Introduction
Prognosis of patients with relapsed/refractory multiple myeloma (RRMM) is poor, particularly in triple-class refractory patients (refractory to a proteasome inhibitor, an immunomodulatory drug and an anti-CD38 monoclonal antibody). Idecabtagene vicleucel (Ide-Cel) is the first chimeric antigen receptor (CAR) T-cell therapy targeting the B-cell maturation antigen (BCMA) approved by both the European Medicines Agency and the US FDA and the only one available in France through the Early Access Program for treatment (≥ 3 lines) of RRMM. Here we present an update of characteristics and outcome of patients enrolled in this program.
Methods
We conducted a multicenter, retrospective, observational study that included all consecutive patients with RRMM registered in the DESCAR-T database of patients treated with CAR T therapy, who underwent apheresis up to November 2022 to be treated by commercial Ide-Cel in France. The main objective was to analyze efficacy in terms of response rates, progression free survival (PFS) and overall survival (OS). Secondary objectives included evaluation of safety in terms of cytokine release syndrome (CRS), neurotoxicity, cytopenia, infections and management of relapse including subsequent anti myeloma therapies (sAMT).
Results
From June 2021 to November 2022, 207 patients were registered in DESCAR-T and underwent leukapheresis to be treated with Ide-cel, 164 (79%) were infused; 43 were not infused: 13 due to rapidly progressive disease or death and 5 due to manufacturing failure. Fifty seven (28%) patients did not meet KarMMa inclusion criteria: cytopenia (n=23), kidney failure (n=13), performance score (PS) >1 (n=15), prior anti BCMA drug conjugate antibody (n=8), prior allograft (n=5).
Median age of the 164 patients was 60.5 years (range 34-82), 59% were male, median number of prior treatment lines was 4 (range 2-12) including prior autologous stem cell transplantation in 147 (90%); 130 (80%) were triple-refractory, 47 (29%) were penta-refractory. At diagnosis, 12 (16%) patients had R-ISS stage III disease (89 missing). High tumor burden (> 30% BMPCs) was present in 71 patients (54%) (33 missing).
High risk cytogenetic abnormalities defined by del(17p) or t(4;14) were present in 63 patients (58%) (56 missing) and 15 (23%) had extramedullary disease (EMD) (98 missing).
One hundred and forty-two (87%) received bridging therapy of which 39 (27%) responded: 25 had partial response (PR); 8 very good partial response (VGPR); 6 complete response (CR). Median time from apheresis to infusion was 62 days.
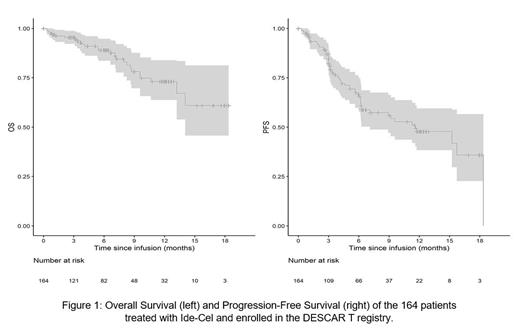
Median follow-up after infusion was 6.2 months.
In the first 3 months, overall response rate was 82% (n=134), best achieved response was CR, VGPR and PR in 60 (38%), 36 (23%) and 38 (24%) respectively. Median PFS was 11.6 months (95% CI (7.1; NA)). The 12-month OS was 73% (95% CI (64; 84)). In the first 6 months post-infusion, MRD was analyzed for 45 patients and reached negativity rate in 77%.
In univariate analysis, only EMD had a significant impact on PFS: median PFS was 6.3 months (95% CI (3; NA)) for patients with EMD (p = 0.003; HR 3.5, 95% CI (1.55; 7.9)).
CRS occurred in 146 (89%) patients, with only 3 cases (2%) of grade ≥ 3. Neurotoxicity occurred in 19 (12%) patients (5 with grade ≥ 3). Ninety-eight (60%) patients received tocilizumab, and 37 (23%) received steroids.
Persistent grade ≥ 3 thrombocytopenia, anemia and neutropenia at M1 were observed in 55 (34%), 20 (12%) and 90 (55%) patients, respectively.
Forty (24%) patients presented infections grade ≥ 3 in the first six months after infusion.
Fifty-two patients progressed during follow-up. 34/52 patients (65%) received a sAMT. Median time from relapse to first administration of sAMT was 0.6 months. Eighteen received bispecific antibodies including 12 anti-BCMA and 6 anti-GPRC5D. Among these 34 patients, a total of 13 (38%) died.
Overall, at data cut-off, 26 patients had died, including 9 after disease progression, 4 of acute toxicity and 2 of sepsis.
Conclusion
This updated study confirms safety and efficacy of Ide-cel in patients with RRMM in real world settings. Response rates and safety were comparable to those reported in the registration trial (Munshi et al., 2021) and American real world study (Hansen et al., 2023). Importantly, patients with EMD had a significant lower PFS. Prognosis data at relapse following ide-cel require a longer follow up and will be presented at ASH.
Disclosures
Karlin:AbbVie, Amgen, Celgene, Janssen, Sanofi, Takeda: Honoraria; Amgen, Celgene, GSK, Janssen, Takeda: Consultancy. Touzeau:Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Leleu:AbbVie: Honoraria; Harpoon Therapeutics: Honoraria; Merck: Honoraria; Amgen: Honoraria; BMS/Celgene: Honoraria; Janssen: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Sanofi: Honoraria; GSK: Honoraria. Perrot:Abbvie, Adaptive, Amgen, BMS, Janssen, Pfizer, Sanofi, Takeda: Honoraria. Bories:Kite Gilead: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Vincent:BMS, Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Financing meeting participation; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financing meeting participation; Pfizer: Other: Financing meeting participation. Mohty:JAZZ PHARMACEUTICALS: Honoraria, Research Funding. Manier:Janssen: Honoraria; BMS: Honoraria; Amgen: Honoraria; Abbvie, Amgen, Celgene/BMS, GlaxoSmithKline, Janssen, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda: Membership on an entity's Board of Directors or advisory committees. Yakoub-Agha:Janssen: Honoraria; Novartis: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Other: Travel Support; Bristol-Myers Squibb: Honoraria. Brisou:Novartis: Consultancy. Decaux:Janssen, BMS, GSK, Sanofi, Takeda, Roche, Gilead: Honoraria. Houot:Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, F. Hoffmann-La Roche Ltd: Honoraria; Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte, Miltenyi: Consultancy. Moreau:GSK: Honoraria, Other: Advisory Board; janssen, celgene BMS, abbvie, sanofi, amgen, takeda, pfizer: Honoraria, Other: advisory boards. Arnulf:Bristol Myers Squibb: Consultancy, Honoraria, Other: Meeting travel payments; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Meeting travel payments, Research Funding; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Meeting travel payments; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Meeting travel payments; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Meeting travel payments.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal