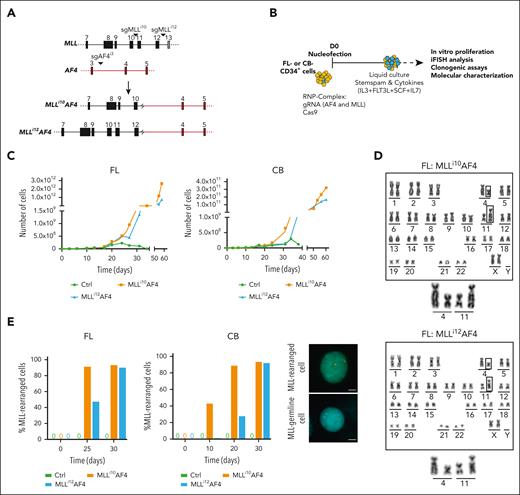
Abstract
Cellular ontogeny and MLL breakpoint site influence the capacity of MLL-edited CD34+ hematopoietic cells to initiate and recapitulate infant patients' features in pro–B-cell acute lymphoblastic leukemia (B-ALL). We provide key insights into the leukemogenic determinants of MLL-AF4+ infant B-ALL.
TO THE EDITOR:
Chromosomal translocations involving the mixed-lineage leukemia (MLL) gene drive leukemia development; key determinants of disease outcome are patient age, MLL fusion partner, and cell-of-origin.1 The MLL::AF4 (also known as KMT2A::AFF1) fusion protein (MA4) is present in 80% of infants with B-cell acute lymphoblastic leukemia (iB-ALLs) and is associated with prenatal origin and poor prognosis.1,2 In fact, the MA4 fusion occurs in early hematopoietic stem/progenitor cells (HSPCs) and is a causal oncogenic driver in iB-ALL.3,4 The prevalence of the MA4 fusion is higher in infant than in noninfant patients with B-ALL and is associated to higher frequency of refractoriness, relapse, and poor prognosis transcriptomic signatures.5 Molecularly, the distribution of genomic breakpoints within the MLL breakpoint cluster region (BCR) varies significantly, with a notable telomeric hot spot in infants and a preference for centromeric breakpoints in noninfants.6,7 Overall, these findings support the notion that age-related mechanisms and inherent biological differences contribute to MA4+ B-ALL. Studying these mechanisms is, however, challenging because of the dearth of human cellular models.8-12 CRISPR-Cas9 gene editing was recently used to recreate the t(4;11)/MLL::AF4 translocation in human HSPCs, mimicking the characteristics of the disease,13,14 but the impact of the cell-of-origin and its functional consequences were not compared nor was the potential to induce pro–B-ALL in vivo determined. Here, we compared the molecular impact and leukemogenic potential of both centromeric (intron 10 [MLLi10]) and telomeric (intron 12 [MLLi12]) MLL breakpoints in human CD34+ HSPCs from samples with different developmental stages, such as second trimester prenatal fetal liver (FL) and postnatal cord blood (CB).
We designed and tested several single-guide RNAs to target intron 10 (centromeric) or intron 12 (telomeric) of MLL, along with AF4 intron 3 (Figure 1A; supplemental Table 1, available on the Blood website). Nucleofected FL and CB cells were cultured with cytokines to promote MLL-edited cell expansion13 (Figure 1B). In contrast to mock cells, edited cells showed exponential growth starting on day 20 to 25 after nucleofection, resulting in billions of MA4-immortalized cells irrespective of their origin (Figure 1C; supplemental Figure 1A-B). Most cells in both cultures harbored MLL rearrangements (Figure 1D; supplemental Figure 1C), although the emergence of MLLi12-rearranged cultures was consistently slower than that of MLLi10-rearranged cultures (Figure 1E; supplemental Figure 1D). We confirmed the presence of the chromosomal translocation, the fusion gene RNA transcript (supplemental Figure 1D-E), and the chimeric protein (supplemental Figure 1F). Furthermore, we found that targeting either intronic regions equally immortalized in vitro both prenatal and neonatal CD34+ cells.
CRISPR-Cas9–induced t(4;11)/MA4 targeting either MLLi10 (Mi10A4) or MLLi12 (Mi12A4) in human FL and CB CD34+ HSPCs causes MA4-driven myeloid immortalization in vitro. (A) Scheme showing the location of the molecular regions targeted by single-guide MLLi10 (sgMLLi10) or sgMLLi12 and sgAF4i3 sgRNAs (top) and the resulting t(4;11)/MA chromosomal translocation (bottom). Filled light or dark gray/orange boxes depict exons and the lines between boxes depict introns. (B) Cartoon of the experimental design for in vitro studies. (C) Representative 60-day cell expansion of control and edited (Mi10A4 and Mi12A4) FL (left) and CB (right) CD34 myeloid progeny (CD45+CD34–CD19–CD33+). Two additional independent experiments for each cell of origin are shown in supplemental Figure 1A-B. (D) Representative G-banding karyotype confirming the presence of chromosomal translocation after 42 days of culture. Enlarged images of the translocated chromosomes are also shown. (E) MLL split-apart iFISH quantification of MLL-edited cells in control and edited cell cultures at the indicated time points, for FL (left) and CB (middle); right panels show representative images of MLLr and MLL germ line cells. The centromeric portion of the MLL gene breakpoint cluster region (bcr) is labeled in green, and the telomeric portion of the bcr is labeled in orange. Scale bars represent 10 μm.
CRISPR-Cas9–induced t(4;11)/MA4 targeting either MLLi10 (Mi10A4) or MLLi12 (Mi12A4) in human FL and CB CD34+ HSPCs causes MA4-driven myeloid immortalization in vitro. (A) Scheme showing the location of the molecular regions targeted by single-guide MLLi10 (sgMLLi10) or sgMLLi12 and sgAF4i3 sgRNAs (top) and the resulting t(4;11)/MA chromosomal translocation (bottom). Filled light or dark gray/orange boxes depict exons and the lines between boxes depict introns. (B) Cartoon of the experimental design for in vitro studies. (C) Representative 60-day cell expansion of control and edited (Mi10A4 and Mi12A4) FL (left) and CB (right) CD34 myeloid progeny (CD45+CD34–CD19–CD33+). Two additional independent experiments for each cell of origin are shown in supplemental Figure 1A-B. (D) Representative G-banding karyotype confirming the presence of chromosomal translocation after 42 days of culture. Enlarged images of the translocated chromosomes are also shown. (E) MLL split-apart iFISH quantification of MLL-edited cells in control and edited cell cultures at the indicated time points, for FL (left) and CB (middle); right panels show representative images of MLLr and MLL germ line cells. The centromeric portion of the MLL gene breakpoint cluster region (bcr) is labeled in green, and the telomeric portion of the bcr is labeled in orange. Scale bars represent 10 μm.
We confirmed similar MA4 expression levels between CRISPR-edited cells and patient-derived cells (supplemental Figure 2A). Transcriptome analysis revealed distinct clustering patterns of MLL-edited HSPCs according to the location of the MLL breakpoint (centromeric-Mi10A4 vs telomeric-Mi12A4) irrespective of the cellular ontogeny (FL or CB; supplemental Figure 2B). Specifically, the expression of HOX cluster genes, known to be involved in MA4-mediated pathogenesis,15,16 exhibited variation dependent on the MLL breakpoint location rather than the tissue of origin (Figure 2A). Notably, crucial HOX/MEIS genes, such as HOXA9, HOXA10, and MEIS1 showed significant upregulation in FL and CB Mi10A4-edited cells but not in Mi12A4-edited cells (supplemental Figure 2C). We performed chromatin immunoprecipitation sequencing analysis to understand how HOXA9, HOXA10, and MEIS1 expression is regulated in gene edited–derived cells, which revealed specific MA4 binding at the promoter and spreading into the gene bodies of HOXA9, HOXA10, and MEIS1 in Mi10A4-edited FL and CB CD34+ cells but not in Mi12A4-edited cells (Figure 2B). Notably, no significant differences were observed in other epigenetic marks across all edited cells. Collectively, these findings confirm the transcriptional regulatory capacity of Mi10A4, but not Mi12A4, in regulating the expression of HOXA9, HOXA10, and MEIS1 through direct and specific binding to their regulatory regions. This recapitulates the HOXAlow transcriptomic signature reported in infant patients.3,15,17
A centromeric location of the MLL breakpoint within the MLL BCR, but not the cellular ontogeny, determines the expression of HOX cluster/MEIS genes in MLL-edited CD34+ cells. (A) Heat map showing clustering of MLL-edited CD34+ HSPCs based on the location of the MLL breakpoint (Mi10A4 n = 6, blue vs Mi12A4 n = 6, green) irrespective of the cellular ontogeny (FL [n = 6, pink] vs CB [n = 6, yellow]) based on significant differentially expressed HOX cluster genes (false discovery rate < 0.05) among the 26 genes included in the HOX cluster. (B) Representative chromatin immunoprecipitation sequencing tracks at the MA4 target genes HOXA9/A10 and MEIS in FL (left) and CB (right) Mi10A4- and Mi12A4-edited cells. Binding of H3K79me3 and H3K27ac to HOXA9/A10 and MEIS was analyzed as a control. (C) Leukemia incidence in primary and secondary recipient mice transplanted with FL- and CB-derived Mi10A4- and Mi12A4-edited HSPCs. Primary mice, n = 57; secondary mice, n = 42. (D) Representative immunophenotype of a non-leukemic (green) and a leukemic (purple) mouse. Leukemic mice recapitulate many immunophenotypic features of primary MA4+ B-ALL, such as pro–B-lymphoid (CD10–)–biased engraftment (practically depleted of myeloid graft) coupled to the expression of the MLL-specific antigen NG2, and some degree of myeloid-lymphoid lineage infidelity/mixed phenotype (CD33+CD19+). Cells in gray are mouse cells. (E) Clinical data from the international MLL Recombinome Taskforce reveals a higher frequency of telomeric (i11-i12) MLL breakpoints among iB-ALLs (0-6 months old); n = 526 patients analyzed. (F) Rates of N/K-RAS mutations determined by the Oncomine childhood leukemia mutational panel in leukemic mice (n = 4 of 9; 44%) reproduce those reported in primary iB-ALLs. PCR, polymerase chain reaction; WT, wild-type.
A centromeric location of the MLL breakpoint within the MLL BCR, but not the cellular ontogeny, determines the expression of HOX cluster/MEIS genes in MLL-edited CD34+ cells. (A) Heat map showing clustering of MLL-edited CD34+ HSPCs based on the location of the MLL breakpoint (Mi10A4 n = 6, blue vs Mi12A4 n = 6, green) irrespective of the cellular ontogeny (FL [n = 6, pink] vs CB [n = 6, yellow]) based on significant differentially expressed HOX cluster genes (false discovery rate < 0.05) among the 26 genes included in the HOX cluster. (B) Representative chromatin immunoprecipitation sequencing tracks at the MA4 target genes HOXA9/A10 and MEIS in FL (left) and CB (right) Mi10A4- and Mi12A4-edited cells. Binding of H3K79me3 and H3K27ac to HOXA9/A10 and MEIS was analyzed as a control. (C) Leukemia incidence in primary and secondary recipient mice transplanted with FL- and CB-derived Mi10A4- and Mi12A4-edited HSPCs. Primary mice, n = 57; secondary mice, n = 42. (D) Representative immunophenotype of a non-leukemic (green) and a leukemic (purple) mouse. Leukemic mice recapitulate many immunophenotypic features of primary MA4+ B-ALL, such as pro–B-lymphoid (CD10–)–biased engraftment (practically depleted of myeloid graft) coupled to the expression of the MLL-specific antigen NG2, and some degree of myeloid-lymphoid lineage infidelity/mixed phenotype (CD33+CD19+). Cells in gray are mouse cells. (E) Clinical data from the international MLL Recombinome Taskforce reveals a higher frequency of telomeric (i11-i12) MLL breakpoints among iB-ALLs (0-6 months old); n = 526 patients analyzed. (F) Rates of N/K-RAS mutations determined by the Oncomine childhood leukemia mutational panel in leukemic mice (n = 4 of 9; 44%) reproduce those reported in primary iB-ALLs. PCR, polymerase chain reaction; WT, wild-type.
The MA4+-edited cells exhibited in vitro expansion with a CD45+CD33+CD34–CD19– myeloid phenotype (Figure 1). Therefore, we next examined the myeloid clonogenic potential of MLL-edited FL and CB CD34+ HSPCs. Both mock-targeted and edited cells showed similar primary colony-forming unit potential and sustained expression of MA4 (supplemental Figure 2D-E); however, only MLLi10-edited cells displayed clonogenic capacity upon serial replating, regardless of their cellular ontogeny (supplemental Figure 2D-E). Notably, these results align with clinical data,18 indicating a higher prevalence of acute myeloid leukemia in patients with a centromeric (42%) rather than a telomeric (30%) MLL breakpoint (supplemental Figure 2F).
We next investigated the impact of cellular ontogeny and the location of the MLL breakpoint on leukemia initiation in vivo by transplanting t(4;11)-CRISPR/Cas9–edited cells into NSG mice (supplemental Figure 2G). No significant bias in the overall engraftment potential was observed across the samples; however, marked differences were noted in the rates of engraftment driven by MA4-rearranged CD34+ cells (from 11% to 70%; Figure 2C). The frequency of Mi10A4+ and Mi12A4+ engraftment was higher when the translocation was generated in CB-CD34+ cells (16 of 28, 57%) than in FL-CD34+ cells (5 of 29; 18%). Furthermore, transplantation of these cells into secondary recipients resulted in human leukemic MA4+, NG2+, lymphoid-based (pro–B and CD19+CD10-–), and multiorgan (bone marrow, peripheral blood, spleen, and liver) grafts, except for Mi10A4 FL–derived cells, which failed to engraft after serial transplantation and consistently exhibited a nonleukemic phenotype (MA−, NG2−, and multilineage; Figure 2D). Of note, clinicobiological data from patients with MLL-rearranged (MLLr) B-ALL studied within the MLL recombinome consortium revealed a lower incidence (30% vs 49%) of centromeric breakpoints (i9-i10) in the MLL BCR among infant patients (<6 months) than among pediatric patients (>6 months)18 (Figure 2E). Collectively, our results show that genome-edited t(4;11)/MA4+ in human FL and CB CD34+ cells are sufficient to induce a transplantable MA4+ pro-B-ALL that recapitulates key phenotypic features of MLLr iB-ALL, but the leukemogenic potential of MA4+ CD34+ cells appear to be influenced by the cellular ontogeny and the MLL breakpoint location within the MLL BCR.
We finally characterized the pro–B-ALL generated from Mi10A4+, Mi12A4+ CB–derived cells, and Mi12A4+ FL–derived cells in secondary mice. Recurrent mutations in K- and N-RAS were found, phenocopying those found in patients with primary iB-ALL (n = 4 of 9, 44%, Figure 2F),3,4 and confirming the leukemogenic potential of t(4;11)/MA4+ (Figure 2F). Differential methylation analysis revealed thousands of hyper and hypomethylated sites (supplemental Figure 3A), and enrichment analyses of transcription factor–binding sites and chromatin states indicated similarities in DNA methylation and chromatin patterns between leukemias from edited cells and primary MLLr iB-ALL (supplemental Figure 3B-C). Transcriptome and methylome analyses revealed that the pro–B-ALL from edited cells clustered with iB-ALL primary MLLr from patients (supplemental Figure 3D-E). Overall, our study provides evidence that pro–B-ALL generated in vivo from MA4-edited CB- or FL-derived CD34+ cells recapitulate key molecular features of iB-ALL MLLr.
Our findings demonstrate that the origin of CD34+ cells does not affect the efficiency of t(4;11)/MA generation. Our human genome editing t(4;11)/MA4+ B-ALL model recapitulates the HOXAlow and HOXAhi transcriptomic signatures reported in patients with t(4;11) B-ALL with telomeric-biased (Mi12A4+) and centromeric-biased (Mi10A4+) MLL breakpoint hotspots, respectively, regardless of the source (prenatal or neonatal) of the HSPCs. We confirmed that Mi10A4, but not Mi12A4, directly and specifically regulates the expression of HOXA9, HOXA10, and MEIS1 by binding to their regulatory regions. Although our ex vivo model failed to replicate the upregulation of other previously reported genes,19 it establishes a link between HOXA cluster gene expression and intronic distribution of MLL breakpoints in patients with t(4;11)/MA4+.
Our model also recapitulates the presence of recurrent mutations exclusively in K- and N-RAS, phenocopying those of patients with primary iB-ALL,3,4 and confirming that t(4;11)/MA4+ is a leukemogenic driver. It is worth noting that targeting the MLL BCR of the centromeric region enhances in vitro myeloid clonogenic replating in MLL-edited CD34+ HSPCs regardless of their cellular origin. These findings align with clinical and biological data,18 and reveal an increased prevalence of acute myeloid leukemia among patients with MLL-r with a centromeric MLL breakpoint. However, all leukemias initiated in mice that received transplantation with either Mi10A4-edited or Mi12A4-edited CD34+ cells were pro–B-ALL, suggesting that the genomic localization of MLL breakpoint influences the resulting leukemia phenotype in cooperation with in vivo–occurring interactions in specific bone marrow niches,20 secondary oncogenic hits,21 and the nature of the cell-of-origin.1 There remain some differences between human leukemias and engineered cells that warrant further exploration.
Human samples were accessed and processed following the institutional guidelines approved by our local institutional review board (HCB/2013/8648).
Acknowledgments
The authors thank the Medical Research Council/Wellcome Trust Human Developmental Biology Resource for providing institutional review board–approved fetal material. The authors thank Centres de Recerca de Catalunya/Generalitat de Catalunya and Fundació Josep Carreras-Obra Social la Caixa for core support.
Financial support for this work was obtained from the Spanish Ministry of Economy and Competitiveness (PID2022-142966OB-100ID2019), Heroes hasta la médula initiative and Instituto de Salud Carlos III (ISCIII)–Redes de Investigación Cooperativa Orientadas a Resultados en Salud within the Next Generation EU program (plan de recuperación, transformación y resiliencia) (P.M.) and the Health Institute Carlos III (ISCIII/Federacion Española de Enfermedades Raras) PI20/00822 (C.B.), PI20/01837 (S.R.-P.) and PI21/01641 (R.T.-R.). Asociación Española Contra el Cancer (PRYGN211192BUEN) (C.B.), AECC-LABAE20049RODR (S.R.-P.), and the Fundación Uno entre Cienmil (C.B.). R.T.-R. and O.M. were supported by investigator fellowships from the Spanish Association of Cancer Research (INVES211226MOLI). R.M. is supported by grants from the Deutsche Forschungsgemeinschaft (Ma 1876/12-1) and Wilhelm Sander foundation (2018.070.2).
Authorship
Contribution: C.B., R.T.-R., T.V.-H., O.M., P.P., A.M., V.R., M.V., S.C., N.V.-G., S.R.-P., J.C.S., O.Q.-B., A.L.S., and J.R.T. performed experiments and interpreted data; O.W., M.F.F., A.R., C.M., R.M., T.A.M., and P.M. supervised research and contributed key knowledge, techniques, and reagents; C.B., R.T.-R., and P.M. conceived the study and funded the research; and all authors have read and agreed to publish the manuscript.
Conflict-of-interest disclosure: P.M. is the founder of the spin-off OneChain Immunotherapeutics, which has no connection with the present research. T.A.M. is a paid consultant for and shareholder in Dark Blue Therapeutics Ltd, a company that has no connection to the results in this paper. The remaining authors declare no competing financial interests.
Correspondence: Clara Bueno, Department of Hematology, School of Medicine Barcelona University, Carrer Casanova 143, 08036 Barcelona, Spain; e-mail: cbueno@carrerasresearch.org; and Pablo Menéndez, Department of Hematology, School of Medicine Barcelona University, Carrer Casanova 143, 08036 Barcelona, Spain; e-mail: pmenendez@carrerasresearch.org.
References
Author notes
∗C.B. and R.T.-R. are joint first authors and contributed equally to this work.
†T.V.-H., O.M., and P.P. are joint second authors and contributed equally to this work.
Reagents/protocols are available to other investigators on request from the corresponding authors, Clara Bueno (cbueno@carrerasresearch.org) and Pablo Menéndez (pmenendez@carrerasresearch.org).
The online version of this article contains a data supplement.

![A centromeric location of the MLL breakpoint within the MLL BCR, but not the cellular ontogeny, determines the expression of HOX cluster/MEIS genes in MLL-edited CD34+ cells. (A) Heat map showing clustering of MLL-edited CD34+ HSPCs based on the location of the MLL breakpoint (Mi10A4 n = 6, blue vs Mi12A4 n = 6, green) irrespective of the cellular ontogeny (FL [n = 6, pink] vs CB [n = 6, yellow]) based on significant differentially expressed HOX cluster genes (false discovery rate < 0.05) among the 26 genes included in the HOX cluster. (B) Representative chromatin immunoprecipitation sequencing tracks at the MA4 target genes HOXA9/A10 and MEIS in FL (left) and CB (right) Mi10A4- and Mi12A4-edited cells. Binding of H3K79me3 and H3K27ac to HOXA9/A10 and MEIS was analyzed as a control. (C) Leukemia incidence in primary and secondary recipient mice transplanted with FL- and CB-derived Mi10A4- and Mi12A4-edited HSPCs. Primary mice, n = 57; secondary mice, n = 42. (D) Representative immunophenotype of a non-leukemic (green) and a leukemic (purple) mouse. Leukemic mice recapitulate many immunophenotypic features of primary MA4+ B-ALL, such as pro–B-lymphoid (CD10–)–biased engraftment (practically depleted of myeloid graft) coupled to the expression of the MLL-specific antigen NG2, and some degree of myeloid-lymphoid lineage infidelity/mixed phenotype (CD33+CD19+). Cells in gray are mouse cells. (E) Clinical data from the international MLL Recombinome Taskforce reveals a higher frequency of telomeric (i11-i12) MLL breakpoints among iB-ALLs (0-6 months old); n = 526 patients analyzed. (F) Rates of N/K-RAS mutations determined by the Oncomine childhood leukemia mutational panel in leukemic mice (n = 4 of 9; 44%) reproduce those reported in primary iB-ALLs. PCR, polymerase chain reaction; WT, wild-type.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/20/10.1182_blood.2023020858/2/m_blood_bld-2023-020858-gr2.jpeg?Expires=1769086507&Signature=fUE2Rp6JDcp8aiEmXO-ZZGa00cLqrHvDYtLM9vhaPP3Y9kEXFfBQdIiZZWel4~LK3N9jRaXaVhhDf2Q07MCqekdBAvD~OtqF62s-VM84OcTNsxRadGGIE21cFBon4RXrnp6FopYkhzRuNqtUQUF6E5A~1Z3iNyRIhrSjxjOlqEmNIyQKOikh29tYgfAiPMrlPm6qLA2K~CJkq8XUeZWIRPtgPP7qJwCaIQkwFHEfdMLaa-KMpCQV~ou6kB0pT-z8AiKIyGCFdVHWvM5ahk0cw~rTmUxWREGQW2C-iTGwKsVfq2hXIN1gjPDtualIPil4DXyLEDnDT2MqjSoQVK~pnQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal