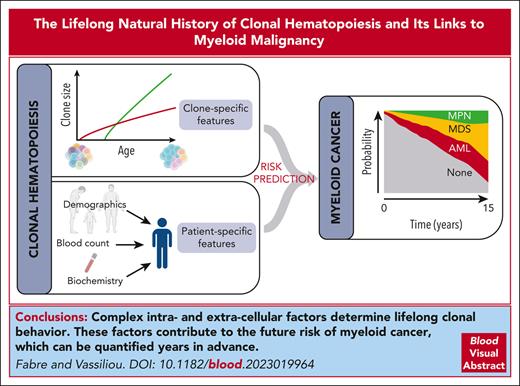
Visual Abstract
The study of somatic mutations and the associated clonal mosaicism across the human body has transformed our understanding of aging and its links to cancer. In proliferative human tissues, stem cells compete for dominance, and those with an advantage expand clonally to outgrow their peers. In the hematopoietic system, such expansion is termed clonal hematopoiesis (CH). The forces driving competition, namely heterogeneity of the hematopoietic stem cell (HSC) pool and attrition of their environment, become increasingly prominent with age. As a result, CH becomes progressively more common through life to the point of becoming essentially ubiquitous. We are beginning to unravel the specific intracellular and extracellular factors underpinning clonal behavior, with somatic mutations in specific driver genes, inflammation, telomere maintenance, extraneous exposures, and inherited genetic variation among the important players. The inevitability of CH with age combined with its unequivocal links to myeloid cancers poses a scientific and clinical challenge. Specifically, we need to decipher the factors determining clonal behavior and develop prognostic tools to identify those at high risk of malignant progression, for whom preventive interventions may be warranted. Here, we discuss how recent advances in our understanding of the natural history of CH have provided important insights into these processes and helped define future avenues of investigation.
Introduction
A pool of long-lived hematopoietic stem cells (HSCs) is at the apex of a differentiation hierarchy that produces hundreds of billions of specialized blood cells every day.1 Aging is associated with the inexorable acquisition of somatic DNA mutations across tissues, and in the blood system, mutations that augment HSC fitness can drive clonal expansion of a mutant HSC and its progeny, a phenomenon known as clonal hematopoiesis (CH).2-5 In this review, key insights into the lifelong natural history of this phenomenon and its relationship with myeloid malignancies will be discussed, and important areas of uncertainty highlighted.
Natural selection in the bone marrow niche
Although Charles Darwin developed his theory of evolution to explain the adaptation of biological organisms to their environment over generations, the same fundamental principles apply to the microcosm in which stem cells, including HSCs, operate over the lifetime of a single individual.6 Like every biological unit in an ecosystem, the fate of each HSC is governed by its ability to thrive in its environment, which depends largely on its fitness in relation to others. Fitness is the product of a complex and dynamic interplay between attributes intrinsic to the cell itself, and the characteristics of its environment.
From the moment a human zygote undergoes its first cell division, its genetic material is susceptible to alteration, such that the DNA sequences of its 2 daughter cells are not identical.7 This process of somatic mutation acquisition continues inexorably throughout life in all tissues, including the hematopoietic system.8-14 In young individuals, only a small number of somatic mutations have been acquired; diversity in the pool is, therefore, low, and the relatively homogeneous HSCs exist in a rich bone marrow environment. In this setting, the forces of Darwinian selection are weak, and many or all of the 50 to 200 000 HSCs are similarly adept at self-renewal and production of mature blood cell progeny. In contrast, by old age, the passage of time has allowed for substantial mutation accumulation: it is estimated that a typical 70-year-old human harbors up to 70 mutations per protein-coding gene across the HSC population, giving rise to considerable diversity.9-12 The combination of functionally variable HSCs and a bone marrow niche altered by aging,15 provides conditions that promote natural selection.16 In line with Darwinian theory, the HSCs best suited to the aging niche “will have the best chance of being preserved in the struggle for life,” and their cognate clones will expand relative to those less fit.6 This relative fitness advantage and subsequent expansion are the defining features of CH.
The fundamentals of clonal expansion
HSCs, like stem cells of other proliferative tissues, are responsible for the generation of mature progeny (differentiation) and maintenance of their own cell numbers (self-renewal). A sustained increase in HSC self-renewal capacity leads, over time, to the generation of an increasing number of clonally derived HSCs17 and associated mature cell progeny. The stronger the increase in self-renewal potential, the greater the advantage and clonal expansion. The precise factors driving a shift toward self-renewal are complex and incompletely understood, yet convergence on this same fundamental tendency is a prerequisite for persistent clonal expansion.
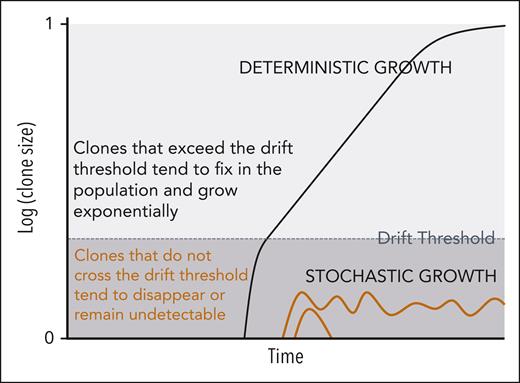
Evolutionary theory can be used to study the dynamics of stem cell populations and infer the evolutionary history of a tumor from molecular data. A framework with strong theoretical foundation that has been applied previously to the mathematical modeling of cancer evolution comprises a two-step clonal growth process with an initial stochastic phase and after the clone has reached a sufficient population size, a deterministic phase (Figure 1).18,19 The likelihood of reaching the threshold for transitioning from stochastic to deterministic growth is proportional to fitness; clones with no or slight fitness advantage are unlikely to reach sufficient size to ensure their persistence, whereas fitter clones are more likely to fix in the population and achieve deterministic growth.
Two-phase model of hematopoietic clonal growth. The likelihood of a clone transitioning from stochastic to deterministic growth is proportional to its fitness. Clones with no fitness advantage (eg, orange trajectories) are unlikely to exceed their drift thresholds and tend to disappear or remain undetectable. Fitter clones (eg, black trajectory) are more likely to reach deterministic growth.
Two-phase model of hematopoietic clonal growth. The likelihood of a clone transitioning from stochastic to deterministic growth is proportional to its fitness. Clones with no fitness advantage (eg, orange trajectories) are unlikely to exceed their drift thresholds and tend to disappear or remain undetectable. Fitter clones (eg, black trajectory) are more likely to reach deterministic growth.
The timing and dynamics of clonal behavior
Three data types have been used to characterize clonal behavior.
First, single-time-point data have been used to infer clonal expansion rates and relative fitness of different CH-driver mutations. Watson et al applied population genetic theory to pooled variant allele fraction data from ∼50 000 individuals to this end.20 Subsequently, Weinstock et al exploited the constant rate of accrual of passenger mutations over time to derive passenger-approximated clonal expansion rates in ∼5000 people with CH.21 The power of these methods lies in their potential applicability to vast cross-sectional data sets, but the granularity of information derived relies on 2 uncertain assumptions: first, that a mutation’s fitness is constant and, second, that the propensity for a mutation to found a clone is uniform throughout life.
Second, serial variant allele fraction measurements from longitudinal blood DNA sequencing data have been used to directly quantify driver-specific changes in clone size over time and the relative contributions of nonmutation factors to clonal growth.18,22 In 1 study, retrograde extrapolation modeling was also used to investigate clonal behavior over the entire period of life before the sampled window.18 Although longitudinal data sets can reveal detailed facets of clonal behavior, their utility is generally limited by their relative rarity and small scale.
Third, hematopoietic phylogenetic trees have been constructed, by whole-genome sequencing of colonies derived from single stem or progenitor cells, using somatic mutations as lineage barcodes.9,18 These capture the global clonal architecture of hematopoiesis in unprecedented detail, allow for reconstruction of clonal growth trajectories, and provide estimates of the timing of driver-mutation acquisition and clonal emergence. However, the labor intensity, high expense, and specific expertise required to use this methodology has limited its application to only a small number of cases.
Together, these 3 broad approaches have transformed our understanding of hematopoietic clonal architecture and dynamics across the human lifespan, converging on a number of key insights:
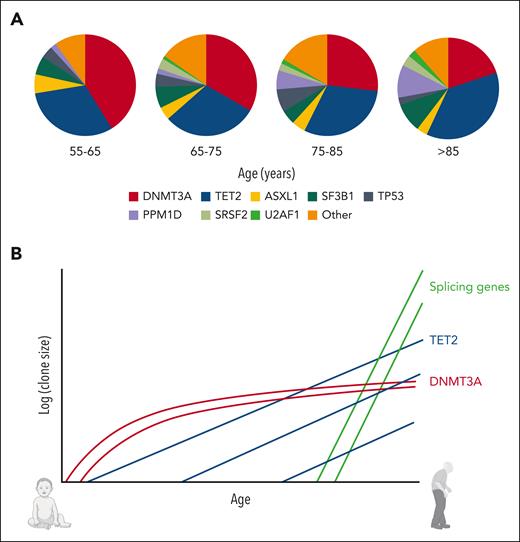
CH drivers are associated with characteristic gene-specific patterns of clonal behavior. An important feature is the lack of uniformity in CH biology with age, which is inferred by differences in mutation prevalence at different stages of life; in younger individuals, for example, DNMT3A is the most prevalent CH driver, but TET2 becomes steadily more prevalent and is the most common CH driver in those aged more than 75 years (Figure 2A).18,23 Similarly, CH driven by splicing gene mutations is rare before the age of 50 years and rises rapidly in prevalence from the seventh decade onwards, providing a compelling reason for the rise in myelodysplastic syndrome (MDS) prevalence beyond this age.4,18 Recent longitudinal and phylogenetic analyses have captured the lifelong clonal behaviors that underpin these prevalence changes, revealing, for example, that DNMT3A-mutant clones preferentially expand early in life and display slower growth in old age (∼5% per year). By contrast, splicing gene mutations only initiate clones in old age, when they drive rapid clonal growth (>50% per year for some SRSF2-mutant clones).18,22,24TET2 displays more uniform behavior throughout life, with mutant clones emerging and growing at moderate rates (∼10% per year) across all ages (Figure 2B).18
By old age, almost all clones expand at a stable exponential rate over time.18 This constancy is compelling, not only from a biological perspective but also because of its translational potential, with predictions of future clonal growth in the clinical setting now feasible. A notable outlier is JAK2-V617F–mutant CH: 1 longitudinal study showed irregular growth trajectories, with only 58% showing stable growth in old age (compared with, eg, stable growth in 99% and 94.3% of DNMT3A- and TET2-mutant clones, respectively).18
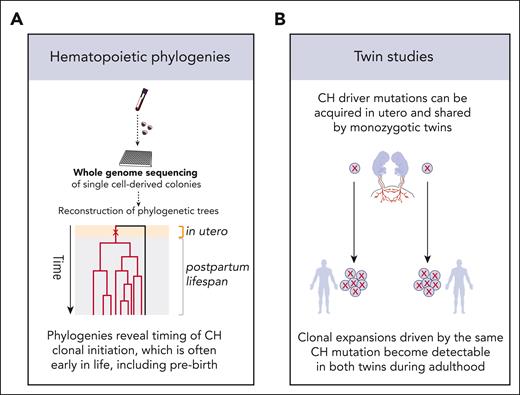
Mutation acquisition and clone foundation occur many years before CH is detected in the blood (Figure 3). For many CH clones, this process begins early in life; even in utero, an observation first made by 2 independent studies of human adult twin pairs.25,26 Across the two studies, both monozygotic twins from each of 3 (of 161) twin pairs were found to harbor identical rare somatic driver mutations, likely acquired just once within each pair in utero, either before twinning or in an HSC whose progeny reached both twins through their shared circulation. Subsequent phylogenetic analyses independently confirmed that mutations driving CH expansions detected during adulthood are often acquired early in life.9,18 Also, phylogenetic investigation of adults with myeloproliferative neoplasms (MPNs) revealed that pathogenic mutations were often acquired in early childhood or prebirth.27 More recently, a study of identical twins aged 37 and 38 years presenting with MPNs, unequivocally traced the origin of the neoplasias to a single cell acquiring a CALR mutation in utero.28 A notable exception to the described long prodrome is CH driven by splicing gene mutations. It has been noted previously that clonal expansions driven by spliceosome mutations only become detectable in old age.2-4 Recent longitudinal and phylogenetic studies have demonstrated that this may not be due to early emergence, followed by latency, and then accelerated growth in old age but is instead due to late emergence and rapid expansion.18
Throughout most of life, hematopoiesis is massively polyclonal, with high diversity and a large stable population of 50 000 to 200 000 stem and progenitor cells contributing to blood production.9 However, clonal diversity decreases profoundly in individuals aged more than 75 years, with just a few independent clones generating the vast majority of mature myeloid cells. Intriguingly, many of these expanded clades do not harbor known CH-driver mutations,9,29 and the emergence and growth dynamics of these unknown-driver clones is not dissimilar to those of clones with known drivers.18
The determinants of clonal behavior
Although substantial progress has been made in describing and characterizing lifelong clonal behavior, our understanding of the mechanisms underlying the observed patterns is limited. Here, we summarize current knowledge and highlight important gaps.
Changing prevalence spectra of CH driver genes with advancing age, and the clonal dynamics underpinning these changes. (A) DNMT3A mutations dominate the landscape of CH in younger individuals, but TET2 becomes the most prevalent driver gene in older people. Mutations in splicing factor genes and PPM1D also become more common in advanced old age. (B) Schematic illustration of gene-specific CH dynamics across the human lifespan. Typical trajectories are shown for each of 3 important CH drivers. DNMT3A-mutant clones preferentially expand early in life and display slower growth in old age; splicing gene mutations drive rapid expansion only later in life; TET2-mutant clones emerge across all ages and tend to drive moderate but stable rates of growth. Data for panel A were from Fabre et al.18
Changing prevalence spectra of CH driver genes with advancing age, and the clonal dynamics underpinning these changes. (A) DNMT3A mutations dominate the landscape of CH in younger individuals, but TET2 becomes the most prevalent driver gene in older people. Mutations in splicing factor genes and PPM1D also become more common in advanced old age. (B) Schematic illustration of gene-specific CH dynamics across the human lifespan. Typical trajectories are shown for each of 3 important CH drivers. DNMT3A-mutant clones preferentially expand early in life and display slower growth in old age; splicing gene mutations drive rapid expansion only later in life; TET2-mutant clones emerge across all ages and tend to drive moderate but stable rates of growth. Data for panel A were from Fabre et al.18
Determining the timing of CH clonal initiation. Two of the approaches used for determining the age at which CH clones emerge are construction of hematopoietic phylogenetic trees using passenger mutations as tracking barcodes (A) and studies of CH mutations in the blood DNA of monozygotic twins (B). These approaches have confirmed that mutations driving clonal growth, which become detectable in adulthood, can be acquired early in life and, in some cases, before birth.
Determining the timing of CH clonal initiation. Two of the approaches used for determining the age at which CH clones emerge are construction of hematopoietic phylogenetic trees using passenger mutations as tracking barcodes (A) and studies of CH mutations in the blood DNA of monozygotic twins (B). These approaches have confirmed that mutations driving clonal growth, which become detectable in adulthood, can be acquired early in life and, in some cases, before birth.
The identity of the CH-driver mutation is clearly influential. The concentration of mutations in a narrow set of driver genes, the fact that most of these genes are the same as recurrent drivers of myeloid cancers, and the observation that different drivers are associated with different patterns of clonal initiation and growth, all suggest that the fate of CH clones depends directly on the driver mutation. However, it is becoming clear that (1) CH mutations themselves are not always inherently advantageous in isolation but become so only under certain conditions and (2) that these conditions can be intrinsic and/or extrinsic to the mutant HSC and are often specific to the mutation type. In other words, driver mutations are necessary but not always sufficient to cause clonal expansion.
This line of thought is supported by calculations based on the number of HSCs, the size of the genome, and the rate of acquisition of somatic mutations as well as studies using exquisitely sensitive error-corrected sequencing demonstrating that essentially all individuals harbor HSCs with coding mutations in CH-driver genes by middle age.17,30 However, only a fraction of mutant HSCs ever expand to a detectable clone size. The impact of context on mutation “fitness” is most evident in situations in which HSCs are under powerful selective pressures. For example, the high prevalence in aplastic anemia (AA)-associated CH of driver mutations in PIGA, BCOR, and BCORL1 genes and in the HLA locus on chromosome 6p is compelling.31-35 In this setting, CH driven by these particular mutations is very common despite the relatively young age of patients with AA (median age, ∼30-45 years across studies), indicating that mutation acquisition is not rate limiting. Instead, it is likely that the aberrant immune environment in AA exerts pressure, selecting HSCs with preexisting mutations that confer resistance to immunological attack. Analogous is the strong selection of TP53-and PPM1D-mutant CH, specifically in the context of systemic chemotherapy,36-38 which contrasts starkly with the low fitness of TP53-mutant clones in elderly individuals not receiving chemotherapy.18
In summary, as particular cellular states or environments arise, previously neutral mutations now confer a growth advantage. These observations provide strong evidence for the premise that the fitness of any somatic mutation is governed, at least in part, by the cellular or environmental state in which it exists. However, the more nuanced questions of which factors influence mutation fitness during normal aging and why these change over life for different drivers are largely unexplored. Important unanswered questions include the following:
Why is the expansion of DNMT3A-mutant clones most rapid in young individuals and then decelerates before old age18? First, it is possible that the inherent absolute fitness conferred by a DNMT3A mutation remains constant, but deceleration results from increasing competition of the mutant clone with other expanding clones (ie, reduction in relative fitness). Indeed, the marked hematopoietic oligoclonality in elderly individuals suggests that the repressive effect of competing clones becomes pervasive, with clonal competition also demonstrated in other tissues.39 Second, it is also possible that the inherent fitness conferred by a DNMT3A mutation wanes with time because of changes in the interaction of the mutation with the aging intracellular or extracellular milieu. We can only speculate at a mechanism here, but it is intriguing that germ line DNMT3A mutations cause Tatton-Brown-Rahman syndrome, in which overgrowth is a cardinal feature,40 perhaps hinting at a relationship between DNMT3A and the action of hormones or processes involved in tissue/organismal growth.
Why do splicing gene mutations drive such rapid clonal expansion specifically during older age? Before discussing, here are 3 points of clarification. First, there is no rationale or evidence to suggest that splicing gene mutations are not acquired throughout all phases of life; instead, it appears that despite democratic accrual, they only confer fitness in the context of older age. Second, we can be confident that the age-association is not due to ascertainment bias. Specifically, it does not result from exclusion of younger individuals with splicing gene mutations from CH cohorts because of the development of hematological cancers, because the occurrence of these is far too rare to be explanatory.2-4 Third, phylogenetic and longitudinal analyses demonstrate that such clones only begin to expand after middle age (∼50 years) and that their expansion rate is rapid and stable after initiation.18 Therefore, factors that facilitate expansion of spliceosome-mutant clones, appear to be operating from middle-age onwards.
Although we have no clear understanding of the mechanism by which spliceosome mutations confer advantage in older age, 1 specific observation points to the possible importance of telomere maintenance. In 1 small study of patients with telomere biology disorders in which pathogenic germ line variants cause abnormally rapid telomere shortening, CH driven by U2AF1 mutation was detected in 12 out of 120 individuals (10%).41 This high rate of spliceosome-mutant CH is staggering, given the young age of patients profiled (median 29 years), when we contrast it with the almost complete absence of this type of CH driver in young individuals without telomere pathology.2-4 One hypothesis is that splicing gene mutations protect HSCs from the replicative senescence induced by age-related telomere shortening or alternatively that they mediate telomere length maintenance.
In contrast to these unknowns, it is now well established that the germ line genome influences the natural history of CH at the level of an individual and this provides some important mechanistic insights.21,42,43 One set of CH-risk alleles is associated with neoplasia in multiple tissues via the altered expression of genes that maintain genome integrity (eg, TERT, CHEK2, ATM, and PARP1). Polymorphisms associated with increased TERT expression displayed the strongest association with CH risk, highlighting the importance of telomere maintenance in CH development. In fact, Mendelian randomization studies propose a causative link between long telomeres and overall CH risk,43 and a recent study showed that individuals with POT1 mutations associated with long telomeres are at a high risk of CH, particularly driven by DNMT3A and JAK2 mutations.44 Collectively, these findings corroborate the importance of telomere biology in CH and may explain some of the distinct clonal behaviors associated with different driver genes.45
A second set of germ line loci is associated with the acquisition of CH mutations in specific driver genes; for example, the JAK2 46/1 haplotype predisposes to JAK2-V617F via a cis-haplotype effect. A third set of risk loci, including TCL1A and CD164, is linked specifically to CH rather than neoplasia more generally. TCL1A is particularly interesting because polymorphisms associated with higher TCL1A expression are associated with faster growth and higher prevalence of CH driven by mutations in many of the common genes, including TET2, ASXL1, SF3B1, SRSF2, and JAK2. In contrast, polymorphisms associated with low TCL1A expression increase the risk of DNMT3A-mutant CH specifically. A recent study of human CD34+ cells showed that TCL1A is aberrantly upregulated through promoter demethylation as a result of mutations in TET2 and ASXL1 (but not DNMT3A), implicating TCL1A as an important driver of clonal expansion downstream of certain common CH genes.21
Less well understood is a postulated role for inflammation in CH behavior, particularly TET2-mutant CH. One hypothesis proposes that although the long-term function of healthy HSCs is perturbed by consistent exposure to proinflammatory cytokines such as tumor necrosis factor α and interleukin-6 (IL-6), TET2-mutant HSCs might be refractory to these signals and maintain functional/proliferative integrity, providing them with a competitive advantage in an inflammatory environment.46 Furthermore, TET2 normally functions as a negative regulator of the inflammatory response in myeloid cells, for example, by recruiting HDAC2 to suppress IL-6 production.47 Therefore, TET2 loss-of-function mutations may set up a positive feedback loop through which the mutant myeloid cells secrete more IL-6, further augmenting their relative advantage over wild-type HSCs. In fact, 1 study suggested that the dependence of TET2-mutant clonal expansion on IL-6 inflammatory signals was active in mice with gut bacterial flora but not in germ-free mice.48 Concurrent evidence is emerging that different proinflammatory cytokines might induce expansion of CH with particular driver mutations, such as positive selection of DNMT3A-mutant clones by interferon gamma signaling during chronic infection.49,50 However, a recent study suggests that inflammation, including that associated with atherosclerosis, might act more broadly, causing indiscriminate expansion of HSC clones, including those with CH-driver mutations.51
Whatever the precise nature of nonmutation factors that influence clonal behavior, it is probably their variation that contributes to differences in growth rate between individuals with the same defined CH-driver mutation. In 1 longitudinal study, interindividual growth rate differences amounted to ∼±5% per year.18 It is likely that differing genetic and epigenetic landscapes also contribute. Indeed, analyses of hematopoietic phylogenies show that pervasive positive selection acts on many more genes than are currently identified9; it follows that the overall fitness of a cell harboring an identified CH-driver mutation, in DNMT3A for example, will depend on the combined fitness effects of the many additional unidentified somatic mutations in the clonal HSCs. The role of the epigenome in CH is relatively understudied, but seminal studies have begun unraveling epigenetic heritability in blood and other cancers,52-54 suggesting that stable chromatin accessibility alterations could provide a heritable substrate for Darwinian selection to operate in hematopoiesis. An intriguing hypothesis accounting for differing clonal growth rates between CH clones with the same mutant epigenetic regulator gene is that the same genetic mutation might be operating via different epigenetic mechanisms in different individuals, because distinct environmental/cellular contexts have favored selection of distinct epigenetic states.
In summary, it is now clear that the role of aging in the context of CH is far more complex than its association with time and that biological aging should be regarded as a progressive and nonlinear form of perturbation that governs the natural history of CH. Although much progress has been made, work to further improve our understanding CH behavior will underpin future efforts to intervene therapeutically.
The relationship between CH and myeloid cancers
Based on an elevated risk of hematological malignancy and the direct demonstration of clonal progression, it is now clear that CH is a premalignant state.2 Although this is unequivocal, the nomenclature and the way in which “premalignancy” is framed might require rethinking in light of the inevitability of CH and the universality of the phenomenon of clonal expansions driven by cancer-associated mutations in all proliferative tissues studied to date.8-14 According to current definitions, at least by old age, all humans live in a perpetual state of premalignancy across tissues. Although this might be true, a descriptor more consistent with the existence of CH as a feature of normal aging may be more appropriate.
The association between CH mutations and myeloid malignancy (including acute myeloid leukemia [AML], MDSs, and MPNs), is extensively observed,55-57 with CH mutations representing the “first hit” on the path to malignant transformation. Indeed, sequential acquisition of cooperating mutations and clonal evolution to cancer is well described.58 In 1 study, the identification of DNMT3A mutations in both AML blasts and sorted T lymphocytes, but the cooperating mutations only in AML blasts, confirmed that the DNMT3A mutation must have been acquired in a preleukemic HSC capable of multilineage differentiation.59 Another study investigating more than 200 000 individuals captured the process of sequential mutation acquisition by identifying low-level NPM1 mutations in 2 individuals with large DNMT3A-mutant clones, both of whom went on to develop AML within 5 months.60
However, 2 important observations imply that the process of malignant progression is often more nuanced than successive mutation acquisition. First, additional driver mutations are not always required for progression. SF3B1, for example, is the sole driver in one-third of SF3B1-mutant MDS cases,57,61 and half of patients with JAK2-V617F–mutant MPN in chronic phase lack additional drivers.55 In these cases, it appears that the expansion of the single-mutant clone may be sufficient to cause disease. Conversely, even among individuals with very large and/or rapidly expanding mutant clones, some maintain normal blood production over many years and are not, based on current definitions, deemed to have a malignancy.18 Especially in light of the prevalence of expansions without identifiable drivers,3,9,62 it appears that as humans age, normal hematopoiesis is maintained by a diminishing number of increasingly expanded clones. However, at least in some cases, the dysfunction defining myeloid cancer appears to be precipitated by the interaction of a mutant clone with factors additional to those promoting expansion in CH, and these might be genetic, epigenetic, or environmental.
Quantifying and dissecting the risk of malignant progression in people with CH has attracted substantial interest, given its potential for developing early-detection/prevention strategies. It has been consistently demonstrated that an individual’s risk of progression is higher if their CH clone is larger.63,64 However, recent evidence suggests that clonal growth rate, which itself correlates with clone size, might be more influential,18 with faster CH growth carrying a higher risk of malignant progression. Indeed, CH clonal growth rate may explain more than 50% of the variance in AML risk progression.18
Although clonal growth rate is clearly important, the overall positive correlation between CH growth and AML risk does conceal some gene-specific detail, and a notable example is DNMT3A. Longitudinal and single–time point studies showed that DNMT3A-R882 mutations do not drive substantially faster clonal growth than some other DNMT3A mutations, at least during old age.18,20 However, the propensity of DNMT3A-R882–mutant clones to progress to AML is higher than that of other DNMT3A mutations. On this note, 1 intriguing observation is the tendency for DNMT3A-R882 mutations to specifically co-occur with NPM1 mutations: in a large AML cohort,56 147 of 211 (69.7%) individuals with DNMT3A-R882 mutations also bore NPM1 gene mutations, compared with only 59of 125 (47.2%) individuals with non–R882 DNMT3A mutations (χ2P = .00004). This suggests that the enrichment of R882 mutations in AML may be driven by a specific synergy between mutant DNMT3A-R882 and NPM1 or by an increased risk of acquisition of NPM1 mutations in DNMT3A-R882 mutant HSCs.
Two independent case-control studies demonstrated that individuals at risk of progression to AML can be identified years in advance.63,64 One of these studies developed a prognostic model that incorporated characteristics of the CH somatic mutation cargo and clone size.63 Mutations in TP53, SRSF2, U2AF1, IDH1, and IDH2 were found to confer a particularly high risk of progression, and the model could identify ∼40% of individuals at highest risk, with a false positive rate ranging from 1% to 2%. Although helpful, this level of discriminatory power is not sufficient for use in population screening, because large numbers of individuals would be falsely categorized as being at high risk. More recently, models were developed for predicting the risk of developing any myeloid neoplasia (CH-risk score)65 or each of the main subtypes (AML, MDS, or MPN) separately (MN Predict; Figure 466). These risk prediction models incorporate characteristics of the expanded clone (driver mutation and clone size), demographic features (age and sex), and routinely measured complete blood count and biochemical parameters. It is noteworthy that although some features add predictive power across the spectrum of myeloid neoplasms (eg, hemoglobin concentration) and are, therefore, included in all 3 disease models, other features are disease subtype-specific and only included in the relevant model (eg, mean red blood cell volume and red blood cell distribution width are included for AML and MDS but not MPN).66
Predicting progression to myeloid malignancy. (A) Four potential outcomes of expanded CH clones are depicted. (B-D) Three examples of risk forecasting by MN-Predict, a recently developed web-based tool for qualification of the future risks of different types of myeloid neoplasm (MN).66 MN-Predict forecasts an individual’s risks of AML, MDS, and MPN over the subsequent 15 years based on their age, sex, genetic data (mutated CH gene and variant allele fraction [VAF]), and specific complete blood count and biochemistry parameters. The examples illustrate baseline risk forecasting for 3 UK Biobank participants with the indicated characteristics who went on to develop the specified MN (panels B, AML; C, MDS; and D, MPN) after the depicted latency.
Predicting progression to myeloid malignancy. (A) Four potential outcomes of expanded CH clones are depicted. (B-D) Three examples of risk forecasting by MN-Predict, a recently developed web-based tool for qualification of the future risks of different types of myeloid neoplasm (MN).66 MN-Predict forecasts an individual’s risks of AML, MDS, and MPN over the subsequent 15 years based on their age, sex, genetic data (mutated CH gene and variant allele fraction [VAF]), and specific complete blood count and biochemistry parameters. The examples illustrate baseline risk forecasting for 3 UK Biobank participants with the indicated characteristics who went on to develop the specified MN (panels B, AML; C, MDS; and D, MPN) after the depicted latency.
Future iterations of predictive models will likely be improved by considering the impact of multiple mutations in the same gene, incorporating a dynamic component such as clonal growth rate in serial samples and adding assays for specific plasma proteins/cytokines. Alternative models will be required in the context of specific perturbations, such as postchemotherapy CH, in which selective landscapes and consequent risk profiling are altered.67 As the field of myeloid cancer prevention advances, predictive models may be used by a broad community of researchers and physicians to help stratify patients for potential clinical interventions. The additional causal role for CH in nonhematological pathology68,69 provides an opportunity for risk reduction across a range of diseases, benign as well as malignant, through therapeutic modulation of CH.
Conclusions
The discovery of CH and the realization that it forms part of a widespread phenomenon of clonal expansions across human tissues has changed the way we view the relationships between aging, premalignancy, and cancer. The investigation of cell-intrinsic and cell-extrinsic mechanisms underpinning clonal behavior is transforming our understanding of what constitutes “normal,” how this relates to cancer, and how we might intervene at a clinically meaningful point during the complex transition between the 2 states. Future preventive and therapeutic advances will rely on an improved understanding of how different CH gene mutations drive clonal expansion, the contexts that facilitate this process, and the overarching influence of aging. Given the dramatic rate of progress since the first definitive descriptions of CH, it is reasonable to expect to see therapeutic interventions entering clinical practice within the next decade.
Acknowledgments
The authors thank Moritz Gerstung and José Guilherme de Almeida for input into ideas and analyses relating to their joint investigation of the longitudinal dynamics of CH and Muxin Gu for help with MN Predict plots in Figure 4.
This work was supported by the Leukemia and Lymphoma Society, Cancer Research UK, European Research Council, The Rising Tide Foundation for Clinical Cancer Research, Wellcome, and Blood Cancer UK.
Authorship
Contribution: M.A.F. and G.S.V. cowrote the manuscript.
Conflict-of-interest disclosure: G.S.V. is a consultant to STRM.BIO and holds a research grant from AstraZeneca for research unrelated to that presented here. M.A.F. is an employee and stockholder of AstraZeneca.
Correspondence: George S. Vassiliou, Department of Haematology, Wellcome-MRC Cambridge Stem Cell Institute, University of Cambridge, Cambridge CB2 0AW, United Kingdom; email: gsv20@cam.ac.uk.

![Predicting progression to myeloid malignancy. (A) Four potential outcomes of expanded CH clones are depicted. (B-D) Three examples of risk forecasting by MN-Predict, a recently developed web-based tool for qualification of the future risks of different types of myeloid neoplasm (MN).66 MN-Predict forecasts an individual’s risks of AML, MDS, and MPN over the subsequent 15 years based on their age, sex, genetic data (mutated CH gene and variant allele fraction [VAF]), and specific complete blood count and biochemistry parameters. The examples illustrate baseline risk forecasting for 3 UK Biobank participants with the indicated characteristics who went on to develop the specified MN (panels B, AML; C, MDS; and D, MPN) after the depicted latency.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/7/10.1182_blood.2023019964/4/m_blood_bld-2023-019964-c-gr4.jpeg?Expires=1769091469&Signature=ZXQjx0jTBiWFiqN4MXiXzd-77IXMNDWRUO2hRPyeOrDTerPYhSlmPCdEFQTRdkxHU9wzuZYP3KHSQZmcS3ZtmTQQe7nIMN4OUgURk9fZYi4yZQIUyYxQvqcW8mF2gNE47mpDpTdiAgJwILxhmOMwMqvFmXsR9vERMhnPAJiy3J2yv1RGkQFh1iw0Fghl7bYhHiX1RLYD1jfHh9cwzCFctxfzvWYalQLDMZCoBJJta9Wp1YsCSCq81Ug4B3mYmt6CVlZZ74~OCoLy-zWSeKpNHGv6eqoZ6Wzv06UThi7E8JaWUuCBBH7S7~fq5WEkoeb5AkUa5LC0dIyX-8K9BdbtSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal