; JCV, JC virus; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; NGS, next-generation sequencing; PCR, polymerase chain reaction; PNH, paroxysmal nocturnal hemoglobinuria.
Step-by-step diagnostic workup depending on ICAHT severity. ∗In case of elevated ferritin and clinical suspicion of MAS, refer to supplemental Table 3 and supplemental Figure 1. AML, acute myeloid leukemia; CMV, cytomegalovirus; CRS/MAS, cytokine release syndrome with macrophage activation syndrome; EBV, Epstein-Barr virus; GPI, glycosylphosphatidylinositol; HHV6, human herpesvirus 6; HSV, herpes simplex virus; IgG, immunoglobulin G; JCV, JC virus; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; NGS, next-generation sequencing; PCR, polymerase chain reaction; PNH, paroxysmal nocturnal hemoglobinuria.
Hemophagocytosis associated with severe hematotoxicity after CAR T-cell therapy
Hemophagocytic lymphohistiocytosis (HLH) is a hyperinflammatory condition resulting from abnormal immune activation, which is associated with high fever, hyperferritinemia, prolonged cytopenia, and eventually multiorgan failure. HLH remains a diagnostic quandary because unique biomarkers are still lacking and/or not readily available. In the context of CAR T-cell therapy, the incidence of HLH-like symptoms ranges from 1% to 3.4%.10,58 Two entities, CRS/MAS and IEC-HS, can be distinguished based on time of onset and presence of concomitant CRS/ICANS symptoms.11,29,59,60 In patients with severe ICAHT that present with aplastic neutrophil recovery and rising serum ferritin, the diagnosis of HLH should be considered as both can present with profound immune dysregulation and increased interferon signaling.42,54 A comprehensive workup is recommended in order to identify additional abnormalities such as new-onset hepatosplenomegaly, hypertriglyceridemia, coagulopathy, and hypofibrinogenemia, as well as hemophagocytosis features on BM biopsy or in other tissues (Figure 2). Existing scoring systems that can guide the diagnosis of HLH in the context of severe ICAHT include HLH-2004 criteria, the H-score, and the optimized HLH inflammatory index.61-63 Furthermore, supplemental Table 3 outlines the MD Anderson criteria,58 EBMT/EHA recommendations,59 and IEC-HS criteria,11 which were deemed more specific to CAR T-cell therapy by the expert panel. In patients in whom ICAHT manifests in the form of HLH, anti-inflammatory measures should be promptly initiated to mitigate cytokine storm and its clinical sequelae. Patients should be treated with anakinra, a recombinant humanized interleukin-1 receptor antagonist, in combination with high-dose corticosteroids (supplemental Figure 1). In refractory cases, ruxolitinib, cytokine adsorption, and emapalumab (an interferon-γ inhibitor) can be considered, albeit data remain scarce.64-66
Management of cytopenias
The management of ICAHT can broadly be separated into an initial phase, which addresses the (expected) early cytopenias and aims to mitigate the risk of infections and/other complications, as well as a later phase that is initiated in case of persistent and/or therapy-refractory cytopenias. An overview of the expert recommendations for early ICAHT management is provided in Table 3.
Short-term management of cytopenias
| . | When . | How . | Precautions . | Comments . |
|---|---|---|---|---|
| pRBC/platelet transfusions | As per institutional standards, based on patient risk profile | As per institutional standards For pRBC: consider using 1 product per time to reduce iron overload68 | Irradiation of blood products; start 7 d before leukapheresis until at least 90 d after CAR T-cell infusion | Because of the use of fludarabine |
| G-CSF | Prophylactic G-CSF: on day +2 in patients with a high-risk profile for ICAHT (eg, high CAR-HEMATOTOX score and risk profile per Table 2) | Based on individual risk profile: consider early G-CSF administration (from day +2) as prophylaxis in those at high risk for ICAHT Dosing: 5 μg/kg once daily | In patients at low risk for ICAHT, G-CSF probably not necessary | Reduced risk of febrile neutropenia (without increasing the risk of severe, or grade ≥3, CRS nor ICANS) No detrimental effect on CAR T-cell expansion kinetics or treatment outcomes73,74 |
| Therapeutic G-CSF: severe neutropenia (ANC < 500/μL) neutropenia with or without infectious complications | In case of prolonged neutropenia with/without infectious complications Dosing: 5 μg/kg once daily, consider increasing dose in case of nonresponse | Patients with intermittent neutrophil recovery often rapidly respond to G-CSF stimulation, whereas those who are aplastic are often G-CSF unresponsive | ||
| Antibacterial prophylaxis | In patients with a low risk for ICAHT, not recommended In patients with a high-risk profile for ICAHT, prophylaxis may be considered once ANC is <500/μL | As per institutional standards (eg, levofloxacin or ciprofloxacin) | Warning in case of colonization by MDR pathogens | Look at local bacterial epidemiology. High local prevalence of MDR GNB might prevent the use of antibacterial prophylaxis. |
| Antiviral | All patients | Start from LD conditioning until 1 y after CAR T-cell infusion AND/OR until CD4+ count is >0.2 × 109/L Valaciclovir 500 mg twice a day or acyclovir 800 mg twice a day | ||
| Antipneumocystis | All patients | Start from LD conditioning until 1 y after CAR T-cell infusion AND/OR until CD4+ count is >0.2 × 109/L Co-trimoxazole 480 mg once daily or 960 mg 3 times each week | In case of co-trimoxazole allergy, pentamidine inhalation (300 mg once every month), dapsone 100 mg daily or atovaquone 1500 mg once daily can be considered | Can be started later depending on center guidelines |
| Systemic primary antifungal prophylaxis | Prophylaxis may be considered in case of severe neutropenia (ANC < 500/μL) and a high-risk profile for ICAHT (eg, CAR-HEMATOTOX score and risk profile per Table 2) and/or prolonged neutropenia | Mold-active prophylaxis for 1-3 mo (depending on the duration of neutropenia and use of steroids): posaconazole (300 mg/d) or micafungin (50 mg per day, IV) | In patients with prior allo-HCT, prior invasive aspergillosis, and those receiving corticosteroids (long-term >72 h, or high-dose), prophylaxis is recommended |
| . | When . | How . | Precautions . | Comments . |
|---|---|---|---|---|
| pRBC/platelet transfusions | As per institutional standards, based on patient risk profile | As per institutional standards For pRBC: consider using 1 product per time to reduce iron overload68 | Irradiation of blood products; start 7 d before leukapheresis until at least 90 d after CAR T-cell infusion | Because of the use of fludarabine |
| G-CSF | Prophylactic G-CSF: on day +2 in patients with a high-risk profile for ICAHT (eg, high CAR-HEMATOTOX score and risk profile per Table 2) | Based on individual risk profile: consider early G-CSF administration (from day +2) as prophylaxis in those at high risk for ICAHT Dosing: 5 μg/kg once daily | In patients at low risk for ICAHT, G-CSF probably not necessary | Reduced risk of febrile neutropenia (without increasing the risk of severe, or grade ≥3, CRS nor ICANS) No detrimental effect on CAR T-cell expansion kinetics or treatment outcomes73,74 |
| Therapeutic G-CSF: severe neutropenia (ANC < 500/μL) neutropenia with or without infectious complications | In case of prolonged neutropenia with/without infectious complications Dosing: 5 μg/kg once daily, consider increasing dose in case of nonresponse | Patients with intermittent neutrophil recovery often rapidly respond to G-CSF stimulation, whereas those who are aplastic are often G-CSF unresponsive | ||
| Antibacterial prophylaxis | In patients with a low risk for ICAHT, not recommended In patients with a high-risk profile for ICAHT, prophylaxis may be considered once ANC is <500/μL | As per institutional standards (eg, levofloxacin or ciprofloxacin) | Warning in case of colonization by MDR pathogens | Look at local bacterial epidemiology. High local prevalence of MDR GNB might prevent the use of antibacterial prophylaxis. |
| Antiviral | All patients | Start from LD conditioning until 1 y after CAR T-cell infusion AND/OR until CD4+ count is >0.2 × 109/L Valaciclovir 500 mg twice a day or acyclovir 800 mg twice a day | ||
| Antipneumocystis | All patients | Start from LD conditioning until 1 y after CAR T-cell infusion AND/OR until CD4+ count is >0.2 × 109/L Co-trimoxazole 480 mg once daily or 960 mg 3 times each week | In case of co-trimoxazole allergy, pentamidine inhalation (300 mg once every month), dapsone 100 mg daily or atovaquone 1500 mg once daily can be considered | Can be started later depending on center guidelines |
| Systemic primary antifungal prophylaxis | Prophylaxis may be considered in case of severe neutropenia (ANC < 500/μL) and a high-risk profile for ICAHT (eg, CAR-HEMATOTOX score and risk profile per Table 2) and/or prolonged neutropenia | Mold-active prophylaxis for 1-3 mo (depending on the duration of neutropenia and use of steroids): posaconazole (300 mg/d) or micafungin (50 mg per day, IV) | In patients with prior allo-HCT, prior invasive aspergillosis, and those receiving corticosteroids (long-term >72 h, or high-dose), prophylaxis is recommended |
LD, lymphodepletion; MDR GNB, multidrug-resistant gram-negative bacteria; pRBC, packed red blood cell.
Transfusions
Because of the frequent nature of severe anemia and thrombocytopenia after CAR T-cell therapy, transfusions are an essential part of supportive care and include either packed red blood cell concentrates or platelet concentrates. Transfusion-associated graft-versus-host disease (ta-GVHD) is a rare complication of transfusion wherein viable donor T lymphocytes in cellular blood products mount an immune response against the recipient.67 Considering the high mortality rate (>90%), prevention of ta-GVHD is recommended, although there is no internationally agreed upon consensus on the duration of the use of irradiated blood products across cellular therapies. In the setting of hematopoietic cell transplantation (HCT), standard practice is to use irradiated blood for (1) at least 2 weeks before stem cell collection until at least 3 months after auto-HCT, and (2) at the start of conditioning at the latest until at least 6 months after allogeneic HCT (allo-HCT), or until immune reconstitution.68 In the context of CAR T-cell therapy, the expert panel recommended the irradiation of blood products from 7 days before leukapheresis until at least 90 days after CAR T-cell infusion unless conditioning, disease, or previous treatment determine indefinite duration (Table 3). Of note, the use of the purine analog fludarabine as a component of lymphodepletion before CAR T-cell infusion may affect local guidance for irradiated blood products.68 Given its relative rarity, we recommend reporting cases of ta-GVHD after CAR T-cell therapy to regulatory authorities.
Growth factor support
GM-CSF
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is typically elevated in patients with CRS and ICANS receiving CAR T-cell therapy. The use of GM-CSF as a growth factor for patients with low blood counts should be avoided as it may promote inflammatory toxicity and induce neuroinflammation after CAR T-cell therapy.69,70
G-CSF
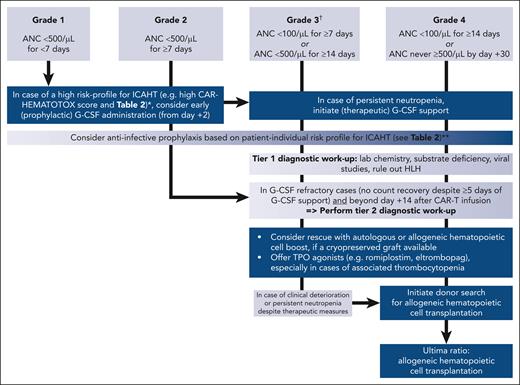
Because of the concerns for the use of GM-CSF and the hypothesized, but largely unknown, risks of exacerbating toxicities, early guidance suggested generally deferring G-CSF until resolution of acute CAR T-cell–related immunotoxicity (typically week 3). However, several recent reports question this as a general rule and point toward an acceptable safety profile for the early use of G-CSF, with no increase of high-grade (grade ≥3) CRS/ICANS.71-75 In the largest retrospective analysis by Miller et al (n = 197), prophylactic G-CSF before CAR T-cell therapy (mostly pegylated G-CSF) was associated with faster neutrophil recovery, comparable treatment outcomes, and similar rates of severe ICANS.74 Although prophylactic G-CSF was associated with a higher rate of grade ≥2 CRS, this observation did not extend to the clinically relevant grade ≥3 CRS. In a subgroup analysis, the authors found that G-CSF did not worsen severity of CRS in patients who already present with low-grade (grade 1) toxicity. In a further study by Lievin et al, early G-CSF administration (from day +2) in patients with neutropenia was associated with a reduced risk of febrile neutropenia without increasing the risk of severe CRS or ICANS.73 Notably, G-CSF was also safe in maintaining CAR T-cell expansion kinetics and antilymphoma activity, without any deleterious impact on the quality of response and outcomes.72,73 Appraising the aforementioned evidence and weighing the benefits and risks, early G-CSF administration on day +2 can be considered in high-risk patients to shorten the length of expected severe neutropenia (see Table 2; Figure 1). Therapeutic G-CSF in case of prolonged severe neutropenia (ANC < 500/μL) can also be considered and can be of diagnostic benefit for identifying the aplastic neutrophil recovery phenotype,13,32 which is often G-CSF unresponsive. The large majority (>80%) of patients receiving CAR T-cell therapy ultimately respond to growth factor support with count recovery.32,34 However, recurrent neutrophil dips (biphasic course) can necessitate intermittent application of therapeutic G-CSF (Figure 3). Finally, a uniform consensus was reached on the necessity of prospective, and ideally multicenter, clinical trials that evaluate the safety and optimal treatment protocol for G-CSF (prophylactic vs early, and pegylated vs nonpegylated) in the context of CAR T-cell therapy and across disease entities (BCP-ALL vs B-NHL vs MM).
Treatment algorithm for immune effector cell–associated hematotoxicity. ∗High risk defined as previous history of hematopoietic stem cell transplantation, baseline cytopenia, high tumor burden, systemic inflammation, and presence of BM infiltration. ∗∗Antifungal prophylaxis particularly recommended in patients with previous invasive fungal disease, previous allo-HCT, and receiving corticosteroids (long-term >72 hour or high dose). Decision for/against antibacterial prophylaxis should incorporate local bacterial epidemiology (eg, prevalence for multidrug-resistant gram-negative bacteria); not recommended for patients with a low-risk profile for ICAHT. †Also extends to late ICAHT if these criteria are met.
Treatment algorithm for immune effector cell–associated hematotoxicity. ∗High risk defined as previous history of hematopoietic stem cell transplantation, baseline cytopenia, high tumor burden, systemic inflammation, and presence of BM infiltration. ∗∗Antifungal prophylaxis particularly recommended in patients with previous invasive fungal disease, previous allo-HCT, and receiving corticosteroids (long-term >72 hour or high dose). Decision for/against antibacterial prophylaxis should incorporate local bacterial epidemiology (eg, prevalence for multidrug-resistant gram-negative bacteria); not recommended for patients with a low-risk profile for ICAHT. †Also extends to late ICAHT if these criteria are met.
TPO agonists
Thrombopoietin (TPO) agonists (eg, eltrombopag and romiplostim) are considered primarily in patients with prolonged and late thrombocytopenia, with the thrombocytopenic nadir typically occurring in the second month after CAR T-cell therapy.12,13 Data supporting the use of TPO agonists in the CAR T-cell setting are extremely limited and are restricted to a few case series from single centers with limited patient numbers.76-78 In these limited reports, improvement in platelets and also hemoglobin and ANC was noted, with some patients becoming transfusion independent both for platelets and packed red blood cell concentrates, similar to improvement in hematopoiesis observed with TPO agonist use in cases of acquired BM failure.79,80 Because of the limited available data, the expert panel advises that the use of TPO agonists should parallel the practice for HCT.81 They can also be used in G-CSF–refractory cases of ICAHT (Figure 3).
Infection prophylaxis
Regarding the administration of anti-infectious prophylaxis during cytopenia, the expert panel broadly recommends adherence to the general EHA/EBMT guidelines for patients receiving CAR T-cell therapy.59 The following specific recommendations were issued (Table 3):
Adherence to current EHA/EBMT guidelines regarding antiviral and antipneumocystis pneumonia prophylaxis, as well as intravenous immunoglobulin substitution for post–CAR T-cell therapy hypogammaglobulinemia.59
The expert panel does not recommend the use of a neutropenic diet to reduce the risk of infection in patients with neutropenia receiving CAR T-cell therapy.82-84
Antibacterial prophylaxis: the panel proposes a risk-adapted strategy based on the patient-individual risk profile for infections including the expected incidence rate of protracted, profound neutropenia (ANC of <100/μL for ≥7 days), in line with the consensus American Society of Clinical Oncology/Infectious Diseases Society of America (ASCO/IDSA) recommendations for adult patients with cancer.85 Antibacterial prophylaxis with a fluoroquinolone (eg, levofloxacin or ciprofloxacin) is not recommended in patients who are at low risk of severe (grade ≥3) ICAHT (Tables 1 and 3) and should be avoided because of fluoroquinolone-specific side effects, the potential emergence of resistant strains, and selection for Clostridium difficile and enterococci.37,86-89 Furthermore, recent publications have demonstrated that antibiotic exposure before CAR T-cell therapy reduces microbiome diversity and is associated with inferior outcomes, potentially because of the multifunctional and immunomodulatory role of the gut microbiome.90-93 In contrast, antibacterial prophylaxis can be considered in patients at high risk once the ANC is <500/μL to mitigate the risk of severe infections. The CAR-HEMATOTOX score may be useful for guidance and identification of candidates at high risk.13,57 In a large retrospective analysis of patients with LBCL receiving CD19 CAR T cells, a significant reduction of severe bacterial infections with fluoroquinolone prophylaxis was observed in patients who were characterized as CAR-HEMATOTOXhigh but not in those characterized as CAR-HEMATOTOXlow, supporting a risk-adapted approach. Importantly, the panel recommends adherence to institutional guidelines that take into account local epidemiology and resistance patterns. In this context, monitoring for multidrug-resistant gram-negative bacteria colonization (ie, active surveillance through rectal swab culture) may be useful both for baseline risk assessment and during prolonged neutropenia.
Antifungal prophylaxis: to reduce the risk of invasive fungal disease, antimold prophylaxis (eg, micafungin or posaconazole) can be considered in patients at high risk for severe ICAHT (grade ≥3) once the ANC is <500/μL (Table 3). Additional risk factors to consider are prior allo-HCT, prior invasive aspergillosis, and receipt of corticosteroids (either long-term, ≥72 hours; or high-dose, eg, >10 mg of dexamethasone or equivalent). The low overall incidence rate for invasive fungal disease in the context of CAR T-cell therapy should be taken into account,94 although fungal infections represent a frequent cause of fatal infectious complications.22,95 Systemic primary antifungal prophylaxis should be continued until stable count recovery (ANC of >500/μL over 3 days) and discontinuation of steroids for CRS/ICANS management.
Hematopoietic cell boost (HCB)
Patients who are unresponsive and/or refractory to G-CSF beyond day +14 after CAR T-cell infusion represent a clinically challenging subgroup of patients at high risk for severe and even fatal infectious complications. Although the evidence remains limited, TPO agonists can be offered in this setting, especially in cases of associated thrombocytopenia.78 In cases of severe ICAHT in which an inflammatory stressor is deemed contributory (severe CRS/ICANS or CRS/MAS), anti-inflammatory strategies such as pulse-dose corticosteroids and/or anticytokine therapies (eg, tocilizumab or anakinra) should be used. A promising strategy pertains to the use of cryopreserved autologous or allogeneic CD34+ hematopoietic cells from prior collection (either prior auto- or allo-HCT).96-98 Three recent case series shed light on both the safety and clinical feasibility of this approach across a broad population of pediatric and adult patients (summarized in supplemental Table 4). High rates of sustained neutrophil and platelet engraftment were noted across studies. Although HCB has been successfully applied during active infection,99 clinicians should be aware of the possibility of immune reconstitution inflammatory syndrome in patients with prolonged BM aplasia.31 Because the earlier application of an available HCB was associated with superior survival outcomes,98 the expert panel recommends considering the application of an HCB without prior conditioning chemotherapy for grade ≥3 ICAHT beyond day +14 if (1) a boost is readily available, and (2) G-CSF refractoriness has been established. At the same time, the survey results highlighted that even when HCB were considered a viable treatment option in a patient with prior auto-HCT, the cell products were often not available. Although prophylactic collection in high-risk candidates has been proposed as a potential mitigating strategy, the panel cautioned that the collection process may add to the already high logistic burden of CAR T-cell therapy (eg, coordination of apheresis slots and storage capacity), which could negatively impact vein-to-vein times in a state of high disease burden. Furthermore, the process could incur unnecessary collection- and storage-associated costs.100,101 Ultimately, it was concluded that further research is needed to assess the number needed to treat for prophylactic stem cell collection.
Allo-HCT
If the above options remain ineffective or elusive and grade 4 ICAHT persists beyond day +30, the expert panel recommends initiating a donor search for a potential allo-HCT as a last resort (ultima ratio). In such cases of life-threatening ICAHT, the benefit and risks of allo-HCT need to be carefully weighed and aligned with the patient’s goals of care. Furthermore, the possibility of spontaneous count recovery needs to be considered seriously.34,102,103 Accordingly, the expert panel suggested that the ultimate trigger for allo-HCT needs to be discussed on a case-by-case basis. Month 3 to 6 after CAR T-cell infusion was deemed a reasonable time frame to balance both the risk of infection and possibility of spontaneous count recovery. Once the decision for allo-HCT has been made, details regarding donor selection, conditioning regimens, and immunosuppression have to be discussed. Experience and evidence are very limited and only general considerations can be reviewed here. As for every allo-HCT, the same basic principles should apply, keeping in mind that the primary indication is severe and persistent cytopenia although essentially all patients currently receive commercially available CAR T cells to treat malignant lymphoid disorders. Most importantly, salvage allo-HCT can also provide tumor control through the conditioning regimen and graft-versus-tumor effects and current standard procedures will most likely lead to eradication of CAR T cells at the latest when full donor chimerism has been established. Therefore, remission status must be determined before allo-HCT and may guide the choice of conditioning regimen and the taper of immunosuppression. As usual, performance status, comorbidities, prior therapies, and expected antitumor activity should be carefully considered when discussing the transplantation modalities, donor choice, and selection.
Conclusions and outlook
Much progress has been made in the last years in defining hematological toxicity as a distinct toxicity entity of CAR T-cell therapy. Although the underlying pathophysiology remains incompletely understood, growing evidence points toward critical interactions between host hematopoiesis and CAR T-cell function and efficacy. By defining ICAHT and delineating a specific grading system, we herein provide a nomenclature that enables crosstrial comparisons and invites severity-based management strategies.
In this international consensus guidelines document, we have proposed a structured approach to diagnosis, grading/staging, and clinical management of ICAHT. This endeavor has also set the stage for areas of future development that will require collaboration between various European and non-European stakeholders involved in CAR T-cell therapy. Structured sample collection across multiple centers represents the basis for translational projects that delineate the underlying mechanisms of ICAHT by leveraging novel technologies such as multiomics and single-cell approaches. One area of particular interest lies in identifying early determinants of ICAHT by studying the peripheral blood immune contexture and/or the local BM microenvironment from pre–CAR T-cell samples. Furthermore, large retrospective real-world analyses may shed light on some of the differences in the clinical management of ICAHT that were identified by the EHA/EBMT survey. Residual questions relate to the optimal timing of G-CSF initiation as well as the optimal protocol to use (eg, prophylactic vs early G-CSF). The question of prophylactic collection of CD34+ hematopoietic cells in candidates at high risk and the optimal trigger time point for both HCB and allo-HCT represent unresolved issues that warrant further systematic study. Ultimately, prospective clinical trials will be needed that determine the potential benefits and evidence-base of treatment strategies that mitigate ICAHT.
Acknowledgment
The authors are grateful for the support by European Hematology Association and European Society for Blood and Marrow Transplantation, without which this work would not have been possible.
Authorship
Contribution: K.R. and M.S. conceptualized the study; K.R., M.S., M.A., E.B., A.B., P.B., B.B., R.B., M.G.C., C.C., F.C., P.C., J.D., R.D.B., R.G., R.H., G.I., U.J., M.J.K., S.M., A.N., F.O., Z.P., C.R., A.R., F.S.-G., I.S.-O., D.S., M.-L.S., J.A.S., C.T., M.T., P.L.Z., J.G.G., C.B., A.S., and I.Y.-A. performed the investigations; K.R., M.S., R.G., R.H., M.J.K., S.M., C.R., F.S.-G., D.S., M.-L.S., C.T., A.S., and I.Y.-A. performed formal analysis and visualization; K.R., M.S., I.S.-O., A.S., and I.Y.-A. developed the methodology; K.R. and M.S. wrote the original manuscript draft; K.R., M.S., M.A., E.B., A.B., P.B., B.B., R.B., M.G.C., C.C., F.C., P.C., J.D., R.D.B., R.G., R.H., G.I., U.J., M.J.K., S.M., A.N., F.O., Z.P., C.R., A.R., F.S.-G., I.S.-O., D.S., M.-L.S., J.A.S., C.T., M.T., P.L.Z., J.G.G., C.B., A.S., and I.Y.-A. wrote, reviewed, and edited the manuscript; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: K.R. reports research funding and travel support from Kite/Gilead; received honoraria from Novartis; consultants for and received honoraria from BMS/Celgene. M.S. receives industry research support from Amgen, BMS/Celgene, Kite/Gilead, Janssen, Miltenyi Biotec, Novartis, Roche, Seattle Genetics, and Takeda; serves as a consultant/adviser to AvenCell, CDR-Life, Ichnos Sciences, Incyte Biosciences, Janssen, Miltenyi Biotech, Molecular Partners, Novartis, Pfizer, and Takeda; and serves on the speakers’ bureau at Amgen, AstraZeneca, BMS/Celgene, Gilead, GSK, Janssen, Novartis, Pfizer, Roche, and Takeda. E.B. received honorarium from, and serves on the advisory board of, Kite/Gilead, BMS/Celgene, Novartis, Pfizer, Incyte, ADC Therapeutics, Roche, and Takeda; receives personal fees from Kite/Gilead, BMS/Celgene, Novartis, and Pfizer; and reports research funding from Amgen and BMS. A.B. serves on the speakers’ bureau of Novartis, Amgen, and Medac; and received meeting support from Novartis, Amgen, Medac, and Neovii. P.B. received honoraria and travel support from Allogene, Amgen, BMS, Kit/Gilead, Incyte, Jazz Pharmaceuticals, Miltenyi Biomedicine, Nektar, Novartis, and Pierre Fabre. R.B. received research funding from Servier and Allogene; and participated in advisory boards for BMS/Celgene, Janssen, Allogene, Takeda, Enara Bio, Securabio, Menarini Stemline, Arovella, and Kite/Gilead. M.C. reports consultancy for Novartis and Janssen. C.C. is a consultant adviser to Bellicum Pharmaceuticals, BMS, Kite/Gilead, Janssen Pharmaceuticals, and Novartis; serves on the speakers’ bureau of BMS, Kite/Gilead, Janssen Pharmaceuticals, Jazz, Miltenyi Biotech, and Novartis; and received travel support from BMS and Kite/Gilead. P.C. received honoraria from AbbVie, Amgen, Celgene, Daiichi Sankyo, Janssen, Kite/Gilead, Novartis, Roche, Sanofi, Servier, and Takeda (consulting, advisory role, or lecturer); and has had travel and accommodations paid by for-profit health care companies during the past 2 years (Novartis, Janssen, BMS/Celgene, Takeda, Kite/Gilead, and Roche). R.D. serves on the scientific advisory board of, and as a conference speaker for, Novartis; serves on the scientific advisory board of, as a conference speaker for, and has received travel accommodation from, Kite/Gilead; serves on the scientific advisory board of Janssen; is a conference speaker for Pfizer; serves on the scientific advisory board of BMS/Celgene; and is a conference speaker for AbbVie and Incyte. R.G. discloses speaking honoraria from Biotest, Pfizer, Medac, and Magenta. R.H. received honoraria from Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, and Roche; and serves in a consultancy role at Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte, and Miltenyi. G.I. received honoraria from BMS/Celgene, Novartis, Janssen, AstraZeneca, AbbVie, Miltenyi, and Roche. U.J. reports consultancy with Novartis, Janssen, and Roche; and having received honoraria from Gilead, BMS/Celgene, and Miltenyi. M.J.K. received honoraria from, and serves in a consulting/advisory role for, Bristol Myers Squibb, Celgene, Kite (a Gilead Company), Miltenyi Biotech, Novartis, and Roche; received research funding from Kite, Roche, Takeda, and Celgene; and received travel support from Kite, Miltenyi Biotech, Novartis, and Roche. S.M. serves, via his institution, on the speakers’ bureau of Celgene/BMS, Novartis, and Janssen; has received travel support from, and serves on the expert panels of, Gilead/Kite; serves on the data safety monitoring boards of Miltenyi and Immunicum/Mendes; is the founder of, and serves in a leadership role in, SWECARNET; and is the spouse of the founder of ScientifyResearch. F.O. declares having had travel, accommodation, and expenses paid for by Takeda, Kyowa Kirin International, and Medac. C.R. received honoraria from BMS, Kite/Gilead, Novartis, and Amgen. F.S.G. received research support from Novartis and Gilead; and honoraria from Novartis, Gilead, Pfizer, and BMS-Celgene. D.S. serves on the advisory board of Autolus. M.L.S. serves on the advisory boards of, and declares consultancy with, Novartis, Kite-Gilead, Takeda, and Janssen; and received honoraria from Kite/Gilead. J.S. received speaker fees from Gilead; and reports consultancy with Kiadis, Medac, and Vertex. C.T. serves on the board of Novartis, Kite/Gilead, BMS, Roche, Takeda, Incyte, and Beigene. M.T. serves on the advisory boards of KITE/Gilead, BMS, Janssen, Novartis, Roche, Incyte, and AstraZeneca; and received research funding from KITE/Gilead, Takeda, Incyte, Roche, and Regeneron. P.L.Z. reports consultancy for MSD, Eusapharma, and Novartis; serves on the advisory boards of Securia Bio, Celltrion, Kite/Gilead, Janssen, BMS/Celgene, Servier, Sandoz, MSD, AstraZeneca, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, ADC Therapeutics, Incyte, and Belgene; serves on the speakers’ bureau of Celltrion, Kite/Gilead, Janssen, BMS/Celgene, Servier, MSD, AstraZeneca, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, Incyte, and Belgene. J.G.G. received honoraria from Amgen, BMS Gilead/Kite, Janssen, and Novartis; and received research funding from AZ, BMS, and Janssen. C.B. is an inventor of different patents on cancer immunotherapy and genetic engineering; is a member of advisory boards of, and consultant for, Intellia, Kite/Gilead, Miltenyi, Kiadis, QuellTx, Janssen, Chroma, Genyo, Pancancer-T, and Alia; and received research support from Intellia Therapeutics. A.S. received honoraria from Takeda, Bristol Myers Squibb, Merck Sharp & Dohme, Celgene, Janssen, Sanofi, Roche, Novartis, Gilead Sciences, and Janssen-Cilag; reports a consulting or advisory role for Takeda, Bristol Myers Squibb, Gilead Sciences, Celgene, Janssen, and Novartis; serves on the speakers’ bureau of Takeda; received travel, accommodations, and expenses funds from Kite/Gilead. I.Y.-A. received honoraria from Novartis, Gilead/Kite, BMS, and Janssen. None of the mentioned conflicts of interest were related to financing or the content of this manuscript. The remaining authors declare no competing financial interests.
Correspondence: Marion Subklewe, Department of Medicine III–Hematology/Oncology, LMU–Klinikum der Universität München, Marchioninistr 15, 81377 Munich, Germany; e-mail: marion.subklewe@med.uni-muenchen.de; and Ibrahim Yakoub-Agha, UAM allogreffes de CSH, CHRU, F-59037 Lille CEDEX, France; e-mail: ibrahim.yakoubagha@chu-lille.fr.
References
Author notes
∗K.R. and M.S. contributed equally to this study.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal