Despite its wide use as a marker for hematopoietic stem cells (HSCs), the function of stem cell antigen–1 (Sca-1) (also known as lymphocyte activation protein–6A [Ly-6A]) in hematopoiesis remains poorly defined. We have previously established that Sca-1−/−T cells develop normally, although they are hyperresponsive to antigen. Here, we report detailed analysis of hematopoiesis in Sca-1–deficient animals. The differentiation potential of Sca-1–null bone marrow was determined from examination of the most mature precursors (culture colony-forming units [CFU-Cs]) to less committed progenitors (spleen CFUs [CFU-Ss]) to long-term repopulating HSCs. Sca-1–null mice are mildly thrombocytopenic with a concomitant decrease in megakaryocytes and their precursors. Bone marrow cells derived fromSca-1−/− mice also have decreased multipotential granulocyte, erythroid, macrophage, and megakaryocyte CFU (GEMM-CFU) and CFU-S progenitor activity. Competitive repopulation assays demonstrated that Sca-1−/−HSCs are at a competitive disadvantage compared with wild-type HSCs. To further analyze the potential of Sca-1−/−HSCs, serial transplantations were performed. While secondary repopulations using wild-type bone marrow completely repopulatedSca-1−/−mice, Sca-1−/−bone marrow failed to rescue one third of lethally irradiated wild-type mice receiving secondary bone marrow transplants from irradiation-induced anemia and contributed poorly to the surviving transplant recipients. These data strongly suggest that Sca-1 is required for regulating HSC self-renewal and the development of committed progenitor cells, megakaryocytes, and platelets. Thus, our studies conclusively demonstrate that Sca-1, in addition to being a marker of HSCs, regulates the developmental program of HSCs and specific progenitor populations.
Introduction
Hematopoiesis proceeds through the differentiation of stem cells into a cascade of committed progenitors and, finally, into all of the terminally differentiated cell lineages of the blood system. At least in C57BL/6 and other Ly-6.2 mouse strains, the cell surface phenotype of the hematopoietic stem cells (HSCs) and primitive progenitors has been well elucidated. One of the markers whose expression defines the HSCs and various developmental intermediates is the glycosyl phosphatidylinositol–anchored protein (GPI-AP) stem cell antigen–1 (Sca-1). Single lineage-negative (Lin−) Thy-1loSca-1+Kit+CD38+CD34−cells fulfill the criteria of HSCs, as they are unable to give rise to short-term repopulating cells but are able to provide long-term repopulation of lethally irradiated mice, including secondary and tertiary transplantations.1,2 Sca-1 is regulated in a complex fashion during hematopoietic differentiation. As HSCs differentiate into common lymphoid progenitors (CLPs) that give rise to T, B, and natural killer (NK) cells but not myeloid lineages, Sca-1 and Kit expression decreases while interleukin 7–receptor (IL-7R) expression increases.3 Sca-1 and anotherLy-6 gene family member, Sca-2, are up-regulated on prothymocytes, which seed the thymic cortex.4 Sca-1 expression is turned off at an early stage of thymocyte differentiation, and Sca-1 is re-expressed by mature single-positive medullary thymocytes and peripheral T cells.5 Transgenic overexpression of Sca-1 during all stages of thymocyte development arrests development at the CD3−CD4−CD8−CD44+CD25+stage, the stage in the thymic cortex at which Sca-1 expression normally ceases.5 Concurrently with differentiation of the HSCs into common myeloid progenitors (CMPs), Sca-1 expression is down-regulated.6 Sca-1 expression is activated in a proportion of spleen colony-forming unit (CFU-S) and culture-CFU (CFU-C) progenitors.7
All functional analysis of Sca-1 has been performed in primary lymphocytes or lymphoid cell lines. The Sca-1 protein (or lymphocyte activation protein–6A [Ly-6A]) was originally identified as an antigen up-regulated on activated lymphocytes.8 GPI-APs are believed to facilitate cell signaling by creating glycosphingolipid cholesterol “rafts” that concentrate or exclude specific signaling molecules.9,10 Consistent with this mechanism, Src family kinase members have been shown to associate with Sca-1.11Several members of the Ly-6 gene family, which share similar structural motifs as well as primary sequence, have identified cognate ligands, such as the Ly-6 protein CD59, which protects cells from complement-mediated lysis by binding the α-chain of complement 8 and the “b” domain of complement 9 (Ninomiya and Sims12). CD59 does not simply act to block the complement cascade, but induces a signaling cascade, which includes activation of the Src family kinase protein Hck, the adapter Src homology and collagen protein (Shc), and Syk following ligand binding.13 Thus, it has long been believed that Sca-1 function is dependent upon interaction with its cognate ligand. Consistent with the expectation that Sca-1 functions as a receptor, an antibody to a 66-kDa GPI-AP immunoprecipitates Sca-114; however, the identity of the purported 66-kDa ligand has yet to be published.
The absence of an identified ligand has made determination of the role of Sca-1 in cell signaling difficult. Simulation of ligand binding by cross-linking Sca-1 with anti–Sca-1 antibodies has generated conflicting results.15-18 However, antibody cross-linking assays are artificial and probably do not represent physiologic ligand-receptor interactions. Therefore, we undertook a genetic approach to determine the function of Sca-1.19 Homologous recombination was used to ablate the Sca-1 gene in 129 ES cells. Homozygous mutant mice were produced at normal Mendelian frequencies, demonstrating that Sca-1 is not required for development. However, T cells from Sca-1–deficient mice demonstrate significantly higher and more prolonged proliferation in response to stimulation through the T-cell–receptor complex as compared with T cells from Sca-1+/+ littermates. Although the mechanism driving hyperproliferation has not been determined, increased cytokine production, skewed thymocyte development, and overall expression of Src family kinases have been ruled out (Stanford et al,19 and W.L.S., unpublished observations, 1998). Consistent with these results, overexpression of Sca-1 in T cells inhibits CD4+ T-cell proliferation induced by T-cell–receptor activation.20 Together, these results suggest that Sca-1 acts to down-modulate an active immune response. Furthermore, we have recently demonstrated that Sca-1 is required for the immunosuppressive function of CD4−CD8− T cells and the induction of major histocompatibility complex (MHC)–mismatched transplantation tolerance.21
To analyze the developmental potential of Sca-1−/−HSCs and progenitors, we generated congenic Sca-1–null mice on the C57BL/6 (Ly-6.2) background. HSCs from Sca-1−/−mice had impaired repopulation potential, as demonstrated by a competitive disadvantage compared with wild-type HSCs. Moreover, the lower engraftment of secondary transplants suggests a defect in Sca-1−/− HSC self-renewal. In addition to HSC deficiencies in Sca-1–deficient mice, specific cell lineages and progenitor subpopulations were also affected. The fact that not all lineages were equally compromised suggests that Sca-1 performs complex and specific functions in hematopoiesis.
Materials and methods
Animals
All mice were maintained in a conventional mouse facility at Mount Sinai Hospital (Toronto, ON, Canada). For all primary assays, 8- to 12-week-old-mice were used. The C57BL/6J-Kit–white-spotted viable(KitW−v) (Wv/+)and C57BL/6J-Gpi1a/a mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred in-house.Wv/+ genotyping was inferred by coat-color analysis or polymerase chain reaction (PCR) analysis.Sca-1+/+ mice (discussed in “Results”) were used as controls for all experiments except competitive and secondary repopulations, in which C57BL/6J-Gpi1a/a mice were used. Clonogenic assays demonstrated no differences betweenSca-1+/+ and C57BL/6J-Gpi1a/a control mice.
Antibodies
Purified rat antimouse monoclonal antibodies (mAbs) for flow cytometry were all purchased from BD PharMingen (San Diego, CA). The following mAbs, conjugated with either fluorescein isothiocyanate (FITC) or phycoerythrin (PE), were used: anti-CD3ε, anti–macrophage adhesion molecule–1 (anti–MAC-1) (CD11b), anti-CD41 (integrin αIIb), anti-B220 (CD45R), anti-CD61 (integrin β3), anti–Thy-1, anti-Kit, anti–IL-7Rα, anti–Sca-1, and anti–TER-119. In some experiments, allophycocyanin-conjugated anti–Gr-1 (Ly-6G) and anti-Kit were used. The appropriate conjugated rat antimouse mAbs were used as negative controls.
Cytokines
Purified thrombopoietin (recombinant human thrombopoietin [rhTPO]), rhIL-6, and rhIL-11 were purchased from PeproTech (Rocky Hill, NJ). Purified rhIL-7 was purchased from Stem Cell Technologies (Vancouver, BC, Canada), and recombinant mouse IL-3 (rmIL-3) was purchased from BD PharMingen.
Flow cytometry
Single cell suspensions were prepared from bone marrow (tibial and femoral). The bone marrow was subjected to red blood cell (RBC) lysis with ammonium chloride, washed once in Iscove Dulbecco modified Eagle medium (DMEM), counted, and resuspended in phosphate-buffered saline (PBS) plus 2% fetal bovine serum (FBS) (staining medium [SM]) at 5 × 106 cells per milliliter. Then, 106 cells were incubated with mAbs for 30 minutes, washed twice in SM, and resuspended in 0.2 mL SM for analysis by means of a Becton Dickinson FACScalibur (Mountain View, CA).
Glucose phosphate isomerase 1 (Gpi1) isoenzyme analysis
The samples were subjected to electrophoresis on cellulose acetate plates (Helena Laboratories, Beaumont, TX), and the Gpi1 isoenzyme bands were visualized as previously described.22 The contribution of mutant cells to the samples was visually estimated by comparing them with a set of standard mixtures of Gpi1-AA and Gpi1-BB cells.
Hematologic analysis
Peripheral blood (50 to 100 μL) from tail bleeds was collected into EDTA (ethylenediaminetetraacetic acid)–coated capillary pipettes (Drummond, Broomall, PA) and transferred to Eppendorf (Hamburg, Germany) tubes. Complete blood counts and differential counts were performed by means of a Coulter Ac-T Differential Hematology Analyzer with veterinary software (Beckman-Coulter, Fullerton, CA).
Bleeding time assay
Six- to 8-week-old mice were anesthetized with the use of 0.15 mg ketamine per gram body weight to reduce influences of heart rate and activity on bleeding time. Approximately a 3-mm portion of the tail was cleanly severed with the use of scissors. The tail was immersed in physiologic saline at 37°C. The time required for the small stream of blood to stop was recorded as the bleeding time.
Culture CFUs
For each experiment, 3 animals of each genotype were used. Each experiment was performed between 3 and 8 times. Single cell suspensions were prepared from bone marrow (tibial and femoral). Cells were counted and diluted to 106 cells per milliliter in Iscove modified Dulbecco medium (IMDM) with 1% FBS. For each assay, 3 aliquots of 104 cells were mixed with the appropriate methylcellulose media, plated, and grown in humidified chambers at 37°C, 5% CO2. To assay megakaryocyte CFU precursors (CFU-Mk), cells were grown in methylcellulose/collagen medium (Stem Cell Technologies) containing 50 ng/mL rhTPO, 10 ng/mL rmIL-3, 20 ng/mL rhIL-6, and 50 ng/mL rhIL-11, and visualized by acetylcholinesterase activity. All other CFU-C colonies were grown in methylcellulose containing IL-3, IL-6, Steel factor (SLF), and erythropoietin (EPO) (M3434; Stem Cell Technologies). Erythroid CFU (CFU-E) precursors were assayed after 2 days by staining in situ with benzidine (Sigma, St Louis, MO) to detect hemoglobin. After 7 to 10 days, erythroid burst-forming units (BFU-Es) and CFU-Cs were counted by colony morphology. Representative colonies were occasionally analyzed by Wright-Giemsa to confirm colony identity.
Spleen CFUs
The results shown are combined from 3 separate experiments. For each experiment, we combined the bone marrow from 3 mice of the same genotype. Single cell suspensions were prepared from bone marrow flushed from both tibiae and femora and diluted to 105 cells per milliliter. Then, 104 or 2 × 104 cells were injected into the tail vein of sublethally irradiated (8.0 Gy, 137C source) mice. For each experiment, 6 recipients were used for each donor genotype. Recipients were humanely killed on day 12 of transplantation, and their spleens were fixed with Bouin solution. Macroscopic colonies were counted. Spleens from irradiated mice that did not receive cells contained on average less than 1 CFU-S colony.
Transplantation assays
For each repopulation experiment, 6 C57BL/6J-Gpi1a/a and 6 C57BL/6J-Gpi1b/b (eitherSca-1−/− or Sca-1+/+used in some control experiments) bone marrow donor mice were treated with 150 μg 5-fluorouracil (5-FU) (Sigma) per gram body weight. At 2 days later, bone marrow (tibial and femoral) was flushed from each donor, and the bone marrow cells were combined according to Gpi1 haplotype. Whole bone marrow cells were then subjected to iso-osmotic Percoll (Pharmacia, Piscataway, NJ) centrifugation. The density-separated (1.077 g/mL) donor bone marrow cells (or “buffy coats”) were then mixed at the following ratios for competitive repopulation: 100%Gpi1a/a to 0% Gpi1b/b,67.7% Gpi1a/a to 33.3%Gpi1b/b, 33.3% Gpi1a/ato 67.7% Gpi1b/b, and 0%Gpi1a/a to 100% Gpi1b/b. Bone marrow recipients (12 for each experiment) were treated with a single-dose, total body lethal irradiation (9.5 Gy, 137C source). Then, 5 × 106 cells of each separated bone marrow mixture (a total of 4 groups) were injected into the tail vein of 3 recipients. Tail bleeds were performed at 2, 4, 8, and 16 weeks for analysis of bone marrow contribution by Gpi1 chimerism. In some experiments, blood samples were obtained at 32 and 52 weeks. No significant differences were observed between 16 and 52 weeks. Bone marrow was isolated from recipients at either 16 or 52 weeks, analyzed for Gpi1 chimerism, and used for secondary repopulations. Bone marrow was harvested from 2 groups of 16-week-long primary bone marrow recipients for secondary transplantation: (1) wild-type(Gpi1a/a) irradiated mice were repopulated withSca-1−/− (Gpi1b/b) bone marrow; and (2) Sca-1−/− (Gpi1b/b)irradiated mice were repopulated with wild-type(Gpi1a/a) bone marrow. For each primary recipient, 5 × 106 Percoll-separated bone marrow cells were injected into the tail veins of 3 lethally irradiated mice of the same genotype (and Gpi1 isotype) as the primary recipient.
Results
Hematologic analysis
To minimize genetic background effects on the analysis of the role of Sca-1 in hematopoiesis, we backcrossed theSca-1–targeted null allele onto the C57BL/6 background for 10 generations. Theoretically, the entire genetic background of these animals is derived from the C57BL/6 strain with the exception of approximately 20 cM flanking the Ly-6 locus, which is derived from the 129 ES cell line used for gene targeting.19 The backcross-10 mice were bred to generateSca-1+/+ and Sca-1−/−progeny, which were subsequently independently bred. Hematopoietic analysis of Sca-1−/− mice began with automated peripheral blood counts (Table 1). The hematocrits, hemoglobin levels, and total RBC and WBC counts were similar in the Sca-1+/+ andSca-1−/− mice. Yet the percentages in the peripheral WBC counts were significantly altered inSca-1−/− mice (P < .05). The percentage of peripheral blood lymphocytes was increased, with a concomitant decrease in both monocytes and granulocytes. These results are consistent with our previous report that lymphocytes derived fromSca-1−/− mice are hyperproliferative in response to antigen.19 Unexpectedly, peripheral blood analysis revealed that Sca-1−/− mice have 31% fewer platelets (P < .01). Next, flow cytometric analysis was performed to compare the number and percentages of bone marrow lineages in Sca-1+/+ andSca-1−/− mice. Bone marrow cellularity was found to be normal in Sca-1−/− mice as were the proportions of monocytes, granulocytes, erythroblasts, and lymphocytes; however, bone marrow from Sca-1−/−mice exhibited a marked reduction in megakaryocytes (by greater than 75%), consistent with the decrease in peripheral blood platelets (Table 2; P < .01). In addition, histologic examination of adult spleens also showed a reduction in megakaryocytes (data not shown).
To determine if the defective megakaryopoiesis resulted in a physiologic defect, bleeding times in response to tail cuts were measured on Sca-1+/+ andSca-1−/− mice. A 69% increase in average bleeding time was observed for Sca-1−/− mice; however, the results were not statistically significant owing to the large variation in bleeding times in both wild-type and mutant mice (data not shown). The limited increase in bleeding time is consistent with the mild thrombocytopenia observed in Sca-1−/−mice.
Precursor analysis
Colony-forming assays were performed to analyze the role of Sca-1 in the development of myeloid-committed precursors. The most primitive in vitro colony-forming cell (granulocyte, erythroid, macrophage, megakaryocyte CFU [CFU-GEMM]) was reduced by more than 60%. As expected by the 4-fold decrease in bone marrow megakaryocytes, there was also a substantial decrease in megakaryocyte precursors (megakaryocyte CFUs [CFU-Mk's]) inSca-1−/− bone marrow (Table3). Consistent with unaltered percentages of RBCs and TER-119+ cells in the periphery and bone marrow, Sca-1−/− bone marrow contained normal percentages of erythroid (both BFU-E and CFU-E) progenitors. Although peripheral blood and bone marrow granulocyte and monocyte numbers are modestly decreased in Sca-1−/−mice, CFU-GM, CFU-M, and CFU-G precursors are all significantly increased in mutant marrow. The CFU-spleen day-12 (CFU-S12) assay is an in vivo clonogenic method that measures a primitive progenitor. As shown in Figure 1,Sca-1−/− bone marrow has a 38% decrease in CFU-S12 progenitors.
Reduction of CFU-S12 precursors in Sca-1–null mice.
CFU-S analysis of Sca-1−/−bone marrow demonstrated a 38% reduction (4.4 CFU-S/104Sca-1+/+cells [▨] versus 3.2 CFU-S/104Sca-1−/−cells [▪];P < .01) in CFU-S12 compared with wild-type bone marrow. The results are shown as the percentage of colonies from wild-type bone marrow from 3 separate experiments.
Reduction of CFU-S12 precursors in Sca-1–null mice.
CFU-S analysis of Sca-1−/−bone marrow demonstrated a 38% reduction (4.4 CFU-S/104Sca-1+/+cells [▨] versus 3.2 CFU-S/104Sca-1−/−cells [▪];P < .01) in CFU-S12 compared with wild-type bone marrow. The results are shown as the percentage of colonies from wild-type bone marrow from 3 separate experiments.
Hematopoietic stem cell analysis
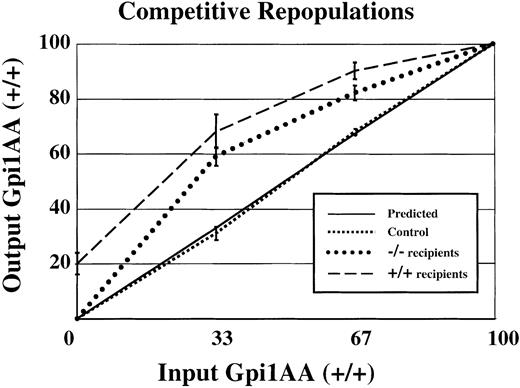
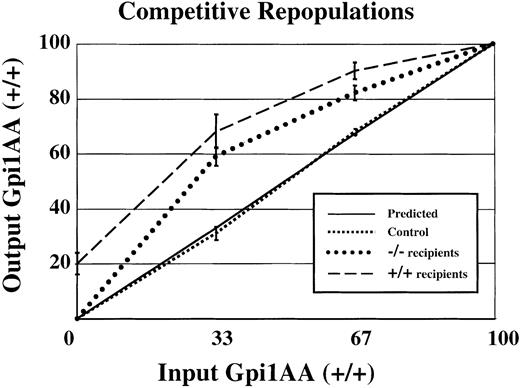
Competitive bone marrow repopulations were performed to measure HSC activity in Sca-1−/− mice. First, lethally irradiated mice received 5 × 106 medium-densitySca-1+/+ and Sca-1−/−bone marrow cells that had been mixed at specific ratios. Wild-type and mutant cells were distinguished by expression of the glucose phosphate isomerase 1 (Gpi1) isoenzyme expressed by all cells, including erythrocytes. Wild-type bone marrow cells (from the C57BL/6J-Gpi1a/a strain) express the Gpi1AA isoenzyme, whereas control Sca-1+/+ andSca-1−/− bone marrow cells express the Gpi1BB isoenzyme. When mixtures of wild-type Gpi1AA and Gpi1BB bone marrow were used to repopulate lethally irradiated wild-type Gpi1AA mice, approximately the same percentage of input donor cells was recovered from the bone marrow of long-term repopulated animals (Figure2, square dotted line), closely resembling the expected values depicted in the solid line. In contrast, when mixtures of wild-type Gpi1AA andSca-1−/− bone marrow cells were used to repopulate lethally irradiated Gpi1AA mice, a significantly greater proportion of Gpi1AA cells were recovered from the bone marrow of long-term repopulated mice (Figure 2, dashed line). For example, mice receiving transplants of bone marrow at a ratio of 2:1 mutant–to–wild-type cells showed reconstitution by wild-type cells at more than twice the expected frequency. In addition, Gpi1 analysis of peripheral blood from mice that have received transplants suggested that Sca-1−/− bone marrow had impaired short-term repopulating activity (data not shown). Yet no differences in Gpi1 ratios were observed in 4-month and 12-month repopulated mice. Surprisingly, the Gpi1AA mice repopulated with 100%Sca-1−/− Gpi1BB bone marrow (BM), showed only an average of 80% Gpi1BB bone marrow. Thus, some endogenous wild-type Gpi1AA HSCs recolonized the bone marrow despite the transplantation of 5 × 106Sca-1−/− bone marrow cells.
Competitive disadvantage for
Sca-1−/− bone marrow compared withSca-1+/+ bone marrow.Sca-1+/+(Gpi1AA) andSca-1−/−(Gpi1BB) bone marrows were mixed at specific ratios and used to repopulate lethally irradiated mice. The x-axis represents input Sca-1+/+cells (Gpi1AA), and the y-axis represents output Sca-1+/+(Gpi1AA) cells. If there is no difference in the ability of HSCs to repopulate irradiated mice, the output percentage of Gpi1AA cells will be the same as the input percentage (solid line, or predicted values). In control experiments, Sca-1+/+Gpi1AA bone marrow was mixed with Sca-1+/+Gpi1BB bone marrow and used to repopulate lethally irradiated Gpi1AA mice. These results (square dotted line) reflect the expected result that there is no competitive advantage for either bone marrow population. The experimental results (dashed and round dotted lines) demonstrate that Sca-1−/−bone marrow has a competitive disadvantage compared with Sca-1+/+cells. The dashed and round dotted lines representSca-1+/+Gpi1AA mice orSca-1−/−mice, respectively, as transplant recipients.
Competitive disadvantage for
Sca-1−/− bone marrow compared withSca-1+/+ bone marrow.Sca-1+/+(Gpi1AA) andSca-1−/−(Gpi1BB) bone marrows were mixed at specific ratios and used to repopulate lethally irradiated mice. The x-axis represents input Sca-1+/+cells (Gpi1AA), and the y-axis represents output Sca-1+/+(Gpi1AA) cells. If there is no difference in the ability of HSCs to repopulate irradiated mice, the output percentage of Gpi1AA cells will be the same as the input percentage (solid line, or predicted values). In control experiments, Sca-1+/+Gpi1AA bone marrow was mixed with Sca-1+/+Gpi1BB bone marrow and used to repopulate lethally irradiated Gpi1AA mice. These results (square dotted line) reflect the expected result that there is no competitive advantage for either bone marrow population. The experimental results (dashed and round dotted lines) demonstrate that Sca-1−/−bone marrow has a competitive disadvantage compared with Sca-1+/+cells. The dashed and round dotted lines representSca-1+/+Gpi1AA mice orSca-1−/−mice, respectively, as transplant recipients.
Reciprocal experiments were conducted in whichSca-1−/− mice were used as host animals for the bone marrow transplants. Again, a significantly higher percentage of wild-type cells repopulated Sca-1−/− mice than were used for the transplant (Figure 2, round dotted line). For example, Sca-1−/− mice that received transplants consisting of 33% wild-type and 67%Sca-1−/− bone marrow cells demonstrated, on average, repopulation by 60% wild-type and 40% mutant cells. In contrast to the incomplete engraftment of Gpi1AA mice bySca-1−/− bone marrow, transplantations of 100% wild-type bone marrow cells into Sca-1−/−mice demonstrated complete engraftment by wild-type cells.
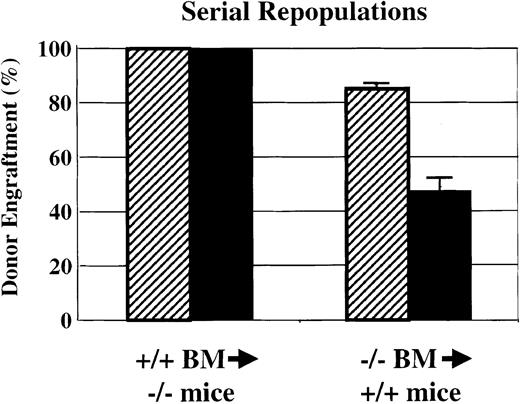
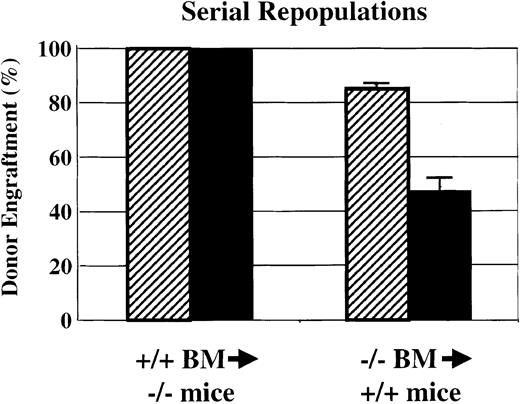
Serial transplantations
HSC self-renewal activity was analyzed by using serial transplantation of Sca-1−/− bone marrow into wild-type Gpi1AA mice and of wild-type Gpi1AA bone marrow intoSca-1−/− mice (Figure3). As shown in Figures 2 and 3, transplantation of 5 × 106 wild-type bone marrow cells into lethally irradiated Sca-1−/− mice demonstrated complete reconstitution by wild-type cells. At 4 months after transplantation, bone marrow was harvested for Gpi1 isoenzyme analysis, and the remaining cells were subjected to gradient centrifugation. For each primary recipient, 5 × 106medium-density bone marrow cells were injected into the tail veins of 3 lethally irradiated Sca-1−/− mice. All of the secondary recipients demonstrated complete reconstitution of wild-type cells into Sca-1−/− mice by 4 months after transplantation. However, repopulation of wild-type mice with the use of Sca-1−/− bone marrow demonstrated remarkably different results. As shown in Figures 2 and 3, primary wild-type mice that received transplants ofSca-1−/− bone marrow exhibited poor donor engraftment. Secondary transplants from these animals into lethally irradiated wild-type mice resulted in about 45% engraftment bySca-1−/− bone marrow in the 6 recipients that survived transplantation. Three of the 9 secondary transplant recipients died within 17 days after transplantation. The moribund animals demonstrated hematocrits of less than 20% measured a week after transplantation.
Serial bone marrow transplantations.
Serial bone marrow transplantations demonstrate a significant defect in HSC competition and self-renewal bySca-1−/−mice. Sca-1+/+BM was able to completely repopulate Sca-1−/− mice in both primary and secondary recipients. However, when Sca-1−/−bone marrow was used to repopulate lethally irradiated Sca-1+/+mice, endogenous Sca-1+/+HSCs were able to compete against donor stem cells in the primary repopulations (▨). In secondary transplantations using bone marrow from primary recipients to repopulate lethally irradiated Sca-1+/+mice (▪), the donor bone marrow could not rescue one-third of the recipients from irradiation-induced anemia. Of the remaining recipients, the Sca-1−/−bone marrow contributed approximately 45% to the recipient bone marrow 4 months following repopulation. Three of 9 secondary recipients died.
Serial bone marrow transplantations.
Serial bone marrow transplantations demonstrate a significant defect in HSC competition and self-renewal bySca-1−/−mice. Sca-1+/+BM was able to completely repopulate Sca-1−/− mice in both primary and secondary recipients. However, when Sca-1−/−bone marrow was used to repopulate lethally irradiated Sca-1+/+mice, endogenous Sca-1+/+HSCs were able to compete against donor stem cells in the primary repopulations (▨). In secondary transplantations using bone marrow from primary recipients to repopulate lethally irradiated Sca-1+/+mice (▪), the donor bone marrow could not rescue one-third of the recipients from irradiation-induced anemia. Of the remaining recipients, the Sca-1−/−bone marrow contributed approximately 45% to the recipient bone marrow 4 months following repopulation. Three of 9 secondary recipients died.
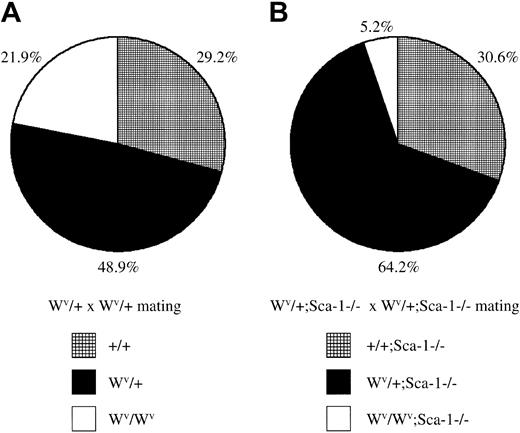
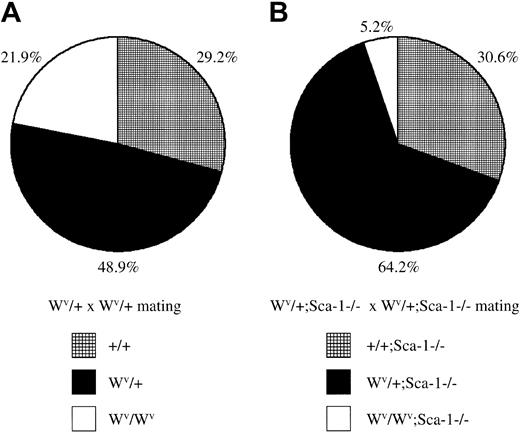
Loss of Sca-1 and diminished Kit signaling reduces embryonic survival
The receptor tyrosine kinase c-Kit encoded by the white-spotted (W) locus23 is required for hematopoietic development and HSC self-renewal. The white-spotted viable (Wv) allele of c-Kit is a homozygous viable mutation that results in a threonine-to-methionine substitution at position 660 in the kinase domain resulting in reduced kinase activity.24 To assess the functional capabilities of Sca-1−/− HSCs in situ (ie, without ex vivo manipulation and transplantation), Sca-1−/−mice were bred with Wv/+ mice to generate Sca-1−/−;Wv/+ mutants, which were then intercrossed. WhileWv/+ intercrosses generated 22%Wv/Wv homozygous pups, which exhibit mild anemia and impaired HSC competition, CFU-S activity, and decreased CFU-C precursors,Sca-1−/−;Wv/+ intercrosses generated only 5%Sca-1−/−;Wv/Wvcompound homozygote pups, indicating that mostSca-1−/−;Wv/Wvcompound homozygotes die in utero (P < .0001; Figure4). Preliminary analysis of embryonic day –14 litters from Sca-1−/−;Wv/+intercrosses indicate that compound homozygotes are very anemic and have dramatically reduced CFU-Cs, suggesting that the cause of embryonic lethality is due to a stem cell or very early progenitor defect (C.Y.J.L., N. Ciliberti, and W.L.S., unpublished data, 2002).
Embryonic lethality in
Sca-1−/−;Wv/Wvcompound homozygotes. (A) The ratio of wild-type,Wv/+, andWv/Wv pups fromWv/+ heterozygous intercrosses. A total of 178 pups were genotyped from 4 breeding pairs. HomozygousWv/Wv pups were born at a slightly lower-than-expected, but not statistically significant, Mendelian ratio for a nonlethal mutation (P > .1). (B) The ratio of Sca-1−/−, Wv/+;Sca-1−/−, andWv/Wv;Sca-1−/−pups from Wv/+;Sca-1−/−heterozygous intercrosses. A total of 288 pups were genotyped from 9 breeding pairs. Compound homozygousWv/Wv;Sca-1−/−pups were observed at an 84% reduction compared withWv/Wv pups fromWv/+ heterozygous intercrosses, demonstrating that mostSca-1−/−;Wv/Wvcompound homozygotes die in utero (P < .0001).
Embryonic lethality in
Sca-1−/−;Wv/Wvcompound homozygotes. (A) The ratio of wild-type,Wv/+, andWv/Wv pups fromWv/+ heterozygous intercrosses. A total of 178 pups were genotyped from 4 breeding pairs. HomozygousWv/Wv pups were born at a slightly lower-than-expected, but not statistically significant, Mendelian ratio for a nonlethal mutation (P > .1). (B) The ratio of Sca-1−/−, Wv/+;Sca-1−/−, andWv/Wv;Sca-1−/−pups from Wv/+;Sca-1−/−heterozygous intercrosses. A total of 288 pups were genotyped from 9 breeding pairs. Compound homozygousWv/Wv;Sca-1−/−pups were observed at an 84% reduction compared withWv/Wv pups fromWv/+ heterozygous intercrosses, demonstrating that mostSca-1−/−;Wv/Wvcompound homozygotes die in utero (P < .0001).
Discussion
The data presented here demonstrate that Sca-1 plays an important role in regulating the repopulating ability of HSCs and the development of committed progenitor cells, megakaryocytes, and platelets. The decrease in CFU-S and CFU-GEMM progenitors may be attributable to deficient stem cell self-renewal and differentiation observed by means of the repopulation assays. The simplest explanation for the dramatic reduction in Sca-1−/− HSC-repopulating ability between primary and secondary BM transplantations is that Sca-1 plays an important role in HSC self-renewal, at least in response to hematologic stress caused by lethal irradiation and transplantation. This explanation is consistent with the regulation of Sca-1 by interferons25,26 and tumor necrosis factor–α (TNF-α),27 which are known to be induced in response to (1) cellular stress; (2) poor competition ofSca-1−/− HSCs with wild-type stem cells in primary transplantations but not a complete lack of competition (Figure 2); and (3) the failure ofSca-1−/−;Wv/Wvcompound homozygotes to survive (Figure 4).
Under homeostatic conditions (ie, Sca-1–null mice), Sca-1 is not required for stem cell maintenance, which is consistent with a role for Sca-1 as a coregulator of cell signaling; in this case, however, a coregulator of the signals that mediate stem cell self-renewal. A postulated molecular role of Sca-1 and other GPI-anchored proteins is to modulate signaling by Src family kinases, receptor tyrosine kinases, and other signaling molecules in lipid rafts. Sca-1 and the receptor tyrosine kinase c-Kit are coexpressed on HSCs, CLPs, early myeloid progenitors, and mast cells.1,3While null Kit mutations lead to embryonic lethality owing to severe anemia caused by decreased stem cells and progenitors, the partial loss of function-W alleles, such asWv/Wv andW41/W41, has impaired HSC competition and CFU-S activity, decreased CFU-Cs, and lead to mild anemia, mast cell deficiencies, and mild thrombocytopenia.28 29 Thus, the HSC and myeloid progenitor phenotype of Sca-1−/− mice raises the intriguing possibility that Sca-1 may play a role in modulating Kit signaling. The observation of severe anemia and embryonic lethality in midgestational Sca-1−/−;Wv/Wvcompound homozygote embryos does not discriminate between the possibility that Sca-1 acts in the Kit signaling pathway or that the lack of Sca-1 signaling and reduced Kit signaling are 2 independent genetic insults to fetal liver HSCs that together are incompatible with cell or population survival. We have initiated more complex mating strategies together with clonogenic assays as well as analysis of Kit signaling in Sca-1−/− mast cells to distinguish between these 2 possibilities.
In addition to biochemical association of Sca-1 and Src family kinases,11 there is genetic evidence that Sca-1 and other GPI-APs are negative regulators of Src family kinases. For example, the lymphoid phenotypes of Sca-1 and Src family kinase gene–targeted mice are diametrically opposite; Sca-1−/− T cells are hyperresponsive to T-cell receptor (TCR) activation,19 while several Src family kinase mutants demonstrate poor TCR activation.30 Interestingly, althoughFyn-null peripheral T cells demonstrate normal TCR activation, they are refractory to activation via the GPI-anchored Thy-1 and Ly-6C.31 Finally, the potential interaction of Sca-1 and Kit may be mediated indirectly via Src family kinase signaling, which is required for ligand-mediated internalization of Kit.32 Unfortunately, HSC analysis of Src family kinase mutant strains has been limited; for example, competitive repopulation and serial transplantations have not been reported. However, the availability of mice with defined mutations in Src family kinases, Kit, or other signaling molecules that affect hematopoiesis will facilitate the molecular analysis of Sca-1 signaling in hematopoiesis and the molecular control of HSC self-renewal and commitment.
Significantly, our finding that Sca-1 plays a functional role in HSC self-renewal is consistent with the pathogenesis of paroxysmal nocturnal hemoglobinuria (PNH), an acquired disease resulting from a clonal expansion of HSCs that harbor a somatic null mutation in the X-linked phosphatidylinositol glycan-class A (Pig-A) gene, which catalyzes the first step in GPI biosynthesis.33 PNH is characterized by complement-mediated intravascular hemolysis and eventual bone marrow failure. Gene-targeting experiments have shown that the lack of the GPI-anchored complement inhibitors CD55 (decay-accelerating factor [DAF]) and CD59 are the primary cause for the complement-mediated hemolysis affecting PNH patients.34,35 However, the mechanism enabling the PNH stem cell to expand within the bone marrow is not due to a loss of GPI-APs. GPI-deficient clones encoding PIG-A mutations have been detected in healthy individuals in trace amounts without the tendency to expand.36 Furthermore, mice chimeric for a germ line–targeted mutation in Pig-A actually losePig-A−/y cells over time,37demonstrating that Pig-A−/y HSCs compete poorly with wild-type HSCs under homeostatic conditions, which is consistent with the role of Sca-1 that we report here.
In addition to HSCs, Sca-1 expression has been reported on muscle, bone, and mammary gland stem and/or primitive progenitors.38-41 Consistent with the function of Sca-1 in HSCs, we have recently found that Sca-1−/−mice develop age-related osteoporosis caused by a cell-autonomous defect in mesenchymal stem cell/primitive progenitor self-renewal (M. Bonyadi, S. D. Waldman, D. Liu, J. E. Aubin, M. D. Grynpas, W.L.S., manuscript submitted, 2002). The ligand/receptor signaling threshold (LIST) model of self-renewal versus differentiation suggests that a threshold level of signaling-competent cytokine-receptor complexes must be activated to propagate a self-renewal, rather than a differentiation, response.42Thus, if stem cells receive self-renewal signals below a certain threshold, both daughter cells differentiate, leading to a reduction in the stem cell pool. The LIST model has recently been shown to account for gp130-mediated embryonic stem cell self-renewal in response to leukemia-inhibitory factor (LIF) and hyper–IL-6.43 We suggest that coreceptors such as Sca-1 mediate self-renewal of stem cells by either increasing the number of functional cytokine-receptor complexes or altering the signaling properties of these complexes via the association of Sca-1 in lipid rafts and secondary signaling molecules sequestered in lipid rafts. Furthermore, we propose that adult stem cells use organ/stem cell–specific primary signaling molecules such as tyrosine kinases, whereas common cosignaling molecules, such as Sca-1, are conserved across many tissues and function to modify the balance between self-renewal and differentiation, especially under conditions of stress such as tissue regeneration. Thus, the absence of Sca-1 lowers the number of functional cytokine-receptor complexes or reduces the signaling capacity of these complexes, leading to a decrease in the stem cell pool. Consistent with this hypothesis, our preliminary biochemical analysis of Sca-1 signaling in mast cells demonstrates thatSca-1−/− cells have altered levels of tyrosine-phosphorylated proteins in response to proliferation signals (N. Ciliberti, M. Ohishi, and W.L.S., unpublished results, 2002). We are now investigating other tissues to determine if Sca-1 can be used to isolate stem cells from these tissues; examining the regeneration potential of these organ systems in theSca-1−/− mice; and analyzing potential Sca-1 signaling pathways that may be used to manipulate stem cells for therapeutic and tissue-engineering ends.
The authors would like to thank Dwayne Barber, Jason Cohn, Norman Iscoves, Tammy Reid, and Peter Zandstra for critical reading and helpful discussions. This work is dedicated to the memory of Ms Karyn Glick.
Prepublished online as Blood First Edition Paper, August 29, 2002; DOI 10.1182/blood-2002-06-1918.
Supported by grants from the Canadian Institutes of Health Research, Ottawa, ON, Canada; the Leukemia and Lymphoma Society, New York, NY; and the Terry Fox Foundation, Vancouver, BC, Canada. C.Y.I. was supported by a National Research Service Award (NRSA) training fellowship (National Institutes of Health [NIH]); and W.L.S. is the Karyn Glick Memorial Special Fellow of the Leukemia and Lymphoma Society of America and the Canadian Research Chair in Stem Cell Biology and Functional Genomics.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
William L. Stanford, Institute of Biomaterials and Biomedical Engineering, University of Toronto, 4 Taddle Creek Rd, Rm 407 Rosebrugh Bldg, Toronto, ON M5S 3G9 Canada; e-mail: william.stanford@utoronto.ca.

![Fig. 1. Reduction of CFU-S12 precursors in Sca-1–null mice. / CFU-S analysis of Sca-1−/− bone marrow demonstrated a 38% reduction (4.4 CFU-S/104Sca-1+/+ cells [▨] versus 3.2 CFU-S/104 Sca-1−/− cells [▪];P < .01) in CFU-S12 compared with wild-type bone marrow. The results are shown as the percentage of colonies from wild-type bone marrow from 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood-2002-06-1918/4/m_h80233713001.jpeg?Expires=1766082450&Signature=ePqyhXJLpoK6jaQQIShYlvoZEY-YUM-8K8u4iiL~F0yDsQSMbenocT-F4OdDHIP784gHy6fGaa2AsyxVIx-I9bx7G-SS9Deeoqg3p5mOJ35z2AKSO4tPOsc3YA5nrYlasoKYG8TSGAvIKjxXr6oMJpXxdE0PIoLCaB8ccbvQVXe6Yzl1TUQE01Y5n9lECnkIRwFIuiHgUhyQtOGyvmEGuCSgfYU6Y49rfMvZQ580f0x006AU2r3eMIoQmLDzemxu8lFNz7C2VRpa3KicDj5f7vkX9j6f30sWvrOneHTbKtZoPSGokoHsvtFWcornbaFqpqoqnYO98CSea7bcP9E9yg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 1. Reduction of CFU-S12 precursors in Sca-1–null mice. / CFU-S analysis of Sca-1−/− bone marrow demonstrated a 38% reduction (4.4 CFU-S/104Sca-1+/+ cells [▨] versus 3.2 CFU-S/104 Sca-1−/− cells [▪];P < .01) in CFU-S12 compared with wild-type bone marrow. The results are shown as the percentage of colonies from wild-type bone marrow from 3 separate experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/2/10.1182_blood-2002-06-1918/4/m_h80233713001.jpeg?Expires=1766503330&Signature=k~KY1Et9-6yWf9JJaAb7Hz30HcG4-SSuE8Y6zTbwIa65xQqPETNEoBna1eE7p1lRfEmvJKdolotX4ys~~L0UufFeISwsrGbO2yKvPSNvexCWOfCCRUXfrMdl~6nA4ucWTD8DGz-R2tc4CuWF26f1T6A9O8DCGghz2HfbqFad98E7u9aA1Q3NHo0ny0wDTb1eHj~2VBo7Bdqn~PrhXgjIXxP2XPxIMQKrvWJJO7Z2jSF7HYilxnE8XEYLpFd8QvdUqCg4bRLGHzHqkSTr1rv1ls8O66rS6JvV0K39XIjAMW0FraUDAlIObvyQhUzBcqcoUj0qcpdhLAaED93DR0EAuQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)