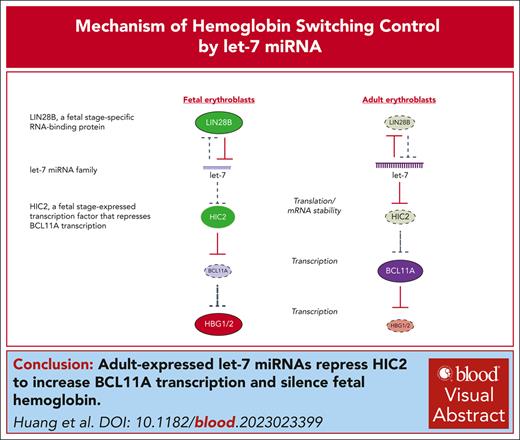
Mechanism by which miRNAs regulate hemoglobin switching.
Adult-specific let-7 miRNAs inhibit the BCL11A repressor HIC2.
Visual Abstract
The switch from fetal hemoglobin (γ-globin, HBG) to adult hemoglobin (β-globin, HBB) gene transcription in erythroid cells serves as a paradigm for a complex and clinically relevant developmental gene regulatory program. We previously identified HIC2 as a regulator of the switch by inhibiting the transcription of BCL11A, a key repressor of HBG production. HIC2 is highly expressed in fetal cells, but the mechanism of its regulation is unclear. Here we report that HIC2 developmental expression is controlled by microRNAs (miRNAs), as loss of global miRNA biogenesis through DICER1 depletion leads to upregulation of HIC2 and HBG messenger RNA. We identified the adult-expressed let-7 miRNA family as a direct posttranscriptional regulator of HIC2. Ectopic expression of let-7 in fetal cells lowered HIC2 levels, whereas inhibition of let-7 in adult erythroblasts increased HIC2 production, culminating in decommissioning of a BCL11A erythroid enhancer and reduced BCL11A transcription. HIC2 depletion in let-7-inhibited cells restored BCL11A–mediated repression of HBG. Together, these data establish that fetal hemoglobin silencing in adult erythroid cells is under the control of a miRNA–mediated inhibitory pathway (let-7 ⊣ HIC2 ⊣ BCL11A ⊣ HBG).
Introduction
The developmental switch in hemoglobin expression from fetal hemoglobin (γ-globin, HBG) to adult hemoglobin (β-globin, HBB) is a highly regulated process, and its reversal is a goal for the treatment of hemoglobinopathies such as β-thalassemia and sickle cell disease. BCL11A is an adult stage–expressed transcriptional repressor acting directly on the fetal HBG genes in adult erythroid cells1,2 and serves as a therapeutic target to treat β-thalassemia and sickle cell disease.3-9 Several prior studies indicated that BCL11A messenger RNA (mRNA) production is the dominant mechanism by which BCL11A levels are developmentally controlled.10-14
We recently identified HIC2 as a direct transcriptional repressor of BCL11A transcription, providing a mechanism by which BCL11A levels and hemoglobin switching are modulated during development.14 Specifically, HIC2 binds to and deactivates BCL11A erythroid-specific enhancers in part by competing with transcription factor GATA1.14 The expression of HIC2 is opposite to that of BCL11A, being high at the fetal stage and low at the adult stage of development.14 However, the mechanisms underlying the developmental regulation of HIC2 expression are unknown.
MicroRNAs (miRNAs) have been shown to play an essential role in governing fetal vs adult gene expression programs.15 A well-studied example is the let-7 miRNA family, with 9 conserved members that are highly expressed in adult erythroid cells.15 The fetal stage-specific RNA-binding protein LIN28B blocks let-7 biogenesis at the fetal stage of development, whereas let-7 miRNAs inhibits LIN28B (and other developmental regulators such as HMGA2) at the adult stage in an autoregulatory feedback loop.16 Both LIN28B and let-7 have been reported to regulate hemoglobin switching,17-19 and previous studies have shown that inhibition of let-7 in adult erythroblasts lowers BCL11A levels and induces HBG expression in a LIN28B-independent manner.18 Although LIN28B has been reported to repress BCL11A mRNA translation,19 multiple lines of evidence support that BCL11A is predominantly controlled at the transcription level.10-15 The mechanistic link between let-7, BCL11A, and hemoglobin switching therefore remains incompletely understood.
Here, we describe the role for global miRNA biogenesis in regulating developmental hemoglobin switching, nominate the let-7 miRNA family as key during this process, and identify the HIC2 mRNA as a direct target for let-7 miRNAs. Thus, we provide a mechanism underlying the developmental regulation of HIC2 control of globin gene regulation by connecting it to the let-7 miRNA pathway in developing erythroid cells.
Methods
HUDEP cell culture
HUDEP1 and HUDEP2 cells were cultured with StemSpan SFEM medium (Stemcell Technologies) supplemented with 1 μg/mL of doxycycline, 3 units/mL of erythropoietin, 1 μM dexamethasone, 100 ng/mL of human stem cell factor (SCF), and 1% penicillin/streptomycin. Cells were differentiated for 1 day in Iscove’s modified Dulbecco’s medium supplemented with 330 μg/mL of holotransferrin, 10 μg/mL of heparin, 10 μg/mL insulin, 5% fetal bovine serum, 3 units/mL of erythropoietin, 1 μg/mL of doxycycline, and 1% penicillin/streptomycin.
Primary human CD34+ HSPC culture
Adult human CD34+ hematopoietic stem/progenitor cells (HSPCs) from peripheral blood were obtained from the Fred Hutchinson Cancer Research Center. Human CD34+ HSPCs were cultured with a 2 phase culture system. For phase 1 medium, Iscove’s modified Dulbecco’s medium was supplemented with 100 ng/mL human SCF, 2% penicillin/streptomycin, 1 ng/mL of interleukin-3, 3 units/mL of erythropoietin, 200 μg/mL of holotransferrin, 10 μg/mL insulin, 5% human AB plasma, and 10 μg/mL heparin. For phase 2 medium, the interleukin-3 was withdrawn. Cells were maintained in phase 1 medium for 9 days and in phase 2 medium for another 4 days. Cells were collected at day 13 for analysis.
let-7a/f inhibition by TuD
The let-7a/f and scramble tough decoy (TuD) sequences are listed in supplemental Table 5 (available on the Blood website). The sequences were synthesized and cloned into a lentiviral vector and driven by the human U6 promoter. HUDEP2 cells and primary HSPCs were transduced with lentivirus and sorted based on GFP reporter 2 days after transduction.
NBSGW xenotransplantation of primary human HSPCs
NBSGW (NOD.Cg-KitW-41JTyr+PrkdcscidIl2rgtm1Wjl/ThomJ) mice were obtained from the Jackson Laboratory. The institutional animal care and use committee at Children’s Hospital of Philadelphia approved all experimental protocols. Human CD34+ HSPCs from adult peripheral blood were obtained from the Fred Hutchinson Cancer Research Center and cultured in StemSpan SFEM medium (Stemcell Technologies) supplemented with 100 ng/mL of human SCF, 100 ng/mL of recombinant human thrombopoietin, 100 ng/mL of recombinant human Flt-3 ligand, and 1% penicillin/streptomycin. On day 1 of culture, CD34+ cells were transduced with either scrambled control or let-7a/f TuD lentivirus. CD34+ cells were then resuspended in 200 μL of phosphate-buffered saline/0.1% BSA and injected retro-orbitally into nonirradiated 6-week-old male and female NBSGW mice at a dose of 7 × 105 cells per mouse. At 16 weeks after transplantation, mice were euthanized and their bone marrow was extracted to assess for percentage of human CD45+ engraftment (human CD45+/[human CD45+ + mouse CD45+] × 100). To assess for globin and BCL11A transcript levels, CD235a+ GFP+ cells were isolated on a FACSAria Fusion (BD). Antibodies are listed in supplemental Table 6.
qRT-PCR and digital droplet RT-PCR (ddRT-PCR)
Cells were homogenized using TRIzol (Invitrogen, #15596026). Total RNA was purified using an RNeasy Mini Kit (Qiagen, #74104) with on-column DNase digestion (Qiagen, #79256). cDNA was prepared using iScript Reverse Transcription Supermix (Bio-Rad). Primers used for quantitative reverse transcription polymerase chain reaction (qRT-PCR) are listed in supplemental Table 2. Reverse transcription and amplification of miRNA were performed using the TaqMan Advanced miRNA Assays kit (Thermo Fisher Scientific) according to manufacturer’s specifications. TaqMan miRNA primers purchased from Thermo Fisher Scientific are listed in supplemental Table 3. Duplicate ddPCR analysis reactions were assembled with ddPCR Supermix for Probes (Bio-Rad), multiplexed FAM-labeled hemoglobin transcript assay, and VIC-labeled AHSP assay using 0.01 to 0.1 ng RNA-equivalent cDNA. Droplets were generated and analyzed using the QX200 AutoDG Droplet Digital PCR system (Bio-Rad).
CRISPR gene knockout in HUDEP2 cells
Single guide RNAs (sgRNAs) cloned into lentiviral vectors are listed in supplemental Table 4. HUDEP2 cells were transduced with spCas9 lentivirus and selected with 2 μg/mL puromycin for 5 days. For gene knockout, HUDEP2/Cas9 cells were transduced with sgRNAs lentivirus and sorted based on the fluorescent reporters 2 days after transduction.
Construction and overexpression of LIN28B resistant clet-7a
A 280 bp fragment spanning the human let-7a-1 gene was amplified from genomic DNA (primers: 5'-TCACACAGGAAACCAGGATT-3' and 5'-GCTGCACTACATCTCTTTAA-3') using PCR. To generate LIN28B resistant let-7a (clet-7a), the let-7a-1 loop structure were replaced with miR-21 loop using a Gibson Assembly kit (New England Biolabs [NEB]). The chimeric clet-7a was cloned into a lentiviral vector and driven by the EF1a promoter. HUDEP1 cells were transduced with lentivirus and sorted based on GFP reporter 2 days after transduction.
Fetal hemoglobin staining and flow cytometry
Cells were fixed with 0.05% glutaraldehyde for 10 minutes at room temperature. After 3 washes, cells were permeabilized with 0.1% Triton X-100 for 3 minutes. Cells were incubated with fetal hemoglobin (HbF)-AF647 conjugated antibody (NB110-41084, Novus Biologicals; 1:250 dilution) for 45 minutes at 4°C. Flow cytometry was performed on a BD FACSCanto instrument. Data were collected with FACSDiva 8 and analyzed using FlowJo software.
Luciferase reporter assay
The 4718 bp HIC2 3' untranslated region (UTR) fragment was PCR amplified from genomic DNA. Firefly luciferase was driven by the EF1a promoter and followed by the HIC2 3’UTR and BGH polyA signal. HIC2 3’UTR with let-7 binding site mutations were cloned using a Gibson Assembly kit (NEB). Renilla luciferase driven by the EF1a promoter was used as the internal control. The plasmids were transfected into HeLa or HEK293T cells using Lipofectamine 2000 (Invitrogen). The luciferase signals were measured 2 days after transfection using the Dual-Glo Luciferase Assay kit (E2920; Promega).
RNA sequencing (RNA-seq)
RNA-seq libraries were prepared from 500 ng of DNase-treated total RNA. Ribosomal RNA was removed using the NEBNext rRNA Depletion Kit (NEB, E6310L). RNA was then prepared with the TruSeq Stranded Total kit (Illumina, 20020598) for cDNA synthesis and library preparation according to manufacturer’s specifications, with 15 cycles of PCR amplification. The quality and size of each library were evaluated using the DNA 7500 kit (5067-1504) on an Agilent Bioanalyzer 2100, and then quantified by real-time PCR using the KAPA Library Quant Kit (KAPA Biosystems, KK4835). Libraries were then pooled and sequenced on the NextSeq 2000 sequencer.
The paired end sequence reads were processed using the ENCODE3 long RNA-seq pipeline (https://www.encodeproject.org/pipelines/ENCPL002LPE/). Differentially expressed genes were identified using DESeq2.
Primary antibodies for western blot
HIC2 (22 788-1-AP, Proteintech; 1:500), BCL11A (19 487, Abcam; clone 14B5; 1:1000), HBG (NB110-41084, Novus Biologicals; 1:2000), LIN28B (4196S, Cell Signaling Technology; 1:2000), DICER1 (3363S, Cell Signaling Technology; 1:1000) and actin (47 778, Santa Cruz Biotechnology; clone C4; 1:500).
Results
Global miRNA perturbation by DICER1 depletion reactivates HBG partially through upregulation of HIC2
The fetal stage-specific expression of HIC2 may be mediated by transcriptional and posttranscriptional mechanisms. When examining HIC2 expression in primary human fetal and adult erythroblasts,11 we noted a greater difference at the protein level (∼9.5-fold) than for mature mRNA (∼4-fold) and primary transcripts (∼2.5-fold) (supplemental Figure 1A), implicating a layer of posttranscriptional regulation of HIC2 expression. Newborn samples, representing a state between fetal and adult, had intermediate values for both protein and mRNA.
As an orthogonal approach we analyzed RNA-seq data sets, which revealed a discrepancy in the differential sequencing read counts between HIC2 exons (representing mature transcripts) and introns (representing primary transcripts) in fetal vs adult cells.11 Reads that mapped to HIC2 exons were approximately threefold more abundant in fetal cells than in adult cells, whereas intronic reads were less significant between the 2 stages (supplemental Figure 1B-D); in contrast, for BCL11A both exon and intron reads were higher in adult erythroblasts (supplemental Figure 1E). Taken together, the variable differentials in expression of protein, mRNA, and nascent RNA between fetal and adult cells, suggest that HIC2 is regulated by posttranscriptional mechanisms.
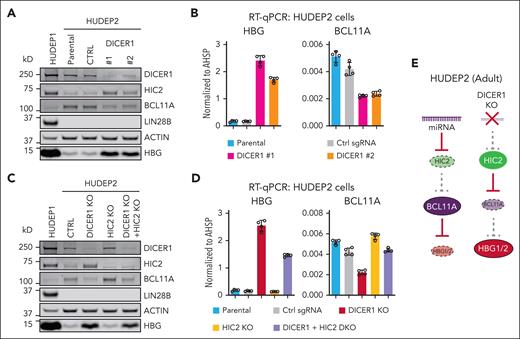
The HIC2 mRNA contains a 4718 bp 3'UTR (supplemental Figure 1B). We examined whether this 3'UTR might convey miRNA–mediated posttranscriptional regulation of HIC2 during erythroid development. First, to globally block the miRNA biogenesis pathway, we depleted the miRNA processing enzyme DICER1 in the adult-type HUDEP2 cell line,20 which expresses low levels of HIC2 and HBG. HIC2 protein levels were strongly induced upon DICER1 depletion with 2 independent sgRNAs, suggesting that HIC2 production is inhibited by miRNAs (Figure 1A). Increased HIC2 levels were accompanied by reduced BCL11A synthesis and induction of HBG but not to the same levels found in the fetal cell line HUDEP1 (Figure 1A-B). Notably LIN28B, the well-known let-7 target and proposed inhibitor of BCL11A mRNA translation, remained undetectable, suggesting that regulation of BCL11A and HBG by miRNAs was LIN28B independent (Figure 1A). Because DICER1 depletion blocks miRNA biogenesis globally, it may affect other BCL11A regulators, BCL11A itself or even the HBG genes. Therefore, we performed an epistasis experiment by depleting HIC2 in addition to DICER1. Codepletion of HIC2 and DICER1 partly restored both BCL11A mRNA and protein (Figure 1C-D). Further, HIC2 and DICER1 codepletion partially resilenced HBG expression (Figure 1C-D). These data suggest that miRNAs contribute to the regulation of BCL11A and HBG, at least partly, by modulating HIC2 expression, although the incomplete rescue implies involvement of other miRNA–regulated pathways.
DICER1 depletion induces HBG partially by upregulation of HIC2. (A) Western blot with indicated antibodies of extracts from DICER1 depleted HUDEP2 cells. Nontargeting sgRNA was used as control. (B) HBG and BCL11A mRNA measured by qRT-PCR in DICER1 depleted HUDEP2 cells. Results are normalized to AHSP and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). (C) Western blot with indicated antibodies of extracts from DICER1 and HIC2 depleted HUDEP2 cells. (D) HBG and BCL11A mRNA measured by qRT-PCR in DICER1 and HIC2 depleted HUDEP2 cells. Results are normalized to AHSP and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). (E) Schematic demonstrating repression of HIC2 in adult cells by miRNAs. Ablation of miRNA processing leads to increased HIC2 expression, decreased BCL11A, and derepression of HBG. SD, standard deviation.
DICER1 depletion induces HBG partially by upregulation of HIC2. (A) Western blot with indicated antibodies of extracts from DICER1 depleted HUDEP2 cells. Nontargeting sgRNA was used as control. (B) HBG and BCL11A mRNA measured by qRT-PCR in DICER1 depleted HUDEP2 cells. Results are normalized to AHSP and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). (C) Western blot with indicated antibodies of extracts from DICER1 and HIC2 depleted HUDEP2 cells. (D) HBG and BCL11A mRNA measured by qRT-PCR in DICER1 and HIC2 depleted HUDEP2 cells. Results are normalized to AHSP and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). (E) Schematic demonstrating repression of HIC2 in adult cells by miRNAs. Ablation of miRNA processing leads to increased HIC2 expression, decreased BCL11A, and derepression of HBG. SD, standard deviation.
let-7 miRNA overexpression represses HIC2 in fetal-type erythroid cells
To uncover the specific miRNAs that target HIC2, we used the miRNA target prediction tool miRDB.21 A total of 303 miRNAs were predicted to have binding sites in the HIC2 3’UTR (supplemental Table 1). As HIC2 is downregulated in adult erythroid cells, we hypothesized that the miRNAs regulating HIC2 are also expressed in a developmental stage-specific manner but opposite to HIC2. Therefore, we examined the expression levels of these 303 miRNAs by interrogating published small RNA-seq data from fetal and adult erythroblasts (Figure 2A).15 This narrowed our search to 7 predominantly adult-expressed miRNAs, which all belong to the 9 member let-7 family and were all strong candidates based on binding site prediction (Figure 2A; supplemental Figure 2).
let-7a overexpression represses HIC2 in HUDEP1 cells. (A) The expression levels of 303 predicted miRNAs targeting HIC2 in fetal and adult erythroblasts. The small RNA-seq data were obtained from published data sets.15let-7 family members are highlighted in red. The balance between let-7 and LIN28B expression is shown for adult and fetal cells. (B) The sequence of LIN28B resistant chimeric let-7a/miR21 (clet-7a). The stem and loop structure from let-7a-1 and miR-21 are highlighted in yellow and blue, respectively. Mature let-7a-5p and miR-21-5p sequences are highlighted in red. (C) The expression levels of let-7a-5p measured by miRNA RT-qPCR upon clet-7a overexpression in HUDEP1 cells. Results are normalized to miRNAs miR-16/103a/191 and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). Other let-7 family members are shown in supplemental Figure 3. (D) HIC2, LIN28B, and BCL11A mRNA measured by qRT-PCR upon clet-7a overexpression in HUDEP1 cells. Results are normalized to AHSP and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). (E) Western blot with indicated antibodies of extracts from clet-7a overexpression in HUDEP1 cells. (F) Schematic of the construct for HIC2 3'UTR luciferase reporter assay. let-7 binding sites are highlighted in green. (G) Sequences of the 4 let-7 binding sites in HIC2 3'UTR. Mutations are highlighted in bold. (H) Luciferase reporter assay of HIC2 3'UTR in HeLa cells. Results are shown as mean ± SD (n = 3). (I) Schematic demonstrating low let-7 levels and high HIC2 expression in fetal-like HUDEP1 cells. Overexpression of a let-7 mimic leads to decreased levels of HIC2 protein and increased level of BCL11A. BS, binding site; pA, BGH polyA signal; RPM, reads per million.
let-7a overexpression represses HIC2 in HUDEP1 cells. (A) The expression levels of 303 predicted miRNAs targeting HIC2 in fetal and adult erythroblasts. The small RNA-seq data were obtained from published data sets.15let-7 family members are highlighted in red. The balance between let-7 and LIN28B expression is shown for adult and fetal cells. (B) The sequence of LIN28B resistant chimeric let-7a/miR21 (clet-7a). The stem and loop structure from let-7a-1 and miR-21 are highlighted in yellow and blue, respectively. Mature let-7a-5p and miR-21-5p sequences are highlighted in red. (C) The expression levels of let-7a-5p measured by miRNA RT-qPCR upon clet-7a overexpression in HUDEP1 cells. Results are normalized to miRNAs miR-16/103a/191 and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). Other let-7 family members are shown in supplemental Figure 3. (D) HIC2, LIN28B, and BCL11A mRNA measured by qRT-PCR upon clet-7a overexpression in HUDEP1 cells. Results are normalized to AHSP and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). (E) Western blot with indicated antibodies of extracts from clet-7a overexpression in HUDEP1 cells. (F) Schematic of the construct for HIC2 3'UTR luciferase reporter assay. let-7 binding sites are highlighted in green. (G) Sequences of the 4 let-7 binding sites in HIC2 3'UTR. Mutations are highlighted in bold. (H) Luciferase reporter assay of HIC2 3'UTR in HeLa cells. Results are shown as mean ± SD (n = 3). (I) Schematic demonstrating low let-7 levels and high HIC2 expression in fetal-like HUDEP1 cells. Overexpression of a let-7 mimic leads to decreased levels of HIC2 protein and increased level of BCL11A. BS, binding site; pA, BGH polyA signal; RPM, reads per million.
To test whether HIC2 is regulated by let-7 miRNA, we aimed to forcibly express let-7a, the isoform that is highly expressed in adult erythroblasts (Figure 2A). However, in fetal human erythroblasts, biogenesis of all tested let-7 isoforms is blocked by LIN28B.17 To bypass this inhibition, we generated a chimeric LIN28B–resistant let-7a pre-miRNA (clet-7a) by fusing the stem structure from let-7a-1 with the loop structure from miR-21 (Figure 2B).22 We then replaced the stem loop sequence of the let-7a-1 primary miRNA with clet-7a, and expressed it in the fetal-type erythroblast cell line HUDEP1 cells. Expression of mature clet-7a was successful as measured by qRT-PCR (Figure 2C) and as reflected in the reduction of protein and RNA of the known let-7 targets LIN28B and IGF2BP1 (Figure 2D-E; supplemental Figure 3B). Notably, other let-7 family members were also upregulated upon clet-7a expression, probably because of the lower LIN28B levels (supplemental Figure 3A). Importantly, both HIC2 mRNA and protein were downregulated upon clet-7a overexpression (Figure 2D-E).
Previously, we demonstrated that HIC2 directly represses BCL11A transcription.14 Accordingly, both BCL11A mature and primary transcript levels were significantly induced by clet-7a overexpression in HUDEP1 cells (Figure 2D-E; supplemental Figure 3B), suggesting the secondary effects owing to inhibition of HIC2. Together, these results suggest that let-7 can inhibit HIC2 expression sufficiently to trigger increased expression of BCL11A.
The HIC2 3'UTR contains 4 predicted perfectly matched let-7 seed sequences (Figure 2F). To test if let-7 directly regulates HIC2 through its 3'UTR, we performed a luciferase reporter assay in HeLa cells, which highly express let-7a/f/i (supplemental Figure 4A).23 We fused the HIC2 3'UTR to firefly luciferase, with either individual or all 4 let-7 binding sites mutated (Figure 2F-G). Mutation of individual let-7 binding motifs had no significant effect on luciferase signal compared to controls. However, luciferase signal was strongly increased when all 4 binding sites were mutated (Figure 2H). Importantly, mutation of these sites had a smaller effect on luciferase expression in HEK293T cells, which express low levels of let-7 (supplemental Figure 4B),23 further confirming let-7 specific regulation of HIC2. Together, these results suggest that let-7 miRNAs can inhibit HIC2 levels through its 3'UTR.
let-7 inhibition induces HIC2 levels in HUDEP2 cells
To directly test if let-7 represses HIC2 in adult stage erythroid cells, we utilized the TuD24 method to inhibit let-7 in the adult-type erythroid HUDEP2 cell line. let-7a/f are the 2 most highly expressed miRNAs of the 9-member let-7 miRNA family in adult erythroblasts (Figure 2A; supplemental Figure 2B).15 Therefore, we designed a TuD lentiviral vector targeting both let-7a/f simultaneously and introduced it into HUDEP2 cells (Figure 3A). Transduction with let-7a/f TuD, but not the scrambled control, strongly decreased the levels of let-7a and let-7f (Figure 3B). Other let-7 family members also showed variable degrees of inhibition, suggesting that TuD can block miRNAs even with several mismatches (supplemental Figure 5). Importantly, we observed increases in HIC2 mature mRNA and protein levels upon let-7a/f inhibition (Figure 3C-E). HIC2 primary transcript levels were unchanged in let-7a/f inhibited cells (Figure 3C), suggesting posttranscriptional regulation of HIC2. Moreover, HIC2 protein levels increased to a greater degree than mRNA levels, suggesting that let-7a/f influence both mRNA turnover and translation (Figure 3D-E).
let-7a/f inhibition induced HIC2 expression in HUDEP2 cells. (A) Schematic of the let-7a/f TuD. let-7a/f binding sites are highlighted in red. (B) The expression levels of let-7a-5p and let-7f-5p measured by miRNA qRT-PCR upon let-7a/f inhibition in HUDEP2 cells. Results are normalized to miRNAs miR-16/103a/191 and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). Other let-7 family members are shown in supplemental Figure 5. (C) HBB, HBG, percentage of HBG, HIC2 and BCL11A mature mRNA, and primary transcript measured by qRT-PCR in let-7a/f inhibited HUDEP2 cells. Results are normalized to AHSP and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). (D). Western blot with indicated antibodies of extracts from let-7a/f inhibited HUDEP2 cells. (E) Quantification of HIC2 and BCL11A protein levels in panel D. Results are normalized to actin and HUDEP2 parental samples and shown as mean ± SD (2 independent biological replicates). (F) Schematic showing high let-7 levels in adult-like HUDEP2 cells repressing HIC2; depletion of let-7 using a tough decoy leads to higher HIC2 levels and resulting decrease in BCL11A.
let-7a/f inhibition induced HIC2 expression in HUDEP2 cells. (A) Schematic of the let-7a/f TuD. let-7a/f binding sites are highlighted in red. (B) The expression levels of let-7a-5p and let-7f-5p measured by miRNA qRT-PCR upon let-7a/f inhibition in HUDEP2 cells. Results are normalized to miRNAs miR-16/103a/191 and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). Other let-7 family members are shown in supplemental Figure 5. (C) HBB, HBG, percentage of HBG, HIC2 and BCL11A mature mRNA, and primary transcript measured by qRT-PCR in let-7a/f inhibited HUDEP2 cells. Results are normalized to AHSP and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). (D). Western blot with indicated antibodies of extracts from let-7a/f inhibited HUDEP2 cells. (E) Quantification of HIC2 and BCL11A protein levels in panel D. Results are normalized to actin and HUDEP2 parental samples and shown as mean ± SD (2 independent biological replicates). (F) Schematic showing high let-7 levels in adult-like HUDEP2 cells repressing HIC2; depletion of let-7 using a tough decoy leads to higher HIC2 levels and resulting decrease in BCL11A.
let-7a/f inhibition reduced both primary and mature BCL11A mRNA, consistent with HIC2 repressing BCL11A gene transcription (Figure 3C), resulting in decreased BCL11A protein levels (Figure 3D-E). Finally, let-7a/f inhibition strongly induced HBG transcription (Figure 3C-D) in agreement with previous reports.17,18 LIN28B levels remained undetectable upon let-7a/f inhibition, suggesting that let-7 inhibition alone is not sufficient to reactivate LIN28B, and that let-7 mediated BCL11A and HBG regulation in adult erythroblasts is LIN28B-independent (Figure 3D). Similarly, HMGA2 was previously shown to be activated by let-7 inhibition and to be involved in HBG upregulation,18,25 but we did not detect HMGA2 in let-7 inhibited HUDEP2 cells. Together, these results suggest that let-7 represses HIC2 levels, contributing to HBG silencing in HUDEP2 cells.
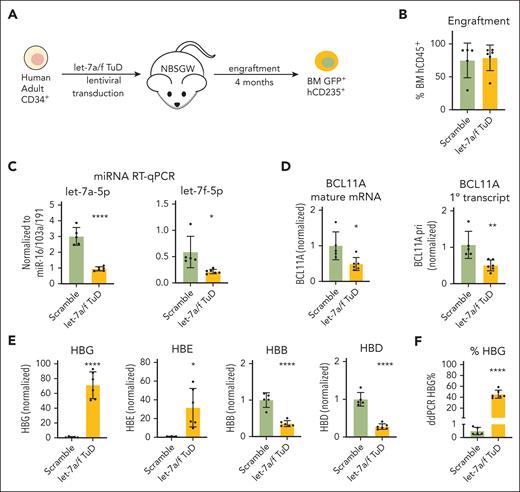
let-7a/f inhibition increases HBG expression in xenografted human primary erythroid cells. (A) Schematic of NBSGW xenograft experiments using transduced primary human CD34+ cells. Transduced GFP+ hCD235+ erythroid cells were purified from mouse BM for analysis. All data are shown as mean ± SD with individual data points, n = 5 for controls and 6 for let-7a/f decoy. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001 by the Student t test. (B) Engraftment analysis measured at 4 months, calculated as human percentage of CD45+ cells. (C) The expression levels of let-7a-5p and let-7f-5p measured by miRNA qRT-PCR upon let-7a/f inhibition. Results are normalized to miRNAs miR-16/103a/191. (D) Real-time RT-PCR of BCL11A mature mRNA and primary transcript in xenografted early-stage erythroid precursors. (E) Real-time RT-PCR of β-globin-like genes in BM CD235ab+ human primary erythroid cells from NBSGW xenografts. (F) ddRT-PCR quantification of HBG transcripts as percentage of total β-like transcripts. BM, bone marrow.
let-7a/f inhibition increases HBG expression in xenografted human primary erythroid cells. (A) Schematic of NBSGW xenograft experiments using transduced primary human CD34+ cells. Transduced GFP+ hCD235+ erythroid cells were purified from mouse BM for analysis. All data are shown as mean ± SD with individual data points, n = 5 for controls and 6 for let-7a/f decoy. ∗P < .05; ∗∗P < .01; ∗∗∗∗P < .0001 by the Student t test. (B) Engraftment analysis measured at 4 months, calculated as human percentage of CD45+ cells. (C) The expression levels of let-7a-5p and let-7f-5p measured by miRNA qRT-PCR upon let-7a/f inhibition. Results are normalized to miRNAs miR-16/103a/191. (D) Real-time RT-PCR of BCL11A mature mRNA and primary transcript in xenografted early-stage erythroid precursors. (E) Real-time RT-PCR of β-globin-like genes in BM CD235ab+ human primary erythroid cells from NBSGW xenografts. (F) ddRT-PCR quantification of HBG transcripts as percentage of total β-like transcripts. BM, bone marrow.
let-7a/f inhibition induces HIC2 in adult primary human erythroblasts
To investigate HIC2 regulation by let-7 miRNAs in primary human erythroblasts, we used a murine xenotransplantation system, in which genetically modified CD34+ HSPCs from healthy human donors are transplanted into immunodeficient NBSGW mice. This system enables erythroid studies under physiologic cytokine and cell growth conditions in vivo.26,27 CD34+ HSPCs were transduced ex vivo with either scrambled or the let-7a/f decoy TuD and subsequently transplanted into NBSGW mice (Figure 4A). Engrafted human erythroid progenitors were sorted from bone marrow and analyzed 16 weeks posttransplantation. HSPCs with let-7a/f inhibition showed similar levels of engraftment compared to controls (Figure 4B). We observed a strong but incomplete reduction of let-7a and let-7f, indicating functionality of the TuD let-7 decoy (Figure 4C). BCL11A mature mRNA and primary transcripts were reduced by ∼50% (Figure 4D), indicating transcriptional control of BCL11A upon let-7 perturbation. Cells expressing TuD displayed strong increases in HBG and HBE1, embryonic globin; whereas adult globin transcripts, HBB and HBD, were decreased (Figure 4E). To compare globin transcript levels more accurately, we utilized ddRT-PCR and found that HBG transcripts increased 90-fold from 0.5% to 45% of total β-like globin transcripts upon let-7a/f inhibition (Figure 4F).
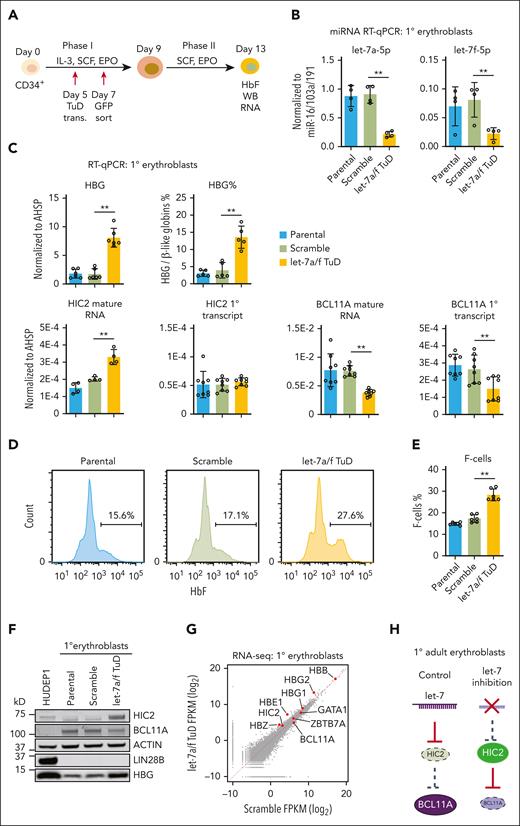
Limiting numbers of successfully transduced and engrafted cells in the NBSGW xenograft system prevented us from precise quantifications of HIC2 and other regulators. Therefore, we carried out subsequent mechanistic studies in ex vivo human erythroid cultures from CD34+ HSPCs. We transduced healthy donor CD34+ HSPCs with scramble or let-7a/f TuD and then sorted cells based on expression of a GFP reporter embedded in the vector (Figure 5A). We observed a strong but incomplete reduction of let-7a and let-7f, indicating functionality of the TuD let-7 decoy (Figure 5B; supplemental Figure 6). TuD expression triggered considerable increases in HBG mRNA and protein levels as well as a significant increase in the number of HbF-expressing cells (ie, F-cells) as measured by flow cytometric analysis (Figure 5C-F). HIC2 mature mRNA and protein levels were strongly induced upon let-7a/f inhibition (Figure 5C,F). Furthermore, both BCL11A mature and primary transcripts as well as BCL11A protein levels were strongly reduced (Figure 5C,F).
let-7a/f inhibition induced HIC2 levels in adult primary human erythroblasts. (A) Schematic of the CD34+ in vitro expansion and erythroid differentiation culture system. (B) The expression levels of let-7a-5p and let-7f-5p measured by miRNA qRT-PCR upon let-7a/f inhibition in primary human adult erythroblasts. Results are normalized to miRNAs miR-16/103a/191 and shown as mean ± SD (2 independent donors with 2 technical replicates each). Other let-7 family members are shown in supplemental Figure 6. (C) HBB, HBG, percentage of HBG, HIC2 and BCL11A mature mRNA, and primary transcript measured by qRT-PCR in let-7a/f inhibited adult human primary erythroblasts. Results are normalized to AHSP and shown as mean ± SD (n = 3 to 4 independent donors). (D) Sample flow cytometry with intracellular HBG staining in primary human erythroblasts. (E) Quantification of HBG–containing F-cells by flow cytometry. Results are shown as mean ± SD (n = 3 independent donors with 2 technical replicates for each donor). (F) Western blot with indicated antibodies of extracts from let-7a/f inhibited primary human adult erythroblasts. (G) Scatterplot of the RNA-seq results in primary human adult erythroblasts. (H) Schematic showing high let-7 levels in adult primary erythroid cells repressing HIC2; depletion of let-7 using a tough decoy leads to higher HIC2 levels and resulting decrease in BCL11A. FPKM, fragments per kilobase per million.
let-7a/f inhibition induced HIC2 levels in adult primary human erythroblasts. (A) Schematic of the CD34+ in vitro expansion and erythroid differentiation culture system. (B) The expression levels of let-7a-5p and let-7f-5p measured by miRNA qRT-PCR upon let-7a/f inhibition in primary human adult erythroblasts. Results are normalized to miRNAs miR-16/103a/191 and shown as mean ± SD (2 independent donors with 2 technical replicates each). Other let-7 family members are shown in supplemental Figure 6. (C) HBB, HBG, percentage of HBG, HIC2 and BCL11A mature mRNA, and primary transcript measured by qRT-PCR in let-7a/f inhibited adult human primary erythroblasts. Results are normalized to AHSP and shown as mean ± SD (n = 3 to 4 independent donors). (D) Sample flow cytometry with intracellular HBG staining in primary human erythroblasts. (E) Quantification of HBG–containing F-cells by flow cytometry. Results are shown as mean ± SD (n = 3 independent donors with 2 technical replicates for each donor). (F) Western blot with indicated antibodies of extracts from let-7a/f inhibited primary human adult erythroblasts. (G) Scatterplot of the RNA-seq results in primary human adult erythroblasts. (H) Schematic showing high let-7 levels in adult primary erythroid cells repressing HIC2; depletion of let-7 using a tough decoy leads to higher HIC2 levels and resulting decrease in BCL11A. FPKM, fragments per kilobase per million.
To assess the global consequences of let-7a/f inhibition, we carried out RNA-seq and observed surprisingly few gene expression changes (Figure 5G). This also indicates lack of global effects on cell differentiation, which may confound our analysis. Using a relatively lenient cutoff (fold change, >1.5 and adjusted P value <.05), we identified 20 downregulated and 105 upregulated genes in the let-7a/f inhibited primary human erythroblasts (supplemental Figure 7A). The differentially expressed genes upon let-7a/f inhibition showed significant correlations with HIC2 levels as determined by GSEA comparison, that is, the upregulated genes were enriched in the HIC2 OE cells (high HIC2 levels), whereas the downregulated genes were enriched in WT cells (low HIC2 levels) (supplemental Figure 7B). These findings suggest that let-7 functions in large part through regulation of HIC2. In concert, these results support a regulatory pathway in which let-7a/f inhibits HIC2 production, facilitating BCL11A transcription and subsequent silencing of HBG production.
let-7 regulates BCL11A and HBG through inhibition of HIC2
Given that perturbation of let-7 changed the mRNA levels of 125 genes and perhaps more at the translational level, we set out to examine whether any of these changes may be involved in the regulation of BCL11A and/or HBG, or whether HIC2 is the sole relevant target. First, we approached this question by characterizing the genome-wide changes in chromatin accessibility via ATAC-seq (assay for transposase-accessible chromatin with sequencing) upon TuD expression in primary human erythroblasts. Consistent with gene expression changes, we observed relatively few chromatin accessibility changes, with 223 and 1035 upregulated and downregulated ATAC-seq peaks, respectively (Figure 6A-B). Notably, the downregulated ATAC-seq peaks were enriched for GATA and HIC2 binding motifs. This suggests that, as we reported for the BCL11A +55 erythroid enhancer,14 HIC2 may interfere with GATA1 binding at numerous sites leading to loss of chromatin accessibility. Indeed, chromatin immunoprecipitation sequencing (ChIP-seq) experiments for HIC2 and GATA1 under conditions of TuD expression revealed that lost ATAC peaks were associated with gains in HIC2 binding and a reduction of GATA1 binding (Figure 6A). Moreover, among the strongly downregulated ATAC-seq peaks was the BCL11A +55 erythroid enhancer (Figure 6B-C). Notably, let-7a/f inhibition triggered increased HIC2 binding and reduced GATA1 binding at the BCL11A +55 enhancer (Figure 6C). Chromatin conformation capture analysis using Capture-C from the viewpoint of the BCL11A promoter showed a reduction in interactions of the promoter with the BCL11A +55 enhancer. We also observed a reduction in ATAC signal at the BCL11A +62 enhancer as well as diminished GATA1 binding at the BCL11A +58 enhancer, consistent with our previous report,14 likely stemming from a broader repressive transcriptional environment caused by HIC2 binding. The upregulated ATAC-seq peaks were enriched for NFE2L2 motifs. The biological significance of this finding is unclear and may be due to indirect effects of let-7a/f inhibition.
let-7 regulates BCL11A and HBG through inhibition of HIC2. (A) Heatmaps of differentially regulated ATAC-seq peaks of let-7a/f inhibited primary human adult erythroblasts. The HIC2 ChIP-seq of newborn erythroblasts was obtained from previously published data.14 Motifs enriched in up- and downregulated peaks are indicated on the right. (B) MA-plot of ATAC-seq peaks of let-7a/f inhibited primary human adult erythroblasts. Up- and downregulated peaks are highlighted in purple and green, respectively. HBG1/2 and BCL11A enhancers are highlighted in yellow. (C) ATAC-seq, HIC2 and GATA1 ChIP-seq tracks of let-7a/f inhibited primary human adult erythroblasts at BCL11A locus. Capture-C demonstrating interactions from a BCL11A promoter viewpoint, with subctraction track highlighting differences between scramble and let-7a/f decoy samples. (D) Western blot from rescue experiments with concurrent HIC2 depletion and let-7a/f decoy expression. (E) BCL11A, HBG, and HIC2 mRNA measured by qRT-PCR from rescue experiments with concurrent HIC2 depletion and let-7a/f decoy expression in HUDEP2 cells. Results are normalized to AHSP and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). RPM, reads per million.
let-7 regulates BCL11A and HBG through inhibition of HIC2. (A) Heatmaps of differentially regulated ATAC-seq peaks of let-7a/f inhibited primary human adult erythroblasts. The HIC2 ChIP-seq of newborn erythroblasts was obtained from previously published data.14 Motifs enriched in up- and downregulated peaks are indicated on the right. (B) MA-plot of ATAC-seq peaks of let-7a/f inhibited primary human adult erythroblasts. Up- and downregulated peaks are highlighted in purple and green, respectively. HBG1/2 and BCL11A enhancers are highlighted in yellow. (C) ATAC-seq, HIC2 and GATA1 ChIP-seq tracks of let-7a/f inhibited primary human adult erythroblasts at BCL11A locus. Capture-C demonstrating interactions from a BCL11A promoter viewpoint, with subctraction track highlighting differences between scramble and let-7a/f decoy samples. (D) Western blot from rescue experiments with concurrent HIC2 depletion and let-7a/f decoy expression. (E) BCL11A, HBG, and HIC2 mRNA measured by qRT-PCR from rescue experiments with concurrent HIC2 depletion and let-7a/f decoy expression in HUDEP2 cells. Results are normalized to AHSP and shown as mean ± SD (2 independent biological replicates with 2 technical replicates each). RPM, reads per million.
Both BCL11A mature mRNA and primary transcripts were repressed upon let-7a/f inhibition (Figure 3C and 4D), suggesting that BCL11A is primarily regulated at the transcriptional level. As miRNAs mostly function through posttranscriptional mechanisms, let-7 likely regulates BCL11A transcription indirectly. Given that HIC2 was upregulated and bound to the BCL11A +55 enhancer upon let-7a/f inhibition, we hypothesized that HIC2 is the pivotal target of let-7 that impinges on BCL11A and ultimately HBG transcription. To test this hypothesis, we depleted HIC2 in let-7a/f inhibited HUDEP2 cells. Loss of both HIC2 and let-7a/f restored the BCL11A mRNA and protein levels compared to that by let-7a/f depletion alone (Figure 6D-E). Accordingly, HBG levels were also resilenced (Figure 6D-E). Together, these results suggest that let-7 regulates BCL11A and hemoglobin switching through direct posttranscriptional inhibition of HIC2 (let-7 ⊣ HIC2 ⊣ BCL11A ⊣ HBG) (Figure 7).
Model of developmental regulation of HIC2 by let-7 miRNAs. During fetal erythropoiesis, LIN28B is highly expressed and blocks let-7 biogenesis. Without the inhibition by let-7, HIC2 represses BCL11A transcription. During adult erythropoiesis, LIN28B is downregulated, derepressing let-7 biogenesis, and inhibiting HIC2. BCL11A is highly transcribed and silences HBG.
Model of developmental regulation of HIC2 by let-7 miRNAs. During fetal erythropoiesis, LIN28B is highly expressed and blocks let-7 biogenesis. Without the inhibition by let-7, HIC2 represses BCL11A transcription. During adult erythropoiesis, LIN28B is downregulated, derepressing let-7 biogenesis, and inhibiting HIC2. BCL11A is highly transcribed and silences HBG.
Discussion
Our recent discovery of HIC2 as a transcriptional regulator of BCL11A provided the first mechanism underlying at least partially the stage-specific transcription of BCL11A and hemoglobin switching.14,28HIC2 itself is developmentally regulated, but how HIC2 is controlled remained unresolved. Here, we showed that HIC2 is posttranscriptionally silenced in adult erythroid cells via let-7, and highlight a miRNA mechanism as a means to control the levels of BCL11A and fetal globin genes during development. Specifically, the adult stage-enriched let-7 miRNAs inhibit HIC2 production to feed into a repressive regulatory network leading to BCL11A and other fetal and adult heterochronic genes controlling the developmental timing of hemoglobin switching (Figure 7). Perturbing the let-7-LIN28B circuitry led to HBG induction through HIC2-mediated repression of BCL11A, but very likely also through additional transcriptional and posttranscriptional pathways, such as those involving LIN28B, IGF2BP1, IGF2BP3, HMGA2, and others.17-19,25 Our results allow for a contribution of BCL11A translational control, but continue to demonstrate that the primary means of regulation is via transcription.
let-7 miRNAs are expressed in a developmental stage-specific fashion and are well-known to maintain the cells in an adult state.29 We previously reported that ectopic expression of HIC2 can promote partial reprogramming of adult cells toward a fetal-like state,14 suggesting HIC2 and let-7 miRNAs play opposite roles during the establishment and maintenance of developmental stages. Indeed, here we showed that let-7 miRNAs repress HIC2 in adult erythroblasts. The high correlation between let-7a/f inhibition-mediated chromatin accessibility changes and HIC2 binding profiles, as well as the significant correlations of mis-regulated genes between HIC2 overexpressing and let-7 inhibited cells (normalized enrichment score 3.54 and –4.09 by GSEA for down- and upregulated genes, respectively) (supplemental Figure 7B), suggest that let-7 miRNAs maintain an adult stage-specific transcription profile in significant part through repression of HIC2.
DICER1 is an essential enzyme that cleaves pre-miRNA to produce miRNAs. DICER1 expression is unchanged between fetal and adult erythroblasts. Hence, expression of its miRNA substrates but not DICER1 itself imparts developmental control. DICER1 depletion blocks global miRNA biogenesis. Thus, the changes of BCL11A and HBG in DICER1 depleted cells could be a combinatorial effect of global impairment of miRNAs, and subsequently misregulation of their targets. Besides let-7, previous reports have implicated other miRNAs regulate hemoglobin switching, for example miR-96,30miR-15a and miR-16-1,31miR-486-3p,32miR-92-3p,33 and the 14q32 miRNA cluster.15 Our studies showed that depletion of HIC2 almost fully restored HBG silencing in let-7a/f inhibited cells, in line with HIC2 being the relevant let-7 target in terms of BCL11A and HBG regulation. However, HIC2 depletion only partially restored HBG silencing in DICER1 KO cells, suggesting that additional miRNAs, such as those listed above, may contribute to HBG silencing.
MicroRNAs can inhibit gene expression at the level of mRNA stability or mRNA translation.34 Indeed, we observed that let-7a overexpression decreased HIC2 and LIN28B protein levels to higher degrees than their mRNA levels (Figure 2D-E), and let-7a/f loss-of-function led to greater upregulation of HIC2 protein than mRNA (Figure 3C-D). This suggests that let-7 inhibits HIC2 by affecting both mRNA turnover and translation.
In summary, our studies uncovered HIC2 as a critical target of the let-7 miRNAs system, providing a mechanism for developmental regulation of HIC2 and ultimately hemoglobin switching.
Acknowledgments
The authors thank the Children's Hospital of Philadelphia flow cytometry core for assistance with cell sorting and all the Blobel laboratory members for helpful comments. The authors acknowledge Ryo Kurita and Yukio Nakamura for sharing the HUDEP1 and HUDEP2 cells. The authors thank the DiGaetano family for their generous support.
This work was supported by grants from the St. Jude Children’s Research Hospital Collaborative Research Consortium on Novel Gene Therapies for Sickle Cell Disease, National Institutes of Health, National Heart, Lung, and Bood Institute (grant R01HL119479) to G.A.B., National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (grant K08DK128571 to E.K. and grant K08DK129716 to S.A.P.), the American Society of Hematology Research Training Award for Fellows (E.K.), and the American Society of Hematology Scholar Awards (E.K. and S.A.P.), as well as research funding from Fulcrum Therapeutics (G.A.B.). The Cooperative Centers of Excellence in Hematology at Fred Hutchinson Cancer Research Center were supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (grant DK106829).
Authorship
Contribution: E.K., P.H., J.S., and G.A.B. conceived the project and designed the experiments; E.K., P.H., S.A.P., V.S., and C.A.K. carried out experiments and analyzed data; B.G. and R.C.H. analyzed data; E.K., P.H., and G.A.B. prepared the manuscript with inputs from R.C.H. and C.A.K.; and all authors had full access to the data in this study and reviewed drafts of the manuscript and approved the final version.
Conflict-of-interest disclosure: J.S. is a scientific consultant for Treeline Biosciences. S.A.P. received research funding from Blueprint Medicines and is a consultant for Bluebird Bio and Agios. The remaining authors declare no competing financial interests.
Correspondence: Gerd A. Blobel, The Children's Hospital of Philadelphia, 3615 Civic Center Blvd, ARC 316, Philadelphia, PA 19104; email: blobel@chop.edu; and Peng Huang, Guangzhou Medical University, Science and Technology Building, Rm708, Xinzao, Panyu District, Guangzhou 511436, People’s Republic of China; email: huangpeng@gzhmu.edu.cn.
References
Author notes
The chromatin immunoprecipitation sequencing, RNA-sequencing, and ATAC-sequencing data have been deposited in the Gene Expression Omnibus database (accession number GSE216197).
Data are available on request from corresponding author Peng Huang (huangpeng@gzhmu.edu.cn).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal