Background
Leukemic stem cells have high expression of CD123 compared to normal hematopoietic stem cells and is therefore a therapeutic target in multiple leukemias including acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), and chronic myelomonocytic leukemia (CMML). Vibecotamab (formally XmAb14045) is a CD3-CD123 bispecific engaging antibody that has shown clinical activity in relapsed/refractory AML, particularly in low-blast disease. We therefore sought to evaluate vibecotamab in other low-blast states, including MDS or CMML after hypomethylating agent failure and MRD-positive AML.
Methods
In this two-arm, open-label, phase II study, adults with either MDS (IPSS-R intermediate or higher risk) or CMML (CMML-1 or CMML-2) after failure of hypomethylating agents or AML in first or second morphologic remission with detectable MRD at a level of ≥0.1% by flow cytometry were eligible. CD123 expression ≥20% on aberrant myeloid blasts was required for enrollment. Vibecotamab was given IV in a ramp-up dose schedule on days 1 (0.43µg/kg), 3 (0.75µg/kg), 5 (1.1µg/kg), and 8 (1.7µg/kg) in cycle 1, followed by weekly doses of vibecotamab at a dose of 1.7µg/kg. Patients (pts) received up to 4 cycles of vibecotamab in 28-day cycles. The primary endpoint of the MDS/CMML cohort was response rate (CR + mCR + PR + HI + clinical benefit) within 4 cycles. The primary endpoint of the AML MRD cohort was the MRD negativity rate within 4 cycles.
Results
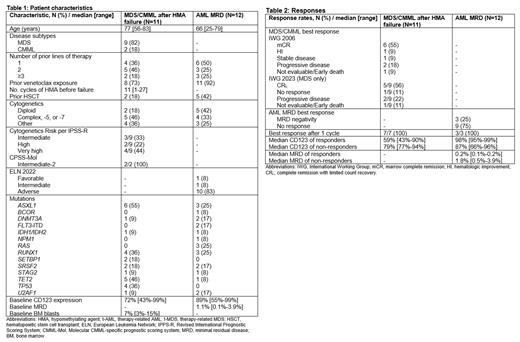
Between May 2022 and July 2023, 23 pts were treated (11 MDS/CMML, 12 AML MRD). Baseline characteristics are shown in Table 1. In the MDS/CMML cohort, 7 pts (63%) had received two or more prior lines of therapy, 8 pts (73%) had prior venetoclax exposure, and 2 pts (18%) had prior hematopoietic stem cell transplant (HSCT). Six MDS pts (66%) were IPSS-R high or very high risk. In the AML cohort, 6 pts (50%) had received two or more lines of prior therapy, 11 pts (92%) had prior venetoclax exposure, and 5 pts (42%) had prior HSCT. Ten pts (83%) were ELN 2022 adverse risk. The baseline CD123 expression was 72% (range, 43%-99%) in the MDS/CMML cohort and 89% (range, 55%-99%) in the AML MRD cohort. The baseline MRD by flow cytometry in the AML MRD cohort was 1.1% (range, 0.1%-3.9%).
Responses are shown in Table 2. In the MDS/CMML cohort, 7 pts responded (64%), with 6 pts (56%) achieving marrow complete remission (mCR) and 1 pt (9%) achieving hematologic improvement (HI) per International Working Group (IWG) 2006 criteria. Among the 9 MDS pts, 4 (44%) achieved mCR + HI (2 HI-N, 1 HI-P, and 1 HI-P + HI-N), and 1 (11%) achieved HI (HI-N + HI-P). Per revised IWG 2023 MDS response criteria, 5 of the 9 MDS pts (56%) achieved complete remission with limited count recovery (CR L). Two of 4 MDS pts (50%) with TP53 mutations achieved CR L. Both CMML pts achieved mCR, with one pt also achieving HI-N. Among 9 pts with baseline bone marrow blasts =>5% at trial enrollment, 6 (67%) achieved a mCR, with or without HI. Best response occurred after the first cycle in all pts. CD123 expression was not associated with likelihood of response. Of the 7 responders, 5 are in ongoing response (range 0.3-6.9 months), one died in CR L from non-hematologic complications (heart failure), and one relapsed 5 months after achieving CR L.
Of the 12 pts in the AML MRD cohort, 3 (25%) achieved MRD negativity, all of which occurred after 1 cycle of vibecotamab. Among the 3 responders, all were ELN adverse risk and had prior venetoclax exposure, 2 had prior HSCT, and 1 had inv(3). The median MRD and CD123 expression in responders was 0.2% (range 0.1%-0.2%) and 98% (range 95%-99%) vs 1.8% (range 0.5%-3.9%) and 87% (range 66%-96%) in non-responders, respectively. At last follow-up, all 3 responders are still in MRD-negative remission (range 3.2-12.8 months).
Vibecotamab was well-tolerated with no pts requiring dose reductions or being taken off study due to adverse events. Ten pts (44%) experienced grade 2 infusion reactions and 1 pt (4%) experienced a grade 3 infusion reaction. Myelosuppression was minimal, consistent with previous studies of vibecotamab.
Conclusion
Vibecotamab was safe and active in low-blast, high-risk myeloid diseases, with a response rate of 64% in MDS/CMML after HMA failure and 25% in MRD-positive AML. The clinical activity of vibecotamab, including in pts with prior venetoclax exposure and/or HSCT, and its lack of clinically significant myelosuppression provide rationale to combine it with other agents in AML, MDS, and CMML.
Disclosures
Ravandi:Syros: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Astex/taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Prelude: Research Funding; Biomea fusion: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; Xencor: Research Funding. Chien:Rigel Pharmaceuticals: Consultancy; AbbVie: Consultancy. Montalban-Bravo:Takeda: Research Funding; Rigel: Research Funding. Issa:Celgene: Research Funding; Kura Oncology: Consultancy, Research Funding; Syndax: Research Funding; Novartis: Consultancy, Research Funding; NuProbe: Consultancy; Merck: Research Funding. Maiti:Celgene: Research Funding; Lin BioScience: Research Funding. Alvarado Valero:Jazz: Research Funding; BerGenBio: Research Funding; Sun Pharma: Consultancy, Research Funding; FibroGen: Research Funding; Daiichi-Sankyo: Research Funding; MEI Pharma: Research Funding; Astex: Research Funding; CytomX Therapeutics: Consultancy. Daver:FATE: Research Funding; Trovagene: Research Funding; Servier: Consultancy, Research Funding; Kite, a Gilead company: Consultancy, Research Funding; Celgene: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Glycomimetics: Research Funding; Novimmune: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Syndax: Consultancy; Jazz: Consultancy; Novartis: Consultancy; AROG: Consultancy; Hanmi: Research Funding; Trillium: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Kronos Bio: Research Funding. DiNardo:AbbVie/Genentech: Honoraria; Notable Labs: Honoraria; Novartis: Honoraria; Astellas: Honoraria; Fogham: Honoraria; BMS: Honoraria; Takeda: Honoraria; Schrödinger: Consultancy; Servier: Honoraria; ImmuniOnc: Honoraria. Jabbour:Adaptive Biotech: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Ascentage Pharma Group: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Hikma Pharmaceuticals: Consultancy, Honoraria, Research Funding. Kadia:Genzyme: Honoraria; Biologix, Cure, Hikma Pharmaceuticals: Speakers Bureau; Jazz Pharmaceuticals, Pfizer, Pulmotect, Inc, Regeneron Pharmaceuticals, SELLAS Life Sciences Group: Research Funding; Amgen, Inc.: Research Funding; Ascentage Pharma Group: Research Funding; Astellas Pharma Global Development: Research Funding; AstraZeneca: Research Funding; Celgene: Research Funding; Cellenkos Inc.: Research Funding; Cure: Speakers Bureau; Cyclacel: Research Funding; Delta-Fly Pharma, Inc.: Research Funding; Genentech: Consultancy, Research Funding; GenFleet Therapeutics: Research Funding; Glycomimetics: Research Funding; Hikma Pharmaceuticals: Speakers Bureau; Iterion: Research Funding; Janssen Research and Development: Research Funding; Liberum: Consultancy; Novartis: Consultancy; Pfizer: Consultancy, Research Funding; Pulmotect, Inc.: Consultancy, Research Funding; Regeneron Pharmaceuticals: Research Funding; Sanofi-Aventis: Consultancy; SELLAS Life Sciences Group: Research Funding; AbbVie, Amgen, Inc, Ascentage Pharma Group, Astellas Pharma Global Development, Astex, AstraZeneca, BMS, Celgene, Cellenkos Inc, Cyclacel, Delta-Fly Pharma, Inc, Genentech, Inc., Genfleet, Glycomimetics, Iterion, Janssen Research and Development: Research Funding; Daiichi Sankyo, Genentech, Inc., Genzyme, Jazz Pharmaceuticals, Liberum, Novartis, Pfizer, PinotBio, Inc, Pulmotect, Inc, Sanofi-Aventis, Servier: Consultancy; Servier: Consultancy; Agios: Consultancy; Pinotb-Bio: Consultancy; BMS: Consultancy, Research Funding; Astex: Honoraria. Pemmaraju:Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Imedex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASH Committee on Communications: Other: Leadership; Physician Education Resource (PER): Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Patient Power: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASCO Cancer.Net Editorial Board: Other: Leadership; Karger Publishers: Other: Licenses; United States Department of Defense (DOD): Research Funding; National Institute of Health/National Cancer Institute (NIH/NCI): Research Funding; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PeerView Institute for Medical Education: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Magdalen Medical Publishing: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; OncLive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Harborside Press: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Menarini Group: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CancerNet: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aplastic Anemia & MDS International Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Pacylex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Curio Science: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Protagonist Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ClearView Healthcare Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ImmunoGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cimeio Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; HemOnc Times/Oncology Times: Other: Uncompensated; Bristol Myers Squibb Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Yilmaz:Daiichi-Sankyo: Research Funding; Pfizer: Research Funding. Bachireddy:Amgen: Current equity holder in publicly-traded company; Agenus: Current equity holder in publicly-traded company; Breakbio Corp: Current equity holder in publicly-traded company; Johnson & Johnson: Current equity holder in publicly-traded company; Exelixis: Current equity holder in publicly-traded company; BioNTech: Current equity holder in publicly-traded company; Allogene Therapeutics: Research Funding. Garcia-Manero:AbbVie: Research Funding; Genentech: Research Funding; Bristol Myers Squibb: Other: Medical writing support, Research Funding. Short:Novartis: Consultancy; AstraZeneca: Consultancy; Takeda: Consultancy, Research Funding; Stemline therapeutics: Research Funding; Astellas: Research Funding; Amgen: Honoraria; Pfizer: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal