Abstract
We have previously demonstrated that young adult DBA/2 (DBA) mice have more stem cells than C57BL/6 (B6) mice, as measured in a cobblestone area-forming cell (CAFC) assay using unfractionated marrow. To study the nature of this difference, we have now compared the proliferative fate of single, highly enriched Sca-1+c-kit+Lin−stem cells from these strains. Although equal in frequency, functional comparison revealed that Sca-1+c-kit+Lin−cells from DBA mice contained twice as many cells with CAFC activity. DBA clones persisted much longer in vitro, and developed later in time. To assess whether these differences were of any functional relevance in vivo, we compared engraftment of lethally irradiated mice transplanted with 1000 B6 or DBA Sca-1+c-kit+Lin−cells. Recipients of enriched DBA cells recovered much faster than animals transplanted with B6 cells. We also studied endogenous hematopoietic recovery after 5-fluorouracil (5-FU) treatment in vivo. Progenitors and peripheral blood cells recovered twice as fast in DBA mice. Thus, DBA stem cells have superior proliferative potential compared with phenotypically identical stem cells obtained from B6 mice. Such genetically determined quantitative and qualitative differences in stem cell behavior likely contribute to the dramatically different hematopoietic recovery rates observed in human transplant patients.
Introduction
Hematopoietic stem cells have the potential to produce mature blood cells of at least 8 different lineages. This impressive proliferative capacity is most convincingly demonstrated by the ability of a single intravenously transplanted stem cell to fully reconstitute the hematopoietic system of a lethally irradiated mouse.1-5 Although several studies suggest that stem cell potential is subject to replicative erosion during ontogeny and aging,6-9 in the mouse, stem cells can be expanded in vivo to an extent that is unlikely to be encountered during normal physiology.10-12 These developmental properties have paved the way for the application of hematopoietic stem cells in a wide variety of clinical situations. Most notably, stem cells harvested from the bone marrow or neonatal and adult peripheral blood can be cryopreserved and used for hematopoietic rescue in allogeneic and autologous transplantation protocols. Stem cell transplantation after high-dose chemotherapy has enabled a significant improvement in the cure of patients affected by a variety of malignancies.13However, the rate of recovery of circulating blood cells in transplanted patients is highly variable, and even with substantial growth factor support some patients fail to normalize blood cell counts in a clinically acceptable time frame.14,15 Undoubtedly, this heterogeneity results partly from prior cytotoxic treatment, disease status, and the number of cells infused,14 but even in patients that have undergone treatments of apparent equal intensity, wide variations in hematopoietic response are not unusual. These data suggest that patient-to-patient variation in hematopoietic recovery after stem cell transplantation may have a genetic basis. Indeed, the heterogeneity in patient responses to therapeutic agents in general, and the genetic basis of this variation, has established the nascent field of pharmacogenomics, which is propelled by advances made in the Human Genome Project.16
The involvement of genetic factors in the regulation of hematopoietic cell production is perhaps most compellingly demonstrated in a series of preclinical mouse studies, partly performed in our own laboratories.17-23 We have shown that the frequency of hematopoietic progenitors and stem cells in unfractionated marrow from DBA/2 (DBA) and AKR/J mice is higher than in all other strains tested, including C57BL/6 (B6).20 We have demonstrated that DBA progenitor cells have a higher proliferative activity than B6 cells,17,21 and embryo-aggregation studies have suggested that this is a cell-intrinsic trait.18
In this study, we have investigated in more detail the functional activity of DBA and B6 stem cells. We compared the in vitro growth kinetics of single, highly enriched Sca-1+c-kit+Lin− stem cells from DBA and B6 mice using the cobblestone area-forming cell (CAFC) assay. To assess whether the distinct in vitro growth properties of these 2 stem cell genotypes had any relevance for hematopoietic cell production in vivo, we subsequently established the engraftment kinetics of lethally irradiated recipient mice transplanted with 1000 of these purified cells obtained from B6 or DBA donors. Finally, we studied the recovery of the hematopoietic system in DBA and B6 mice after myeloablation with 5-fluorouracil (5-FU). Overall, our results show that stem cells from DBA mice exhibit more “primitive” developmental characteristics than their phenotypically identical counterparts from B6 mice. Such genetically determined quantitative and qualitative differences in stem cell behavior likely contribute to the dramatically different outcomes observed in human transplant patients.
Material and methods
Mice
C57BL/6J and DBA/2J mice were obtained from The Jackson Laboratories (Bar Harbor, ME) and from Charles River/Broekman Instituut (Someren, the Netherlands). Mice were used at 8 to 10 weeks of age, except for one experiment in which mice were 10 months old. These mice were aged in the animal facilities of the University of Kentucky under specific pathogen-free conditions.
Enrichment of hematopoietic stem cells
Bone marrow cells were flushed from the femora and tibiae of B6 or DBA mice using a 21-gauge needle and syringe into Hank's balanced salt solution (HBSS) containing 2% fetal calf serum (HF medium). Erythrocytes were eliminated by hypotonic lysis, and the remaining leukocytes incubated with saturating amounts of biotinylated rat monoclonal antibodies (MoAbs) specific for murine CD3 (clone KT3.1), CD5 (clone 53-7.3), CD8 (clone 53-6.72), CD11b/Mac-1 (clone M1/70), CD45R/B220 (clone RA3-6B2), and Ly-6G/Gr-1 (clone RB6-8C5). The labeled cells were then incubated with goat antirat IgG paramagnetic beads (Dynal, Lake Shearer, NY) at a bead:cell ratio of approximately 4:1, and mature lymphoid and myeloid cells were removed by exposure to a magnetic field. Lineage-depleted cells were blocked with anti-CD16/32 (clone 2.4G2, Fc Block), and then stained with phycoerythrin (PE)-conjugated anti-Ly-6A/E (Sca-1; clone E13-161.7), allophycocyanin (APC)-conjugated anti-CD117 (c-kit; clone 2B8) MoAbs, and streptavidin∼FITC (all from Pharmingen, San Diego, CA). Stained cells were washed and resuspended in HF containing 5 μg/mL propidium iodide (PI). Unstained cells or lineage-depleted cells stained with appropriate fluorochrome-conjugated isotypic control MoAbs (Pharmingen, San Diego, CA) or streptavidin∼FITC were used as background controls. Viable (PI−) Sca-1+c-kit+Lin− cells were sorted into 96-well plates for CAFC assays using a dual-laser FACS Vantage instrument equipped with an automated cell deposition unit (ACDU) (Becton Dickinson Immunocytometry Systems, San Jose, CA). Reanalysis of the sorted cells immediately after sorting indicated a purity typically more than 90%.
Measurement of in vivo hematopoietic reconstitution kinetics
The 1000 enriched B6 or DBA stem cells were intravenously injected into 21 (Ly-5 congenic) B6.SJL or 33 syngeneic DBA hosts, respectively. Recipient mice were exposed to 9 Gy total body γ-irradiation administered in 2 doses of 4.5 Gy approximately 3 hours apart just before transplantation. Mice were bled from the retro-orbital sinus 6, 9, 12, 15, 18, 25, 32, and 42 days later. Until day 25, only half of the mice in each cohort was analyzed alternately at each time so that no individual animal was bled more frequently than every 7 days. Circulating leukocyte, erythrocyte, and platelet counts were measured by analysis of 40 μL of blood using a System 9118+ Hematology Series Cell Counter (BioChem ImmunoSystems Inc, Allentown, PA).
Administration of 5-fluorouracil
Mice were subcutaneously injected with 5-FU (Fluracedyl, Pharmachemie, Haarlem, The Netherlands) at a dose of 200 mg/kg in saline. Three mice were killed per timepoint. Mice were bled from the retro-orbital venous plexus, and white blood cells were quantified using an automated cell analyzer (Coulter Model ZF, Coulter Electronics Ltd, Dunstable, Beds, England). Hematocrit values were calculated after centrifugation of approximately 100 μL of anticoagulated blood in microcapillary tubes.
Hematopoietic cell assays
The CAFC assay was performed exactly as detailed previously.20 In short, confluent monolayers of FBMD-1 stromal cells were established in 96-well tissue culture-treated plates. After 10 to 14 days, wells were seeded either with sorted Sca-1+c-kit+Lin− cells at a dose of 1, 3, 10, or 30 cells per well using an automated cell deposition unit, or with unfractionated marrow at a dose of 81 000, 27 000, 9 000, 3 000, 1 000, or 333 cells per well. Typically 30 replicate wells per dilution were evaluated. However, in experiments with sorted cells 120 replicate wells were seeded with 1 cell, 60 wells with 3 or 10 cells, and 10 to 30 replicate wells were seeded with 30 cells. Individual wells were screened every 3 to 4 days for the presence of a “cobblestone area,” defined as a colony of at least 5 cells growing underneath the stroma, according to definitions established by Ploemacher et al.24 Frequency estimates were calculated using maximum likelihood analysis,25 considering at least 3 cell doses that yielded both negative and positive wells.
Results
Cobblestone area-forming cell frequencies in purified DBA and B6 Sca-1+c-kit+Lin−stem cell populations
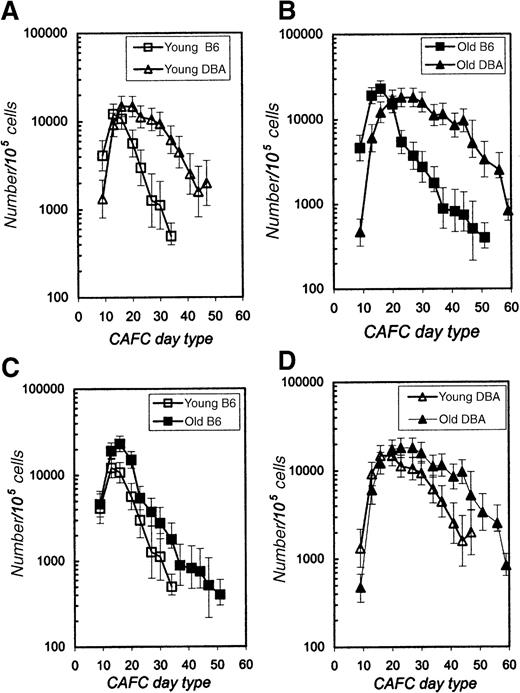
We have previously demonstrated that the frequency of late-appearing (day 28-35) CAFC subsets in unfractionated marrow of young DBA mice is approximately 3-fold higher than in B6 mice.20,26 To investigate this variation in more detail, we compared the CAFC activity present in highly enriched Sca-1+c-kit+Lin− cells isolated from the bone marrow of both strains. Two independent sorting experiments were performed. In the first experiment, bone marrow was harvested from a single 10-month-old mouse of each strain. In the second sorting experiment, marrow cells were pooled from 3 8-week-old mice of each strain. The immunophenotype of both young and old, as well as of DBA and B6 cells, was very comparable, and thus the same sorting gates were used throughout the study. Six percent to 12% of marrow cells were lineage negative. Approximately 0.3% to 0.5% of the Lin− cells expressed both c-kit and Sca-1, and this fraction was sorted and evaluated for CAFC activity. Sorted cells were seeded in a limiting-dilution fashion (1, 3, 10, or 30 cells per well) onto preestablished stromal cell layers in 96-well plates, as described in the “Materials and methods.” Figure1 depicts the CAFC frequency as a function of culturing time. DBA cells are compared with B6 cells obtained from young (Figure 1A) or old (Figure 1B) donors. It is evident that the general pattern for both experiments was very similar. During the first 9 days of culture, no cobblestone areas were detected in any well, indicating that the stringent selection criteria that were used in the sorting protocol depleted the most mature progenitor cells that typically appear between days 5 and 7.20,24 In young animals, CAFC activity in the Sca-1+c-kit+Lin− fraction peaked at day 14 in B6 mice, and at day 21 in DBA mice, reaching values in both strains of approximately 104 CAFC per 105(ie, 10%) sorted cells (Figure 1A). CAFC frequency in B6 sorted cells decreased rapidly thereafter and no positive wells were detected after day 35. In contrast, assay of sorted DBA cells showed that the decline in CAFC frequency was slower, and some wells remained positive even after day 50. We have previously measured CAFC activity in unfractionated marrow from these strains, and found that the frequency of CAFC day 35 is approximately 1 per 105 B6 cells and approximately 3 per 105 DBA cells.26 The current experiments reveal that this variation in stem cell frequency is more pronounced when purified cells are studied. Table1 compares CAFC frequencies in unfractionated marrow and the stem cell–enriched fraction from young mice. CAFC day 35 frequency was 497 per 105 cells for sorted B6 cells, and 6172 per 105 cells for sorted DBA cells, ie, the frequency of this stem cell subset was more than 10-fold higher among Sca-1+c-kit+Lin− DBA cells than in phenotypically identical B6 cells.
CAFC frequency in purified Sca-1+c-kit+Lin−cells obtained from young (8-10 weeks) and old (10 months) B6 and DBA mice.
In panel A, cells from young B6 and DBA mice are compared, whereas panel B shows results obtained with cells from old mice of both strains. In panels C and D the same data are shown, but to allow detailed comparison of intrastrain age effects, data for young and old B6 (panel C) and young and old DBA (panel D) are plotted in one graph. Error bars indicate 95% confidence limits for the limiting-dilution analysis.
CAFC frequency in purified Sca-1+c-kit+Lin−cells obtained from young (8-10 weeks) and old (10 months) B6 and DBA mice.
In panel A, cells from young B6 and DBA mice are compared, whereas panel B shows results obtained with cells from old mice of both strains. In panels C and D the same data are shown, but to allow detailed comparison of intrastrain age effects, data for young and old B6 (panel C) and young and old DBA (panel D) are plotted in one graph. Error bars indicate 95% confidence limits for the limiting-dilution analysis.
CAFC frequency in unfractionated marrow and sorted Sca-1+c-kit+Lin−stem cells from young B6 and DBA mice
| . | CAFC d7/105 cells . | CAFC d14/105cells . | CAFC d21/105 cells . | CAFC d28/105cells . | CAFC d35/105 cells . |
|---|---|---|---|---|---|
| B6 unfractionated | 156 | 19.4 | 8.6 | 2.7 | 1.5 |
| B6 Sca-1+c-kit+Lin− | nd | 12 177 | 5 645 | 1 265 | 497 |
| B6 fold enrichment | <1× | 627× | 656× | 469× | 331× |
| DBA unfractionated | 237 | 57 | 23 | 8.6 | 3.4 |
| DBA Sca-1+c-kit+Lin− | nd | 9 197 | 14 782 | 10 534 | 6 172 |
| DBA enrichment | <1× | 161× | 642× | 1 225× | 1 815× |
| . | CAFC d7/105 cells . | CAFC d14/105cells . | CAFC d21/105 cells . | CAFC d28/105cells . | CAFC d35/105 cells . |
|---|---|---|---|---|---|
| B6 unfractionated | 156 | 19.4 | 8.6 | 2.7 | 1.5 |
| B6 Sca-1+c-kit+Lin− | nd | 12 177 | 5 645 | 1 265 | 497 |
| B6 fold enrichment | <1× | 627× | 656× | 469× | 331× |
| DBA unfractionated | 237 | 57 | 23 | 8.6 | 3.4 |
| DBA Sca-1+c-kit+Lin− | nd | 9 197 | 14 782 | 10 534 | 6 172 |
| DBA enrichment | <1× | 161× | 642× | 1 225× | 1 815× |
nd = not detected. The frequency of Sca-1+c-kit+Lin− cells in unfractionated marrow was approximately 30 to 40 per 105 in both strains (or 0.03%-0.04% of the total population).
Intrastrain comparisons of young and old mice, as shown in Figure 1C and D for B6 and DBA respectively, revealed that late-appearing CAFC activity in sorted cells from old mice was higher than in cells from young mice. This is in agreement with our previous finding when we quantified CAFC subsets using unfractionated marrow from young and 1-year-old mice.20 27 In addition, CAFC activity of old cells could be detected at later timepoints (more than 50 days) than that of young cells.
The fate of single, purified DBA and B6 Sca-1+c-kit+Lin−stem cells
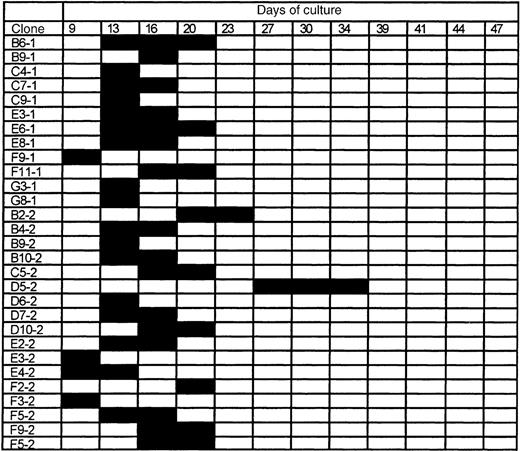
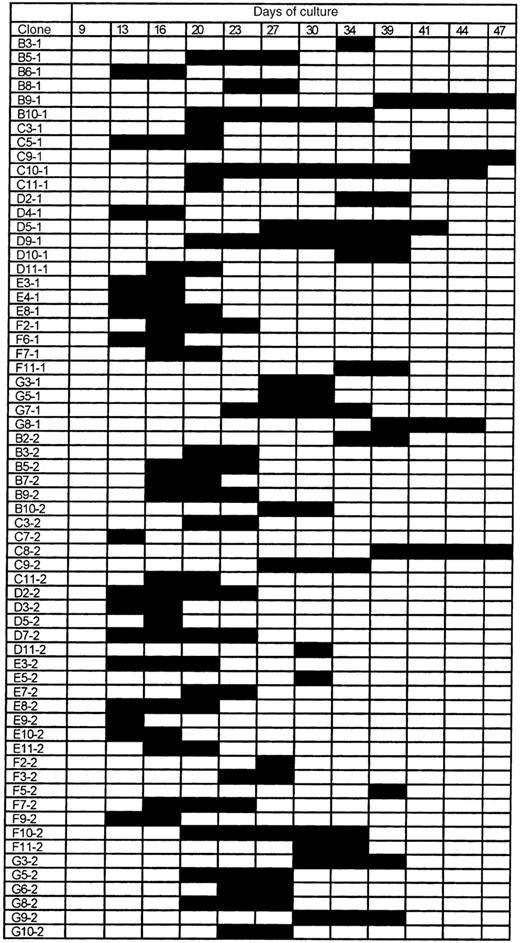
To further characterize these functional differences, we investigated the growth properties of 120 single Sca-1+c-kit+Lin− cells from both young and old DBA and B6 mice in the CAFC assay system. Every 3 to 4 days, all 480 (4 × 120) wells were screened for cobblestone activity to track the proliferative fate of each individual cell. Figures2 and 3show the results for young B6 and DBA cells, respectively. The data obtained for single sorted cells from 10-month-old mice were very similar (data not shown).
The proliferative fate of individual Sca-1+c-kit+Lin− bone marrow cells from young B6 mice.
A total of 120 individual cells were analyzed for CAFC potential, of which 29 (here shown) started to proliferate within a period of 50 days. Black bars indicate the timepoints at which cobblestone areas were detected. Clone numbers refer to the address of wells in which cell growth was observed.
The proliferative fate of individual Sca-1+c-kit+Lin− bone marrow cells from young B6 mice.
A total of 120 individual cells were analyzed for CAFC potential, of which 29 (here shown) started to proliferate within a period of 50 days. Black bars indicate the timepoints at which cobblestone areas were detected. Clone numbers refer to the address of wells in which cell growth was observed.
The proliferative fate of individual Sca-1+c-kit+Lin−cells from young DBA mice.
A total of 120 individual cells were analyzed for CAFC potential, of which 64 (shown here) started to proliferate within a period of 50 days. Black bars indicate the timepoints at which cobblestone areas were detected. Clone numbers refer to the address of wells in which cell growth was observed.
The proliferative fate of individual Sca-1+c-kit+Lin−cells from young DBA mice.
A total of 120 individual cells were analyzed for CAFC potential, of which 64 (shown here) started to proliferate within a period of 50 days. Black bars indicate the timepoints at which cobblestone areas were detected. Clone numbers refer to the address of wells in which cell growth was observed.
Of a total of 120 young B6 stem cells, 29 (ie, 24%) gave rise to a cobblestone area at some time during the culture period. For old cells, this number was 43 per 120, ie, 35%. The first wells4became positive at day 9, and the last positive well was observed at day 34. Colonies persisted for a maximum interval of 3 observation points (ie, approximately 8 days).
In contrast, when the 120 DBA stem cells were evaluated, major differences compared with B6 cells were observed (Figure 3). The total number of cells with cobblestone area-forming potential was 64% or 53%. For old DBA cells, this number was 66% or 55%. No well with visible hematopoietic activity was detected before day 13. Although only a minority (3 of 29) of the B6 colonies remained for a maximum of 3 timepoints, most DBA colonies persisted for at least this long, and clone C10-1 remained for 8 timepoints (approximately 25 days).
Overall, Sca-1+c-kit+Lin− cells from DBA mice: (a) contained twice as many cells with cobblestone area-forming activity, (b) persisted much longer in vitro, and (c) initiated hematopoiesis later in time. Interestingly, in contrast to our predictions, there was no significant correlation between the time of first appearance of a colony and its persistence in culture (r2 = 0.13 and 0.002 for B6 and DBA, respectively). Instead, the proliferative fate of individual clonogenic cells was unpredictable in both strains.
Engraftment kinetics after transplantation of 1000 purified DBA and B6 Sca-1+c-kit+Lin−stem cells
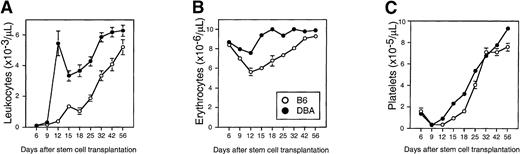
To examine whether the distinct in vitro growth characteristics of stem cells had any in vivo relevance, we transplanted 1000 DBA or B6 Sca-1+c-kit+Lin− cells into syngeneic and congenic recipients, respectively, and monitored the regeneration of circulating leukocytes, erythrocytes, and platelets for 2 months (Figure 4). White and red blood cell counts in mice transplanted with enriched DBA stem cells recovered to normal values in approximately 12 days (Figure 4A and B). Leukocyte engraftment was biphasic, so counts did drop transiently to approximately 50% of normal on day 15 before recovering permanently by day 32. In contrast, mice transplanted with enriched B6 stem cells showed delayed recovery as blood cell counts failed to normalize until approximately 30 to 40 days. Platelet counts recovered significantly faster in DBA-stem cell–transplanted recipients as well, but the effect was less pronounced as for the other blood cell lineages (Figure 4C).
Differential engraftment kinetics of enriched stem cells isolated from B6 or DBA mice.
Sca-1+c-kit+Lin− bone marrow cells were sorted from B6 (open circles) or DBA (closed circles) marrow and transplanted into lethally irradiated Ly-5 congenic (B6.SJL) or syngeneic DBA recipients, respectively (1000 cells per mouse). Peripheral blood leukocytes (panel A), erythrocytes (panel B), and platelets (panel C) were counted on the indicated days. Shown are the mean ± SEM number of blood cells for pooled data from 2 independent experiments comprising a total of 21 (B6) or 33 (DBA) mice. Error bars not shown are too small for the scale used. DBA values are significantly greater than B6 values (P < .01) at all times after day 9 (day 6 for red cells), except for platelets on day 32. Normal blood counts for B6 and DBA mice, respectively, are leukocytes, 6 to 9 × 103 and 4 to 7 × 103; erythrocytes, 9 to 11 × 106 and 7 to 10 × 106; platelets, 11 to 14 × 105, and 10 to 11 × 105.
Differential engraftment kinetics of enriched stem cells isolated from B6 or DBA mice.
Sca-1+c-kit+Lin− bone marrow cells were sorted from B6 (open circles) or DBA (closed circles) marrow and transplanted into lethally irradiated Ly-5 congenic (B6.SJL) or syngeneic DBA recipients, respectively (1000 cells per mouse). Peripheral blood leukocytes (panel A), erythrocytes (panel B), and platelets (panel C) were counted on the indicated days. Shown are the mean ± SEM number of blood cells for pooled data from 2 independent experiments comprising a total of 21 (B6) or 33 (DBA) mice. Error bars not shown are too small for the scale used. DBA values are significantly greater than B6 values (P < .01) at all times after day 9 (day 6 for red cells), except for platelets on day 32. Normal blood counts for B6 and DBA mice, respectively, are leukocytes, 6 to 9 × 103 and 4 to 7 × 103; erythrocytes, 9 to 11 × 106 and 7 to 10 × 106; platelets, 11 to 14 × 105, and 10 to 11 × 105.
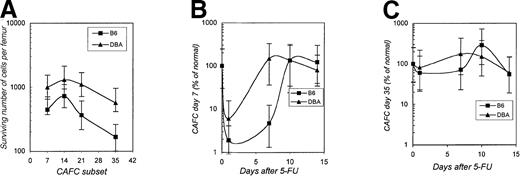
Stem cell pool size correlates with hematopoietic recovery rate after myeloablation
Finally, we compared the tempo of recovery of several hematopoietic parameters in DBA and B6 mice after administration of a single dose of 5-FU (200 mg/kg). Figure5A depicts the number of various CAFC subsets surviving 24 hours after 5-FU administration in DBA and B6 mice. Because of the larger total stem cell pool in normal DBA mice (Table 1), the absolute number of all CAFC subsets that survived 5-FU treatment was higher in this strain. Next, the recovery kinetics of the bone marrow CAFC day-7 and day-35 compartments was determined from days 1 to 14 after 5-FU. CAFC day-7 numbers recovered approximately twice as fast in DBA mice than in B6 mice (Figure 5B). At day 7, the number of CAFC day-7 numbers in B6 mice was still less than 5% of normal, whereas progenitors in DBA mice had already normalized. Similarly, the recovery kinetics of more primitive CAFC day 35 were very different in the 2 strains (Figure 5C). In B6 mice, stem cell numbers showed a nadir between 2 and 7 days after 5-FU treatment, and exhibited a profound overshoot of normal numbers around day 10. Stem cells in DBA mice were also reduced initially, but to a much lesser extent than in B6, and, perhaps as a result, the overshoot was less dramatic.
Progenitor and stem cell pool size after administration of 5-FU to B6 and DBA mice.
Panel A depicts the absolute number of CAFC day 7, day 14, day 21, and day 35 per femur that remain viable 24 hours after 5-FU treatment. Panel B and C show recovery kinetics expressed as percentage of normal values of respectivily marrow CAFC day 7 and day 35 numbers after administration of 5-FU. Normal values for B6 and DBA mice, respectively, are CAFC day-7, 20 to 25 × 103 and 14 to 18 × 103; CAFC day-35, 250 to 300 and 600 to 800. Error bars indicate 95% confidence intervals.
Progenitor and stem cell pool size after administration of 5-FU to B6 and DBA mice.
Panel A depicts the absolute number of CAFC day 7, day 14, day 21, and day 35 per femur that remain viable 24 hours after 5-FU treatment. Panel B and C show recovery kinetics expressed as percentage of normal values of respectivily marrow CAFC day 7 and day 35 numbers after administration of 5-FU. Normal values for B6 and DBA mice, respectively, are CAFC day-7, 20 to 25 × 103 and 14 to 18 × 103; CAFC day-35, 250 to 300 and 600 to 800. Error bars indicate 95% confidence intervals.
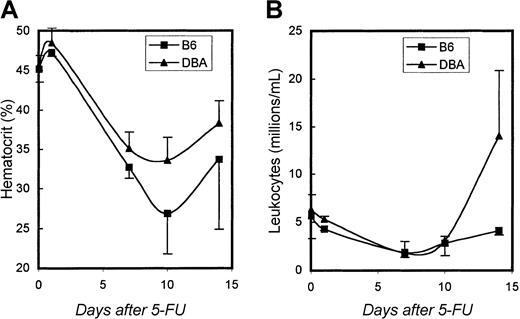
To determine whether these CAFC recovery differences were of any relevance for mature blood cell production, we also measured peripheral blood cell counts in these same mice. Figure6A and B show hematocrit values and white blood cell counts, respectively. During the first week after 5-FU, no significant differences were detected between the 2 strains. However, consistent with the pattern of progenitor cell recovery shown in Figure5B, hematocrit values began to recover faster in DBA mice than in B6 mice, and DBA leukocyte counts exhibited a strong overshoot that was not observed in B6 animals.
Hematocrit and leukocyte counts.
Recovery of hematocrit (A), and leukocyte counts (B) after administration of 5-FU to B6 and DBA mice. Data are shown as mean values ± 1 SD.
Hematocrit and leukocyte counts.
Recovery of hematocrit (A), and leukocyte counts (B) after administration of 5-FU to B6 and DBA mice. Data are shown as mean values ± 1 SD.
Discussion
Variation in the frequency, proliferative activity, and mobilization potential of mouse and human hematopoietic stem cells is well described.20-23,26,28 Clinically, this is manifested in a multitude of parameters, ranging from differences in response to hematopoietic growth factors, to hemotoxicity after chemotherapy and speed of recovery after stem cell transplantation. Elucidation of the mechanisms contributing to such variation are of significant importance to the field of stem cell biology and transplantation. To address these issues, we have previously focused on 2 strains of mice that possess widely disparate hematopoietic stem cell characteristics; B6 mice have relatively few stem cells with a low proliferative rate, whereas DBA mice have several-fold more cells with a significantly higher cycling activity.20-22,26 Chromosomal loci have been identified that are involved in some of the traits in which these 2 strains differ.21,22 26 In parallel with genetic approaches ultimately directed at identifying genes involved in this variation, this study was conducted to characterize in detail differences in the functional behavior in vitro and in vivo of stem cells from these 2 model strains.
DBA mice have been shown to have a much larger late-appearing CAFC and LTC-IC compartment than B6 mice.22,26 Therefore, we anticipated that the number of Sca-1+c-kit+Lin− cells, a population that is known to be highly enriched for cells with long-term repopulating ability29 would be higher in DBA than in B6 mice. Our results did not support this; rather, in both strains, the fraction of these cells was approximately 0.04% of the total bone marrow cells. However, when we compared these highly enriched cells functionally in vitro and in vivo, large strain variation became evident, even more than observed in our previous studies with unfractionated bone marrow cells. The CAFC day-35 frequency within the purified population was more than 10-fold higher in DBA than in B6 mice. The single cell experiments revealed particularly different growth properties between DBA and B6 stem cells. Individual DBA Sca-1+c-kit+Lin− cells proliferated in vitro at a higher frequency than B6 cells, they started to divide later, and colonies growing underneath the stroma persisted for longer periods. Because a single cell was deposited in each well, it is likely that these differences in growth kinetics were stem cell intrinsic. An additional finding of our study was that the proliferative behavior of an individual cell, whether from B6 or DBA mice, was highly unpredictable. The issue of stochastic stem cell properties has been addressed repeatedly over many years,30-34 and our current results are consistent with such an interpretation when these highly purified cells are considered in the context of uniform conditions provided by a cloned stromal cell line. Obviously, we cannot exclude the possibility that functional heterogeneity within these purified populations is related simply to not using sufficiently stringent stem cell sorting criteria. However, several lines of reasoning argue against this. First, we routinely use this sorting protocol to enrich primitive stem cells with long-term repopulating potential. In vivo limiting-dilution analysis indicates that competitive repopulating units (CRU) represent 1 per 15 Sca-1+c-kit+Lin− cells (95% confidence limits, 1 per 12 to 23 cells), demonstrating the high purity of this stem cell fraction.35 Our current CAFC analysis of individual cells with this phenotype also shows that they are highly enriched for cobblestone area-forming activity (1 CAFC per 2 to 4 Sca-1+c-kit+Lin− cells). A simple calculation shows that the Sca-1+c-kit+Lin− cell population in fact must contain almost all of the CAFC day-35 activity present in whole bone marrow cells. In the 2 experiments performed with young and old DBA marrow, 15 and 13 cells, respectively, of a total of 240 single stem cells analyzed generated a cobblestone area on day 35. This represents a cloning efficiency of approximately 11%. The frequency of Sca-1+c-kit+Lin− cells in DBA marrow was 0.04% or 40 per 105 bone marrow cells. Because approximately 11% of these 40 cells read out as CAFC day 35, the frequency of this subset in unfractionated marrow should be approximately 4 per 105 marrow cells. Table 1 shows that the frequency of 3.4 per 105 cells measured using unfractionated marrow is very close to this value. For B6 mice, a similar finding was made. In this strain, the percentage of single Sca-1+c-kit+Lin− cells from young and old donors that resulted in CAFC day 35 was 2% (1 and 4 cells, respectively, of 240 cells analyzed). The frequency of Sca-1+c-kit+Lin− cells in whole B6 marrow was equal to that in DBA, ie, 40 per 10,5 and thus, on the basis of this finding, the frequency of CAFC day 35 in unfractionated B6 marrow should be approximately 0.8 per 105 bone marrow cells (2% of 40 cells). Table 1 shows that this is clearly within the range of what is observed when unfractionated marrow is used in the CAFC assay.
Our data reveal not only quantitative differences between these 2 inbred mouse strains (ie, larger stem cell pool size in DBA mice), but also distinct qualitative effects (ie, single DBA stem cells exhibit more “primitive” growth kinetics in vitro than B6 stem cells). In keeping with these in vitro observations, lethally irradiated recipient mice transplanted with 1000 DBA Sca-1+c-kit+Lin− cells showed dramatically more rapid multilineage reconstitution compared with mice transplanted with an equivalent number of B6 Sca-1+c-kit+Lin− cells. In addition, DBA mice recovered approximately twice as fast as B6 mice after in vivo administration of 5-FU. These findings may have important clinical consequences if one considers that, in humans, recovery from myeloablation or after stem cell transplantation is highly variable, costly, and potentially life-threatening. Our data suggest that a measurement of stem cell pool size before chemotherapy or transplantation may provide an important tool to predict whether hematopoietic recovery might occur in a clinically acceptable time frame. Unfortunately, at present it is not clear how one could quickly quantify stem cell pool size in humans. Our current data in the mouse suggest that even a detailed FACS phenotype may not be very meaningful, as it may not adequately predict stem cell function. An alternative method may be to assess progenitor cell mobilization potential. Recent studies have shown that in various strains of mice granulocyte colony-stimulating factor (G-CSF)–induced progenitor cell mobilization is highly variable. C3H/He and B6 mice mobilize poorly, whereas DBA mice do so very efficiently.23 Qualitatively and quantitatively, similar observations have been made in healthy human volunteers treated with G-CSF.28 Intriguingly, mobilization patterns observed in mice accurately reflect marrow stem cell pool size, as we have previously shown that DBA mice have many more stem cells than B6 and C3H mice.20 Preliminary data in our laboratory indicate that AKR mice, which we had previously identified as a strain with high stem cell numbers,20 are also good mobilizers (GdH, March 1999).
In summary, it may be of significant clinical value to identify, prospectively, patients with a relatively small stem cell compartment who may experience more severe hemotoxicity during cancer therapy. Our study underscores this point in a preclinical mouse model, and offers a first approach to address this issue experimentally.
Supported by funds provided by NIH grants RO1 AG16653 to G.V.Z. and RO1 HL61392 to S.J.S., the Lucille P. Markey Cancer Center, the University of Kentucky Hospital and the Department of Internal Medicine. G.d.H. is a fellow of the Netherlands Organization for Scientific Research (NWO) and of the Royal Netherlands Academy of Arts and Sciences (KNAW). S.J.S. is the recipient of a Junior Faculty Scholar Award from the American Society of Hematology.
G.d.H. and S.J.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gerald de Haan, Department of Cell Biology, University of Groningen, A. Deusinglaan 1, 9713 AV Groningen, The Netherlands; e-mail: g.de.haan@med.rug.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal