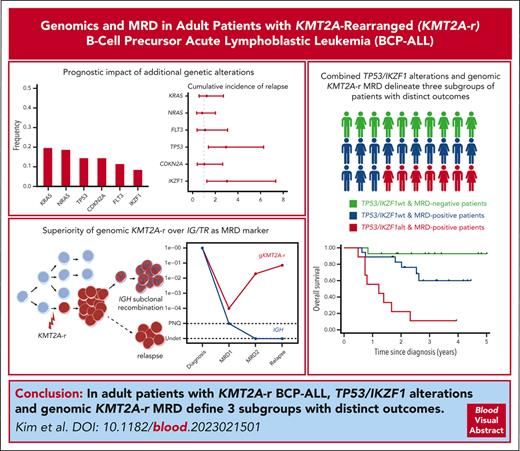
Key Points
In adult KMT2A-r BCP-ALL, TP53 and IKZF1 alterations are associated with very poor outcome.
KMT2A genomic fusion should be the preferred MRD marker over IG/TR to assess early treatment response and predict long-term outcome.
Abstract
KMT2A-rearranged (KMT2A-r) B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is widely recognized as a high-risk leukemia in both children and adults. However, there is a paucity of data on adults treated in recent protocols, and the optimal treatment strategy for these patients is still a matter of debate. In this study, we set out to refine the prognosis of adult KMT2A-r BCP-ALL treated with modern chemotherapy regimen and investigate the prognostic impact of comutations and minimal residual disease (MRD). Of 1091 adult patients with Philadelphia-negative BCP-ALL enrolled in 3 consecutive trials from the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL), 141 (12.9%) had KMT2A-r, with 5-year cumulative incidence of relapse (CIR) and overall survival (OS) rates of 40.7% and 53.3%, respectively. Molecular profiling highlighted a low mutational burden in this subtype, reminiscent of infant BCP-ALL. However, the presence of TP53 and/or IKZF1 alterations defined a subset of patients with significantly poorer CIR (69.3% vs 36.2%; P = .001) and OS (28.1% vs 60.7%; P = .006) rates. Next, we analyzed the prognostic implication of MRD measured after induction and first consolidation, using both immunoglobulin (IG) or T-cell receptor (TR) gene rearrangements and KMT2A genomic fusion as markers. In approximately one-third of patients, IG/TR rearrangements were absent or displayed clonal evolution during the disease course, compromising MRD monitoring. In contrast, KMT2A-based MRD was highly reliable and strongly associated with outcome, with early good responders having an excellent outcome (3-year CIR, 7.1%; OS, 92.9%). Altogether, our study reveals striking heterogeneity in outcomes within adults with KMT2A-r BCP-ALL and provides new biomarkers to guide risk-based therapeutic stratification.
Introduction
B-cell precursor acute lymphoblastic leukemia (BCP-ALL) harboring a rearrangement of KMT2A (KMT2A-r), formerly MLL, represents a distinct subtype accounting for 75% of infant, 2% to 5% of childhood noninfant, and 5% to 10% of adult BCP-ALL.1-5 Historically, all these patients have experienced poor outcomes, leading to the inclusion of KMT2A-r as a high-risk criterion in all risk-adapted treatment protocols.6-9 The high prevalence and poor prognosis of KMT2A-r within BCP-ALL in infants has generated considerable efforts to improve the clinical management of these patients.10-12 By contrast, there is a paucity of studies specifically addressing this subgroup in adults, and the optimal treatment strategy for these patients is still a matter of debate.6-9 Similarly, the biology of KMT2A-r BCP-ALL has been extensively studied as an infant leukemia.13-19 In this context, it was proposed that specific properties of the cell-of-origin at the early developmental stage may confer prominent susceptibility to leukemogenesis induced by KMT2A-r.14,20 Moreover, the landscape of genomic alterations in infant KMT2A-r leukemia has been shown to be exceptionally limited, in agreement with a 1-hit model of oncogenesis.19,21,22 Notably, the presence of additional genetic alterations and their possible role in leukemogenesis and prognosis have not been investigated in adult KMT2A-r BCP-ALL.
Over the past 2 decades, the use of more intensive regimens in young adults has yielded significant improvements in outcomes, challenging the conventional risk stratification criteria.23-28 Like previously in children, the assessment of minimal residual disease (MRD) at early stages of treatment has allowed for the refinement of risk groups within adult BCP-ALL.24,25,28-30 Interestingly, studies in childhood BCP-ALL using large data sets have demonstrated that distinct genetic subtypes are typically associated with different response kinetics, suggesting that MRD results should be interpreted in the context of subtype-specific patterns.31 Although MRD has proven to have prognostic value within infant KMT2A-r BCP-ALL,32,33 no study has investigated the significance of MRD in the setting of adult KMT2A-r BCP-ALL, likely owing to limited patient numbers in each study. Thus, KMT2A-r BCP-ALL is usually regarded as a high-risk leukemia regardless of other risk criteria.
Considering the current outcomes of BCP-ALL in young adults, we hypothesized that additional criteria may allow for refinement of the prognosis of KMT2A-r BCP-ALL. In this study, we conducted a comprehensive analysis of a large cohort of patients treated in 3 consecutive trials from the Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL). Through analyses of genetic comutations and MRD, we could identify distinct subsets of patients having markedly heterogeneous clinical outcomes. Overall, our study highlights the significant heterogeneity in clinical outcomes within this population and provides criteria for further treatment stratification.
Patients and methods
Patients and treatment
Between 2003 and 2020, a total of 1163 patients aged between 15 and 59 years with newly diagnosed Philadelphia-negative (Ph–) BCP-ALL were treated in the GRAALL-2003 (n = 149), -2005 (n = 525), and -2014 (n = 489) trials (clinicaltrials.gov numbers: NCT00222027, NCT00327678, and NCT02617004, respectively).23,26 This study included 1091 of these patients with a known status for 11q23/KMT2A rearrangement, of whom 141 (12.9%) tested positive. All patients or their relatives provided informed consent according to the Declaration of Helsinki, and the study was approved by the local ethics committee. The 3 GRAALL protocols used similar intensive pediatric-inspired chemotherapy regimens. In the GRAALL-2003 and -2005 protocols, all patients with 11q23/KMT2A rearrangement were eligible to allogeneic hematopoietic stem cell transplantation (HSCT). In the GRAALL-2014, chemotherapy intensity was reduced in patients aged ≥45 years, and HSCT indication was restricted to patients with suboptimal early MRD response, regardless of 11q23/KMT2A status. In addition, a nested phase 2 substudy introduced blinatumomab in consolidation for patients who were at higher risk, including those with a KMT2A-r BCP-ALL (n = 16 patients).
Genetic characterization
The presence of a 11q23/KMT2A rearrangement was determined at the diagnosis in local centers through cytogenetic analysis, including karyotype and KMT2A break-apart fluorescence in situ hybridization. For 97 KMT2A-r cases with available material (n = 7 of 21, 34 of 64, and 56 of 56 patients in GRAALL-2003, -2005, and -2014 trials, respectively), molecular analyses were performed centrally. Briefly, mononuclear cells were isolated from bone marrow or blood specimens through Ficoll centrifugation, quantitation of leukemia cells percentage was performed by flow cytometry, and extractions of DNA and RNA were performed using standard procedures. Targeted screening for recurrent KMT2A fusion transcripts was conducted using reverse transcription quantitative polymerase chain reaction (qPCR) or reverse transcription mixed ligation probe assay.34 Screening for additional alterations was performed through targeted DNA sequencing of a custom panel consisting of 189 known or putative target genes in BCP-ALL, as previously described.35 Data processing and visualization were performed using R software version 4.2.3 and ggplot2 version 3.4.1.
MRD assessment
MRD evaluation was performed in the national reference laboratories using qPCR of clono-specific immunoglobulin or T cell receptor (IG/TR) genes rearrangements, according to EuroMRD guidelines.36 For KMT2A-r BCP-ALL treated in the GRAALL-2014 trial, the KMT2A breakpoint genomic sequence was retrospectively assessed as an additional target for MRD evaluation.32 The cutoff for MRD positivity was set at 10–4.
Statistical analysis
Complete remission (CR) was defined by <5% blasts in the bone marrow. Disease-free survival (DFS) was calculated as the time from the first CR to relapse or death in CR and censored at the last follow-up for patients without events. Overall survival (OS) was defined as the time from diagnosis to death. Kaplan-Meier estimators were used for DFS and OS. Cumulative incidence of relapse (CIR) was defined as the time from CR to first relapse considering death in CR as a competing event. A cause-specific cumulative incidence function was used to estimate CIR. For OS and DFS, univariate analyses of covariate effects were performed using a Cox model. The Fisher exact and Mann-Whitney tests were used for clinical, biological, and mutational analyses. The role of HSCT in KMT2A-r BCP-ALL was evaluated using 4-month landmark intention-to-treat analyses for CIR, DFS, and OS from CR, excluding patients who died or relapsed before the first 4 months after CR achievement. Statistical analyses were performed with STATA/SE software (version 17.0, StataCorp LLC, College Station, TX). All tests were 2-sided, and P < .05 was considered statistically significant.
Results
Baseline characteristics and early response to treatment of KMT2A-r BCP-ALL
The baseline characteristics of the 141 patients with KMT2A-r BCP-ALL compared with those of patients with other Ph-negative BCP-ALL are presented in Table 1. Patients with KMT2A-r BCP-ALL were significantly older (median age, 42.0 vs 36.8 years; P = .02) and had a higher proportion of females (male-to-female ratio, 0.78 vs 1.3; P = .005). They also presented significantly higher white blood cell (WBC) counts at diagnosis (median, 94.4 vs 6.3 x 109/L; P < .0001) and predominantly a CD10– pro-B immunophenotype (74.8% vs 14.3%; P < .0001). The most common KMT2A rearrangement was the translocation t(4;11)(q21;q23), resulting in the KMT2A::AFF1 fusion, identified in 121 out of 141 (86%) patients, whereas the t(11;19)(q23;p13.3)/KMT2A::MLLT1 fusion was identified in 12 patients (8.5%). The only significant difference in presenting features between those with KMT2A::AFF1 and non-AFF1 KMT2A-r BCP-ALL was a lower WBC count in the latter (median, 116 vs 14.7 x 109/L; P = .0002). Early response to corticosteroids, as evaluated based on peripheral blood blast clearance on day 8 of the prephase, was similar between those with KMT2A-r and other BCP-ALL. Surprisingly, patients with KMT2A-r BCP-ALL had a lower rate of poor bone marrow blast clearance on day 15 (26.6 vs 42.0%; P = .001). No difference was observed between the 2 groups regarding the CR rate, and only 2 out of 131 patients with KMT2A-r (1.5%) required a second induction phase to achieve CR, as compared with 3.9% of the others. Therefore, although patients with KMT2A-r display baseline features historically associated with higher risk (ie, older age, higher WBC, and pro-B phenotype), they do not show evidence for early treatment resistance.
Characteristics, treatment response, and outcomes of the study cohort
| . | All patients with Ph– BCP-ALL (n = 1091) . | Patients with KMT2A-r BCP-ALL (n = 141) . | ||||
|---|---|---|---|---|---|---|
| KMT2A-r BCP-ALL . | Other BCP-ALL . | P value . | KMT2A::AFF1 . | Other KMT2A-r . | P value . | |
| Number of patients (%) | 141 (12.9) | 950 (87.1) | 121 (85.8) | 20 (14.2) | ||
| Patient-related characteristics | ||||||
| Median age, y (range) | 42.0 (18.4-59.4) | 36.8 (15.2-59.9) | .02 | 41.7 (18.4-59.4) | 46.2 (21.1-58) | .24 |
| Sex, M:F (ratio) | 62:79 (0.78) | 542:408 (1.3) | .005 | 53:68 (0.8) | 9:11 (0.8) | .99 |
| Disease-related characteristics | ||||||
| Median WBC, x 109/L (range) | 94.4 (1-712) | 6.3 (0-396) | <.0001 | 116 (2-712) | 14.7 (1-216) | .0002 |
| CNS involvement (%) | 10/140 (7.1) | 60/944 (6.4) | .71 | 8/120 (6.7) | 2/20 (10.0) | .64 |
| EGIL classification | 119 (100) | 805 (100) | <.0001 | 103 (100) | 16 (100) | .53 |
| Pro-B (I) | 89 (74.8) | 115 (14.3) | 78 (75.7) | 11 (68.8) | ||
| Common (II) | 4 (3.4) | 538 (66.9) | 3 (2.9) | 1 (6.2) | ||
| Pre-B (III) | 25 (21.0) | 138 (17.1) | 21 (20.4) | 4 (25.0) | ||
| Mature (IV) | 1 (0.8) | 14 (1.7) | 1 (1.0) | 0 (0.0) | ||
| Response-related characteristics | ||||||
| Poor early PB blast clearance | 22/141 (15.6) | 151/945 (16.0) | .99 | 19/121 (15.7) | 3/20 (15.0) | .99 |
| Poor early BM blast clearance | 34/128 (26.6) | 376/895 (42.0) | .001 | 31/110 (28.2) | 3/18 (16.7) | .40 |
| Late CR (achieved after induction 2) | 2/131 (1.5) | 34/875 (3.9) | .22 | 1/112 (0.89) | 1/19 (5.3) | .27 |
| CR (after induction 1 or 2) | 131/141 (92.9) | 875/950 (92.1) | .87 | 112/121 (92.6) | 19/20 (95.0) | .99 |
| Postremission treatment | ||||||
| HSCT in CR1 | 64/131 (48.9) | 265/875 (30.3) | <.0001 | 57/112 (50.9) | 7/19 (36.8) | .32 |
| Postremission outcome | ||||||
| 5-y CIR, % (95% CI) | 40.7 (32.7-49.9) | 33.7 (30.3-37.2) | .02 | 43.0 (34.2-52.9) | 27.6 (12.4-54.5) | .18 |
| 5-y DFS, % (95% CI) | 50.3 (41.1-58.8) | 55.5 (51.7-59.0) | .07 | 49.3 (39.4-58.5) | 56.5 (31.2-75.5) | .36 |
| 5-y OS, % (95% CI) | 53.3 (44.5-61.4) | 59.9 (56.2-63.3) | .02 | 51.4 (41.9-60.2) | 64.6 (39.7-81.3) | .21 |
| . | All patients with Ph– BCP-ALL (n = 1091) . | Patients with KMT2A-r BCP-ALL (n = 141) . | ||||
|---|---|---|---|---|---|---|
| KMT2A-r BCP-ALL . | Other BCP-ALL . | P value . | KMT2A::AFF1 . | Other KMT2A-r . | P value . | |
| Number of patients (%) | 141 (12.9) | 950 (87.1) | 121 (85.8) | 20 (14.2) | ||
| Patient-related characteristics | ||||||
| Median age, y (range) | 42.0 (18.4-59.4) | 36.8 (15.2-59.9) | .02 | 41.7 (18.4-59.4) | 46.2 (21.1-58) | .24 |
| Sex, M:F (ratio) | 62:79 (0.78) | 542:408 (1.3) | .005 | 53:68 (0.8) | 9:11 (0.8) | .99 |
| Disease-related characteristics | ||||||
| Median WBC, x 109/L (range) | 94.4 (1-712) | 6.3 (0-396) | <.0001 | 116 (2-712) | 14.7 (1-216) | .0002 |
| CNS involvement (%) | 10/140 (7.1) | 60/944 (6.4) | .71 | 8/120 (6.7) | 2/20 (10.0) | .64 |
| EGIL classification | 119 (100) | 805 (100) | <.0001 | 103 (100) | 16 (100) | .53 |
| Pro-B (I) | 89 (74.8) | 115 (14.3) | 78 (75.7) | 11 (68.8) | ||
| Common (II) | 4 (3.4) | 538 (66.9) | 3 (2.9) | 1 (6.2) | ||
| Pre-B (III) | 25 (21.0) | 138 (17.1) | 21 (20.4) | 4 (25.0) | ||
| Mature (IV) | 1 (0.8) | 14 (1.7) | 1 (1.0) | 0 (0.0) | ||
| Response-related characteristics | ||||||
| Poor early PB blast clearance | 22/141 (15.6) | 151/945 (16.0) | .99 | 19/121 (15.7) | 3/20 (15.0) | .99 |
| Poor early BM blast clearance | 34/128 (26.6) | 376/895 (42.0) | .001 | 31/110 (28.2) | 3/18 (16.7) | .40 |
| Late CR (achieved after induction 2) | 2/131 (1.5) | 34/875 (3.9) | .22 | 1/112 (0.89) | 1/19 (5.3) | .27 |
| CR (after induction 1 or 2) | 131/141 (92.9) | 875/950 (92.1) | .87 | 112/121 (92.6) | 19/20 (95.0) | .99 |
| Postremission treatment | ||||||
| HSCT in CR1 | 64/131 (48.9) | 265/875 (30.3) | <.0001 | 57/112 (50.9) | 7/19 (36.8) | .32 |
| Postremission outcome | ||||||
| 5-y CIR, % (95% CI) | 40.7 (32.7-49.9) | 33.7 (30.3-37.2) | .02 | 43.0 (34.2-52.9) | 27.6 (12.4-54.5) | .18 |
| 5-y DFS, % (95% CI) | 50.3 (41.1-58.8) | 55.5 (51.7-59.0) | .07 | 49.3 (39.4-58.5) | 56.5 (31.2-75.5) | .36 |
| 5-y OS, % (95% CI) | 53.3 (44.5-61.4) | 59.9 (56.2-63.3) | .02 | 51.4 (41.9-60.2) | 64.6 (39.7-81.3) | .21 |
Poor early PB blast clearance at day 8 is defined by ≥1x 109/L blast. Poor early BM blast clearance at day 15 is defined by ≥5% blasts.
BM, bone marrow; CNS, central nervous system; EGIL, European Group for the Immunological Characterization of Leukemias; F, female; M, male; PB, peripheral blood.
Outcome according to baseline and early response risk criteria
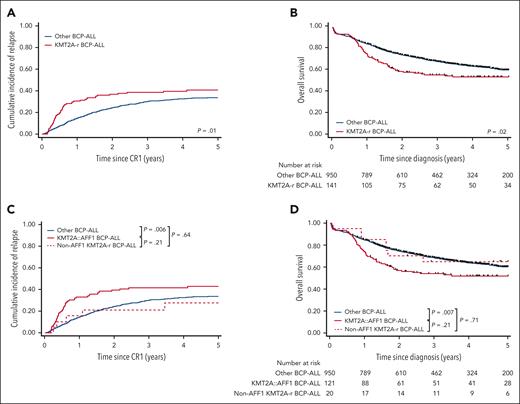
The median follow-up was 4.3 years. The 5-year CIR, DFS, and OS rates for patients with KMT2A-r were 40.7% (95% confidence interval [CI], 32.7-49.9), 50.3% (95% CI, 41.1-58.8), and 53.3% (95% CI, 44.5-61.4), as compared with 33.7% (95% CI, 30.3-37.2; P = .02), 55.5% (95% CI, 51.7-59.0; P = .07), and 59.9% (95% CI, 56.2-63.3; P = .02), respectively, for other patients (Table 1; Figure 1A-B). A higher percentage of patients undergoing HSCT in first remission was observed in patients with KMT2A-r BCP-ALL (48.9% vs 30.3%; P < .0001). With a median time from CR to HSCT of 3.8 months (95% CI, 3.2-4.2) for these patients, we performed a 4-month landmark intention-to-transplant analysis to evaluate the impact of HSCT on the first CR. We did not find evidence for an improvement in the CIR, DFS, or OS (supplemental Table 1, available on the Blood website). These intermediate survival rates of patients with KMT2A-r prompted us to search for prognostic criteria within the KMT2A-r group. Strikingly, age, sex, central nervous system infiltration, pro-B phenotype, and WBC (either as continuous or with different cutoff values) were not found to be associated with outcomes (supplemental Table 1). No significant impact of the KMT2A-r partner gene, whether AFF1 or non-AFF1 genes could be demonstrated (Figure 1C-D), although this may be related to the low number of non-AFF1 cases. Finally, poor responses to corticosteroids on day 8 or to chemotherapy on day 15 did not serve as predictors for poorer survival. Therefore, historical risk criteria could not predict distinct clinical outcomes within KMT2A-r BCP-ALL.
Outcomes of all adult patients with Ph– BCP-ALL. CIR (A) and OS (B) in adult patients with Ph– BCP-ALL according to KMT2A-rearrangement status, and CIR (C) and OS (D) according to KMT2A gene fusion partners, either AFF1 or others.
Outcomes of all adult patients with Ph– BCP-ALL. CIR (A) and OS (B) in adult patients with Ph– BCP-ALL according to KMT2A-rearrangement status, and CIR (C) and OS (D) according to KMT2A gene fusion partners, either AFF1 or others.
Landscape of additional genetic alterations
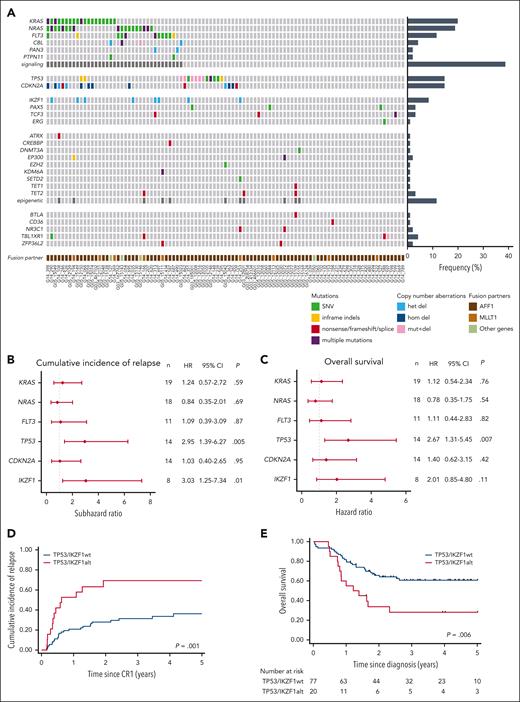
We then characterized the spectrum of additional genetic alterations in patients with KMT2A-r BCP-ALL, through targeted DNA sequencing of a comprehensive panel of genes (Figure 2A; complete list of mutations in supplemental Table 2). Overall, after excluding KMT2A partial gains or losses directly resulting from 11q23 unbalanced rearrangements, 32 patients (33%) had no detectable alterations, 30 (31%) had a single altered gene, 18 (19%) had 2 altered genes, and 17 (17%) had ≥3 altered genes. The most frequent genetic alterations were activating mutations in the NRAS and KRAS genes, detected in 20% and 19% of patients, respectively. Mutations in the FLT3 gene were observed in 11 (11%) patients, encompassing internal tandem duplications and point mutations in the tyrosine kinase and juxta-membrane domains. Collectively, mutations affecting the receptor tyrosine kinase (RTK)-RAS signaling pathways were present in 37 (38%) of these patients. Notably, multiple mutations in signaling factors were often identified within the same case, with low variant allele frequencies (median, 6%; range, 2%-47%) contrasting with high leukemic infiltration (median, 89%), indicating subclonality. TP53 mutations were detected in 14 (14%) of patients, with half of them also exhibiting copy-number losses related to 17p abnormalities. Only 1 patient displayed a high variant allele frequency consistent with TP53 biallelic alteration in the major clone, whereas in most cases, alterations were likely subclonal. CDKN2A alterations were found in 14 patients (14%), including biallelic deletions (n = 8), monoallelic deletions (n = 3), and truncating mutations (n = 3). IKZF1 deletions were observed in 8 patients (8%), all of which were related to gross abnormalities of chromosome 7, predominantly isochromosome 7q. No PAX5 deletions were found, but a unique PAX5 mutation (p.S133R) was identified in 3 patients. It is noteworthy that no KMT2A-r cases met the definition of IKZF1plus ALL.37 Recurrent mutations were also identified in TBL1XR1 (n = 4 cases), TCF3 (n = 3 cases), and TET2 (n = 3 cases). No other gene alterations were found in >2 cases. Alterations affecting factors involved in epigenetic modifications were collectively observed in 11 patients (11%). To summarize, adult KMT2A-r BCP-ALL exhibit a specific pattern of additional genetic alterations being infrequent and subclonal, and primarily targeting the RTK-RAS pathway and the TP53, CDKN2A, and IKZF1 tumor-suppressor genes.
Landscape and prognostic significance of additional genetic alterations in adult KMT2A-r BCP-ALL. (A) Heatmap of recurrent gene alterations, including mutations and deletions, detected by targeted sequencing. (B-C) Forest plots showing the HRs of CIR (B) and OS (C), estimated by univariable Cox proportional-hazards model, for genes found altered in >5% of cases. (D-E) CIR (D) and OS (E) rates in patients according to TP53 and IKZF1 alteration status.
Landscape and prognostic significance of additional genetic alterations in adult KMT2A-r BCP-ALL. (A) Heatmap of recurrent gene alterations, including mutations and deletions, detected by targeted sequencing. (B-C) Forest plots showing the HRs of CIR (B) and OS (C), estimated by univariable Cox proportional-hazards model, for genes found altered in >5% of cases. (D-E) CIR (D) and OS (E) rates in patients according to TP53 and IKZF1 alteration status.
Prognosis of additional genetic alterations
Next, we assessed the impact of recurrent alterations on CIR, DFS, and OS (Figure 2B-C; supplemental Table 3). Mutations in RTK-RAS pathway genes and CDKN2A deletions did not show any significant association with clinical outcomes. In contrast, TP53 alterations were associated with significantly worse CIR (subhazard ratio [SHR], 2.95; 95% CI, 1.39-6.27; P = .005), DFS (hazard ratio [HR], 2.20; 95% CI, 1.06-4.58; P = .034) and OS (HR, 2.67; 95% CI, 1.31-5.45; P = .007) rates (Figure 2B-C; supplemental Figure 1). Of the 14 patients with TP53 alterations, 10 succumbed to their disease within 2 years from diagnosis, either because of primary resistance (n = 1) or early relapse (n = 9), indicating an exceptionally aggressive disease course. Interestingly, analysis of relapse samples in 6 of these patients demonstrated an expansion of the TP53-mutant clone under treatment and/or an additional alteration of the second allele, in all cases (supplemental Figure 2). IKZF1 deletions also appeared to be linked to a higher CIR (SHR = 3.03; 95% CI, 1.25-7.34; P = .01), with a smaller number of patients (n = 8) limiting the statistical significance on DFS (HR, 2.31; 95% CI, 0.99-5.57; P = .053) and OS (HR, 2.01; 95% CI, 0.85-4.80; P = .11). These results, thus, suggest that additional alterations in KMT2A-r BCP-ALL confer disease heterogeneity which translates in heterogeneous responses to treatment.
Overall, of the total 97 patients, 20 (20.6%) had either TP53 or IKZF1 alterations (hereafter named “Onco+” status), forming a subgroup of patients who experienced significantly poorer outcomes (CIR, 69.3% vs 36.2%; SHR = 3.06; 95% CI, 1.55-6.04; P = .001; DFS, 30.7% vs 49.8%; HR = 2.18; 95% CI, 1.14-4.14; P = .02; and OS, 28.1% vs 60.7%; HR, 2.47; 95% CI, 1.30-4.69; P = .006; Figure 2D-E).
Prognostic significance of IG/TR MRD
Next, we evaluated the prognostic value of MRD within KMT2A-r BCP-ALL, as measured by quantification of clonal rearrangements of IG/TR at the end of induction (MRD1) and after the first consolidation (MRD2). With a cutoff value of 10−4, 35 out of 78 patients (44.2%) had positive MRD1, and 20 out of 74 patients (27.0%) had positive MRD2. Both MRD1 and MRD2 positivity were significantly associated with poorer outcomes (Figure 3; supplemental Table 4). Hence, MRD evaluation in KMT2A-r BCP-ALL reveals heterogeneous responses to treatment that are associated with long-term outcomes, suggesting that MRD evaluation may provide additional value in refining risk assessment for adult KMT2A-r BCP-ALL.
Prognostic significance of IG/TR MRD. CIR and OS in patients at the end of induction (MRD1) (A-B) and after first consolidation (MRD2) (C-D). The cutoff for MRD positivity was set at 10−4.
Prognostic significance of IG/TR MRD. CIR and OS in patients at the end of induction (MRD1) (A-B) and after first consolidation (MRD2) (C-D). The cutoff for MRD positivity was set at 10−4.
Comparison between IG/TR and KMT2A-based MRD
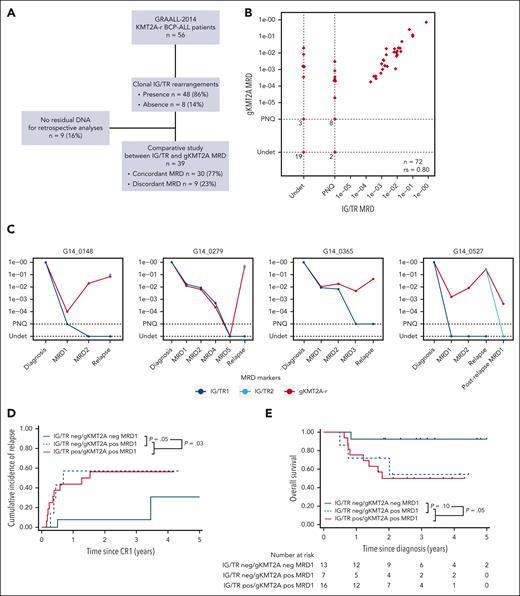
Studies in infant KMT2A-r ALL have shown that they usually have few IG/TR rearrangements, and these are frequently immature and oligoclonal, most likely because of the rapid transformation of an immature B-lineage progenitor cell. As a consequence, these rearrangements are not always present in the entire leukemic clone or stable during the disease course, resulting in false-negative or underestimated MRD values when used as MRD markers.32 To circumvent this issue, the breakpoint genomic sequence of KMT2A-r (gKMT2A) is used in infants as the preferred MRD marker. We questioned whether adult patients with KMT2A-r ALL would display a similar pattern of IG/TR rearrangements, leading to similar limitations. We conducted a retrospective analysis in 56 patients with available diagnostic material in order to compare IG/TR and gKMT2A markers (Figure 4A). First, in 8 of 56 patients (14.3%), no clonal IG/TR rearrangement could be detected. In comparison, this situation was seen in only 14 of 433 patients with non-KMT2A-r BCP-ALL (3%) in the GRAALL-2014 cohort. By contrast, a specific and sensitive qPCR assay for gKMT2A was applicable to virtually all patients. Then, a comparison of MRD1 and/or MRD2 results was feasible for 39 patients with leftover follow-up samples. Although most of the results demonstrated high concordance, 13 results (7 MRD1 and 6 MRD2) in 9 of 39 patients (23.1%) exhibited discrepancies regarding the critical cutoff of 10−4. These discrepancies were attributed to IG/TR markers being undetectable or at significantly lower levels as compared with those of gKMT2A (Figure 4B). Moreover, analysis of IG/TR rearrangements at relapse showed, in some cases, a complete modification of IG/TR clonality, that is, the absence of initial IG/TR clonal sequences and the emergence of new ones (Figure 4C). Altogether, these findings show that IG/TR rearrangements in adult KMT2A-r BCP-ALL do not confidently allow for the tracking of the whole leukemic clone, and suggest that gKMT2A-based MRD may predict outcome more accurately.
KMT2A genomic fusion is a more reliable MRD marker than IG/TR to assess treatment response. (A) Flowchart of the study design. (B) Comparison of the MRD levels between IG/TR and KMT2A genomic fusion (gKMT2A) measured at MRD1 and MRD2. (C) Illustrative cases showing discordant MRD results between gKMT2A and IG/TR markers CIR and OS in patients at the end of induction (MRD1) according to IG/TR and gKMT2A markers (D-E) and according to gKMT2A marker only (F-G), including 5 additional patients without IG/TR MRD because of the lack of clonal IG/TR rearrangement. PNQ, positive not quantifiable; Undet, undetectable.
KMT2A genomic fusion is a more reliable MRD marker than IG/TR to assess treatment response. (A) Flowchart of the study design. (B) Comparison of the MRD levels between IG/TR and KMT2A genomic fusion (gKMT2A) measured at MRD1 and MRD2. (C) Illustrative cases showing discordant MRD results between gKMT2A and IG/TR markers CIR and OS in patients at the end of induction (MRD1) according to IG/TR and gKMT2A markers (D-E) and according to gKMT2A marker only (F-G), including 5 additional patients without IG/TR MRD because of the lack of clonal IG/TR rearrangement. PNQ, positive not quantifiable; Undet, undetectable.
We, thus, evaluated the prognostic significance of MRD using gKMT2A compared with IG/TR markers. The median follow-up of patients with KMT2A-r enrolled in the GRAALL-2014 was 3.3 years. As expected, patients with discordant MRD, that is gKMT2A-positive and IG/TR-negative, behaved similarly to those with positive MRD for both markers, with significantly higher CIR and lower OS (Figure 4D-E), in agreement with false negativity of IG/TR-based MRD. Considering all the 41 patients with gKMT2A MRD data regardless of IG/TR MRD, 27 (66%) had positive MRD1 and 17 (41%) had positive MRD2. Both MRD1 and MRD2 had a strong impact on CIR, DFS, and OS, and the superiority of gKMT2A over IG/TR was further confirmed by a C-Harrell concordance test (Figure 4F-G; supplemental Figure 3; supplemental Table 4). Moreover, gKMT2A MRD1 allowed for the identification of a subgroup of patients who were “true” good responders, having low CIR and good OS rates (3-year CIR, 7.1% vs 61.7% [P = .02] and OS, 92.9% vs 41.9% [P = .03]). Notably, only 1 of these good responders underwent HSCT, owing to the GRAALL-2014 eligibility criteria solely based on MRD response. Altogether, our results demonstrate that MRD measurement using the gKMT2A marker allows us to reliably assess early response to treatment in adult KMT2A-r BCP-ALL, which is dramatically associated with long-term outcomes.
Prognostic value of the combination of TP53/IKZF1 status and KMT2A-based MRD
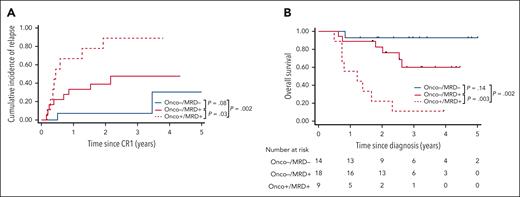
Finally, we aimed to further improve the prognostic stratification of adults with KMT2A-r BCP-ALL by combining oncogenetics and gKMT2A MRD1 (Figure 5). No TP53 or IKZF1 alterations were observed within the good responders (ie, no Onco+/MRD–). By contrast, the presence of TP53 or IKZF1 alterations within poor responders distinguished a subgroup of patients having significantly worse CIR (SHR = 3.04; 95% CI, 1.12-8.28; P = .03), DFS (HR, 2.77; 95% CI, 1.05-7.34; P = .04), and OS (HR, 5.28; 95% CI, 1.77-15.8; P = .003). Bivariate analysis confirmed the strong impact of oncogenetics, with less significance for MRD in this model because all the patients with TP53/IKZF1 alterations were poor responders (supplemental Table 5). Thus, the combination of oncogenetics and MRD response allowed for the identification of three subgroups of patients, that is, Onco–/MRD–, Onco–/MRD+, and Onco+/MRD+, having markedly distinct 3-year CIR (7.1%, 47.6%, and 88.9% respectively), DFS (92.9%, 41.9%, and 11.1%, respectively), and OS rates (92.9%, 60.0%, and 11.1%, respectively).
Combined genetics and MRD define 3 subsets of adult patients with KMT2A-r BCP-ALL with distinct outcomes. CIR (A) and OS (B) in patients according to oncogenetics and MRD. Patients with Onco+ were defined as having at least 1 alteration in TP53 and/or IKZF1, Onco– as having no alteration in both genes. MRD is based on gKMT2A measured at the end of induction (MRD1).
Combined genetics and MRD define 3 subsets of adult patients with KMT2A-r BCP-ALL with distinct outcomes. CIR (A) and OS (B) in patients according to oncogenetics and MRD. Patients with Onco+ were defined as having at least 1 alteration in TP53 and/or IKZF1, Onco– as having no alteration in both genes. MRD is based on gKMT2A measured at the end of induction (MRD1).
Discussion
This study represents the most comprehensive study of a large cohort of adults with KMT2A-r BCP-ALL who were treated with modern intensive protocols. As previously reported, KMT2A-r was associated with typical high-risk presenting features (older age, high WBC counts, and pro-B phenotype), but this did not translate into a higher risk for failure to achieve CR after induction course. Moreover, survival rates of patients with KMT2A-r BCP-ALL (DFS, 50.3%; OS, 53.3%) were only moderately lower than those of patients with other BCP-ALL in our cohort. These results are in sharp contrast with other studies that reported 5-year OS rates ranging from 20% to 35% for similar KMT2A-r cohorts.6-9 Notably, the proportion of patients with KMT2A-r undergoing HSCT in first CR in this study was not different from that in other cohorts, and no benefit of HSCT could be observed for those patients, suggesting that the GRAALL pediatric-inspired chemotherapy effectively contributed to outcome improvement. These results prompted us to investigate disease or patient heterogeneity within this subtype, in order to recognize patients at high risk of relapse and those who may achieve long-term remission. Strikingly, no classical risk factors, namely, age, WBC, pro-B phenotype, and early blast clearance in blood and bone marrow were associated with outcome. Although studies in children reported a better outcome associated with KMT2A-r involving non-AFF1 partners,38 we were unable to demonstrate this in our adult cohort, and this may need larger cohorts. We, therefore, analyzed other characteristics that could serve as prognostic markers for risk stratification, namely comutations and MRD.
First, we hypothesized that the presence of comutations may affect prognosis. Hence, there are many instances in other leukemia subtypes in which comutations detected in addition to the major driver lesion can act as modifiers of subtype-associated prognosis.39-42 By performing genomic characterization of a large cohort of adults with KMT2A-r BCP-ALL, we could depict its specific pattern of comutations. Strikingly, we observed an exceptionally low mutational burden, with a median of 1 altered gene per patient. Although we used a targeted approach on a large panel of genes involved in BCP-ALL, the actual number of alterations might be slightly higher. In addition, the most frequent mutations involved the RTK-RAS pathway and most mutations were present in minor clones. Altogether, this genomic pattern is very similar to that previously observed in infant KMT2A-r.19,22 Interestingly, although it has been suggested that the low mutational burden and short latency of infant KMT2A-r ALL may be related to a more permissive state of the fetal target cell of transformation, our results in adults argue for the ability of KMT2A-r to induce overt leukemia without the need of cooperating events, regardless of developmental stage. This is also consistent with the short latency of therapy-induced KMT2A-r leukemia.43 Another important finding of our study is the presence of TP53 alterations in a significant fraction of adult KMT2A-r BCP-ALL, whereas it has not been observed in children. Moreover, such alterations, although often present in minor clones, dramatically worsened the prognosis of patients. IKZF1 deletions were also observed and they tended to confer poorer prognosis. Thus, the acquisition of TP53 and IKZF1 alterations in KMT2A-r leukemia cells may create intraclonal and interindividual disease heterogeneity, with consequences on the response to treatment and outcome. Importantly, the subset of patients with TP53 or IKZF1 alterations had a particularly aggressive course, with most relapses occurring on-therapy within 6 months and being resistant to further treatments. These results call for the implementation of rapid screening for these alterations at KMT2A-r BCP-ALL diagnosis in order to propose early investigational therapeutic interventions to those patients at very high risk of resistant disease.
The second part of this study investigated the relevance of MRD level assessment in adults with KMT2A-r BCP-ALL. MRD has been shown to be the most important prognostic parameter in ALL and is widely used in contemporary protocols for risk stratification and treatment adaptation. However, it has never been examined within defined genetic subtypes in adults. Virtually all adult ALL protocols regard KMT2A-r as a very high-risk feature and propose HSCT in first CR regardless of the MRD status.44 Although this is reasonable when survival rates barely reach 30%, our improved results in the GRAALL protocols warranted further risk stratification. Studies in infant and noninfant children with ALL showed that MRD assessment can unveil heterogeneity in treatment response within KMT2A-r BCP-ALL and identify distinct subsets of patients with different outcomes.32,33,38 In this study, we showed that MRD, indeed, had a strong prognostic significance. We then investigated the value of using the KMT2A-r genomic sequence as an MRD marker, as compared with IG/TR markers. As previously shown in infants,32,33 we report that IG/TR rearrangements are frequently absent or subclonal in adult KMT2A-r, in agreement with malignant transformation of an early lymphoid precursor preceding IGH complete rearrangement.45 Consequently, we demonstrated the superiority of gKMT2A-based MRD over IG/TR in evaluating response and predicting treatment outcome. Notably, although flow-based MRD may be considered an alternative approach, it should be noted that it can also prove defective owing to phenotypic heterogeneity and plasticity of KMT2A-r, especially in the context of B-cell–directed therapies favoring loss of B-cell antigens.15,46
By combining oncogenetics and gKMT2A-based MRD, we highlighted 3 subgroups of patients with markedly distinct outcomes. Although the limited number of patients in each subgroup calls for further validation, these results pave the way for future risk-adapted treatment stratification of adult KMT2A-r BCP-ALL. Remarkably, we could identify patients who were good MRD responders as having excellent outcomes, without undergoing HSCT. Whether HSCT benefits adult patients with KMT2A-r has been a longstanding matter of debate, with limited data available in the literature.47 Although pediatric protocols have reduced indications of HSCT based on additional prognostic criteria,11,12 virtually all adult protocols still rely on HSCT for patients with KMT2A-r.44 However, the benefit of HSCT was considered in a context in which poor results were obtained with respective suboptimal chemotherapy regimens.6-9 Our results suggest that intensive pediatric-inspired ALL regimens are able to cure at least a subset of patients. We recognize that these findings were obtained from a group of young adults who underwent intensive treatment. Consequently, the relevance of our results may not be directly transferrable to other contexts, such as those involving older patients or implementing new treatment strategies. For patients who are not eligible to intensive chemotherapy and/or those who have a suboptimal response to chemotherapy, additional treatment strategies, including B-cell–directed immunotherapies48 or menin inhibitors,49 are urgently needed.
Acknowledgments
The authors thank the patients and all the GRAALL investigators and biologists who contributed samples and data for this study. The authors thank the Saint-Louis molecular hematology laboratory for technical support, especially Hélène Boyer, Emilie Gaudas, Agnès Pérus, Chantal Simon, Edwige Sylva, and Sophie Theunissen.
This work was supported by the Institut Hospitalo-Universitaire THEMA, the French national center for precision medicine in leukemia.
Authorship
Contribution: R.K., H.D., N.B., and E.C. conceived and designed the research and oversaw the project; C.P., F.P., E.R., N.G., E.D., S.K., A.T.-B., M. Balsat, M.E.-B., S.B., M. Baumann, A.B., N.S., M.-P.G.-H., Y.C., C.G., J.S., T.L., M.H., F.H., N.B., and E.C. provided study materials or patients; R.K., H.B., C.P., L.L., M.P., N.G., E.D., S.K., A.C.-E., C.M., R.M., and E.C. performed molecular analyses; R.K., H.B., V.L., N.B., and E.C. collected and assembled data; R.K. and N.B. performed statistical analysis; R.K., H.B., N.B., and E.C. analyzed and interpreted data; and R.K., H.B., J.S., H.D., N.B., and E.C. wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Emmanuelle Clappier, Laboratoire d’Hématologie, Hôpital Saint-Louis, 1 Ave Claude Vellefaux, 75010 Paris, France; e-mail: emmanuelle.clappier@aphp.fr; and Nicolas Boissel, Unité Adolescents et Jeunes Adultes, Service Clinique des Maladies du Sang, Hôpital Saint-Louis, 1 Ave Claude Vellefaux, 75010 Paris, France; e-mail: nicolas.boissel@aphp.fr.
References
Author notes
∗R.K. and H.B. contributed equally to this study.
†N.B. and E.C. are joint last authors.
Data are available on request from corresponding author Emmanuelle Clappier (emmanuelle.clappier@aphp.fr).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal