Abstract
The transplantation of primitive human cells into sublethally irradiated immune-deficient mice is the well-established in vivo system for the investigation of human hematopoietic stem cell function. Although mast cells are the progeny of hematopoietic stem cells, human mast cell development in mice that underwent human hematopoietic stem cell transplantation has not been reported. Here we report on human mast cell development after xenotransplantation of human hematopoietic stem cells into nonobese diabetic severe combined immunodeficient
Introduction
Mast cells are recognized as the principal cells which initiate immunoglobulin E (IgE)–dependent immediate hypersensitivity and also as the cells which contribute to innate immunity and tissue remodeling.1,2 There are 2 phenotypically distinct mast cell subpopulations in rodents: connective tissue-type mast cells (CTMCs) and mucosal-type mast cells (MMCs). These populations differ in location, cell size, staining characteristics, ultrastructure, mediator content, and T-cell dependency.3 Proliferation of rodent MMCs is dependent on T-cell–derived cytokines,3,4 whereas that of CTMCs is supported by stem cell factor (SCF). In humans, mast cells are distinguished on the basis of their protease composition,5,6 MCTC contains tryptase and chymase in its granules and is predominant in skin and intestinal submucosa, like CTMCs in rodents. MCT also contains tryptase, but lacks chymase, and is predominant in the alveolar wall and gastric mucosa, similar to MMCs in rodents. Human mast cells were reported to develop only under the influence of SCF, but T-cell–derived interleukin 3 (IL-3) has little affect on their differentiation.7 Recently, human intestinal mast cells were reported to respond to IL-3 by enhancing their growth,8 but SCF is still an indispensable factor for human mast cells. Mast cells are the progenies of hematopoietic stem cells (HSCs).9,10 In mice, the progenitor cells capable of becoming mast cells leave the bone marrow and enter the circulation but complete their differentiation into mast cells only after arriving in peripheral tissues such as lung, bowel, and skin.10,11 Unfortunately, the developmental mechanism of human mast cells remains far less clear, possibly because the lack of an appropriate in vivo assay system.
The transplantation of primitive human cells into immune-deficient C.B-17-Prkdcscid (scid)12,13 and into NOD/LtSz-scid or NOD/Shi-scid (nonobese diabetic severe combined immunodeficient [NOD/SCID]) mice14,15 is thought to constitute an appropriate functional in vivo system for human HSCs. However, it has been suggested that residual natural killer (NK) cell activity in NOD/SCID mice might interfere with engraftment.16,17 Recently, we developed
This is, therefore, the first report of human mast cell development in mice after transplantation of human HSCs, with NOG mice as recipients. Moreover, development of human mast cells in NOG mice was supported by the murine environment, and, depending on their protease compositions, the distribution of human mast cells was similar to that in the human body.
Materials and methods
Mice, human cell preparation, and xenotransplantation
NOG mice were established at the Central Institute of Experimental Animals (Kawasaki, Japan) by backcrossing
Human cord blood was collected from healthy full-term deliveries after obtaining informed consent. Mononuclear cells were isolated on Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) after phagocyte depletion with silica (ImmunoBiological Laboratories, Gunma, Japan).23 CD34+ cell fractions were further isolated by using AutoMACS (Miltenyi Biotec, Bergisch Gladbach, Germany). After the enrichment, assessment of their purity by flow cytometry showed that approximately 95% of the cells were CD34+ cells. In the experiments using lineage-depleted cells (lin–/CD34+ cells), cord blood mononuclear cells were treated with StemSep (Stem Cell Technologies, Vancouver, Canada), followed by CD34+ selection.
Xenotransplantation of purified human cells into NOG mice was also described previously.18,21 Mice were irradiated at 8 to 12 weeks of age with 240 cGy. Enriched CD34+ cells (50 000) were injected intravenously through the tail vein. After the transplantation, mice were given sterile water containing prophylactic neomycin sulfate (Invitrogen, Carlsbad, CA). The experimental protocol was approved by the Human Studies Internal Review Board at Kyoto University (no. 322).
Flow cytometry
Human cell development in NOG mice was periodically monitored with a flow cytometer (FACS Calibur; BD Cytometry, San Diego, CA) with fluorescein isothiocyanate (FITC)–conjugated antihuman CD45 monoclonal antibody (mAb) and allo-phycocyanin (APC)–conjugated antimouse CD45 mAb (BD Pharmingen, San Diego, CA), as previously reported.18,21 The lineage analysis was performed with APC-conjugated antihuman CD45; phycoerythrin (PE)–conjugated anti-CD3, anti-CD33 (BD Pharmingen), and anti-CD203c mAb which recognized both human mast cells and basophils24,25 ; PC5-conjugated anti-CD19, anti-CD56, and anti-Kit (CD117) mAb (Immunotech, Marseille, France); and biotin-conjugated anti-CD123 (IL-3 receptor α-chain) and streptavidin-FITC (BD Pharmingen).
Murine mast cell determination
Tissue samples were frozen in O.C.T. Tissue-Tek compound (Miles Labs, Elkhart, IN) or fixed in 10% buffered formalin or Carnoy solution (60% ethanol, 30% chloroform, and 10% acetic acid). Those sections were stained with acidic toluidine blue. Carnoy fixed preparations were used for safranin-O and Alcian blue staining.
To collect mast cells from the peritoneal cavity, 5 mL prewarmed Hanks balanced salt solution containing 1% fetal calf serum was injected into the mouse peritoneal cavity. The abdomen was gently massaged for 1 minute, after which the peritoneal cavity was carefully opened, and the fluid containing peritoneal cells was collected with a pipette. One part of the collected cell suspension was used for direct counting of living cells, and the remaining cells were used for staining with toluidine blue or with safranin-O and Alcian blue on the cytospin preparations and for cytometry. On the cytospin preparation, a proportion of the positively stained cells among the 200 nucleated cells was determined.
Immunochemistry
To detect human mast cells, acetone-fixed frozen sections were blocked with donkey serum before incubation with antihuman CD45 mAb (Nichirei, Tokyo, Japan) and then incubated with Cy3-conjugated 2nd Ab (Jackson, West Grove, PA), FITC-conjugated avidin bound to mast cells,26,27 and Hoechst 33342 (Molecular Probes, Eugene, OR). Specificity of avidin binding to mast cells was confirmed with both human and mouse tissue preparations from skin, lung, and gastric stomach.
We used acetone-fixed frozen sections for chymase, because the routinely used Carnoy solution reduces the number of chymase+ cells.28 Antihuman chymase mAb (Chemicon, Temecula, CA) labeled slides were stained with alkaline phosphatase (AP)–conjugated 2nd Ab (Vector, Burlingame, CA). For tryptase, formalin-fixed paraffin-embedded sections and methanol-fixed cytospin preparations were incubated with antitryptase mAb (Chemicon) and with AP-conjugated 2nd Ab. The color was developed with naphthol AS-BI/new fuchsin. In some experiments, biotin-conjugated antichymase mAb-labeled cells were incubated with horseradish peroxidase–conjugated streptavidin, and the color was developed with 3-amino-9-ethylcabbazole (Vector). The cells were sequentially labeled with AP-conjugated antitryptase mAb, and the color was developed with fast blue substrate (Vector). We used healthy parts of skin obtained after mastectomy as positive controls.
For SCF distribution in NOG mouse skin, we sequentially incubated acetone-fixed frozen sections with antimouse SCF polyclonal Ab (R&D systems, Minneapolis, MN) and Cy3-conjugated 2nd Ab (Jackson) and observed with a confocal laser microscopy (Olympus).
RNA purification and RT-PCR (reverse transcription–polymerase chain reaction)
Cellular total RNA was isolated with the phenol/guanidine isothiocyanate method using a Trizol reagent (Invitrogen) and reverse-transcribed to complementary (cDNA) with oligo dT primers and SuperScript Synthesis System (Invitrogen). Reaction mixtures were amplified with 0.2 U Taq polymerase (Sigma) using 25 cycles for glyceraldehyde-3-phosphate dehydrogenase (G3PDH) and 40 cycles for others under the following conditions: denaturation at 95°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 30 seconds. Oligonucleotide primers for SCF which recognized both human and mouse SCF, 5′-TCTTCAGCTGCTCCTATTT-3′ and 5′-ACTGCTACTGCTGTCATTC-3′; human tryptase, 5′-GGAAAACCACATTTGTGACG-3′ and 5′-ATTCACCTTGCACACAGGG-3′; and human chymase, 5′-AAGGAGAAAGCCAGCCTGACC-3′ and 5′-TCCGACCGTCCATAGGATACG-3′ were synthesized.
To evaluate the species origin of SCF, PCR products were purified with the QIAquick PCR purification kit (Qiagen, Valencia, CA) and digested for 1 hour with restriction enzymes, XmaI and NsiI. Mouse keratinocyte Pam212 and human keratinocyte DJM-1 were positive controls.
Human mast cell culture in vitro
Human cord blood CD34+ cells were cultured in AIM-V medium (Invitrogen) with either human SCF (Amgen, Thousand Oaks, CA) or murine SCF (Kirin Brewery, Gunma, Japan) at concentration of 10 ng/mL (suboptimal) or 100 ng/mL (optimal), as described previously but with a minor modification.29-31 During the first week, 50 ng/mL human IL-6 (Kirin Brewery) was also added. Flow cytometry at the constant flow rate was used to assess viable cell number with propidium iodide and mast cell percentage with anti-Kit mAb.
Statistical analysis
Data are presented as the mean ± SD values. Statistical significance was determined with the Student t test, and P < .01 was considered significant.
Results
Murine mast cell distribution in NOG mice
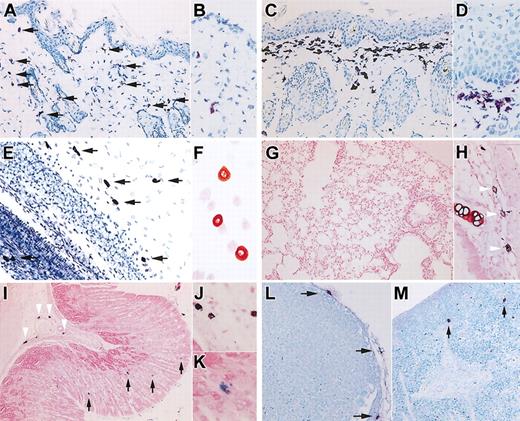
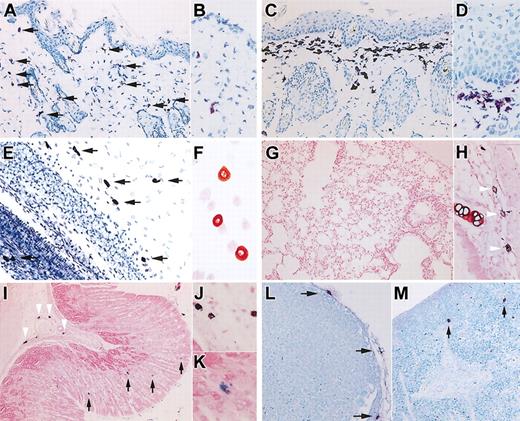
Compared with age-matched control B6 mice, the dermis of 12-week-old NOG mice (n = 6) has a normal concentration of mast cells (Figure 1A-B), but, surprisingly, in the skin of 20-week-old NOG mice (n = 6), we observed a substantial number of toluidine blue+ cells in the upper dermis (Figure 1C-D). The pathologic findings also showed epidermal hyperplasia.
Murine mast cell distribution in NOG mice. (A-D) Toluidine blue staining of the skin of NOG mice. Thin frozen sections from 12-week-old (A-B) and 20-week-old (C-D) NOG mice were stained with acidic toluidine blue. Arrows in the picture from 12-week-old NOG mice (A) indicate metachromatically stained cells. The upper dermis of 20-week-old NOG mice shows bandlike proliferation of toluidine blue+ cells and hyperplasia of epidermis. Magnification, × 200 (A,C) and × 400 (B,D). (E) Toluidine blue staining of mesentery of 20-week-old NOG mice, and arrows indicate mast cells. Magnification × 200. (F) Safranin-O staining of peritoneal cells obtained from 20-week-old NOG mice. Magnification, × 400. (G-K) Alcian blue and safranin-O staining in Carnoy fixed preparations from 20-week-old NOG mice. In lung, a few safranin+ CTMCs were recognized around major airways (H), whereas safranin-negative, Alcian blue+ MMCs were not observed in alveoli (G). In the gastric stomach, NOG mice showed safranin+ CTMCs in the submucosa (arrowheads in I and image with hypermagnification in J), and safranin-negative, Alcian blue+ MMCs in mucosa (arrows in I and image with hypermagnification in K). Magnification, × 100 (G,I), × 200 (H), and × 400 (J-K). (L-M) Toluidine blue staining of Carnoy fixed preparations from lymph node (L) and spleen (M) from 20-week-old NOG mice. Mast cells located only at the capsule of the lymph nodes. In the spleen, a few mast cells were present as shown with arrows in M. Magnification, × 200 (L-M).
Murine mast cell distribution in NOG mice. (A-D) Toluidine blue staining of the skin of NOG mice. Thin frozen sections from 12-week-old (A-B) and 20-week-old (C-D) NOG mice were stained with acidic toluidine blue. Arrows in the picture from 12-week-old NOG mice (A) indicate metachromatically stained cells. The upper dermis of 20-week-old NOG mice shows bandlike proliferation of toluidine blue+ cells and hyperplasia of epidermis. Magnification, × 200 (A,C) and × 400 (B,D). (E) Toluidine blue staining of mesentery of 20-week-old NOG mice, and arrows indicate mast cells. Magnification × 200. (F) Safranin-O staining of peritoneal cells obtained from 20-week-old NOG mice. Magnification, × 400. (G-K) Alcian blue and safranin-O staining in Carnoy fixed preparations from 20-week-old NOG mice. In lung, a few safranin+ CTMCs were recognized around major airways (H), whereas safranin-negative, Alcian blue+ MMCs were not observed in alveoli (G). In the gastric stomach, NOG mice showed safranin+ CTMCs in the submucosa (arrowheads in I and image with hypermagnification in J), and safranin-negative, Alcian blue+ MMCs in mucosa (arrows in I and image with hypermagnification in K). Magnification, × 100 (G,I), × 200 (H), and × 400 (J-K). (L-M) Toluidine blue staining of Carnoy fixed preparations from lymph node (L) and spleen (M) from 20-week-old NOG mice. Mast cells located only at the capsule of the lymph nodes. In the spleen, a few mast cells were present as shown with arrows in M. Magnification, × 200 (L-M).
Murine mast cells collected from the peritoneal cavity were safranin+ CTMCs, as from the dermis. The number of peritoneal mast cells from 12-week-old NOG mice (0.51 ± 0.07 × 106 cells/mouse, mean ± SD values from 6 mice) was not different from that from control B6 mice (0.49 ± 0.05 × 106 cells/mouse). Although peritoneal mast cells in B6 mice had a tendency to increase in number as the mice became older, 20-week-old NOG mice had significantly fewer peritoneal mast cells (0.28 ± 0.05 × 106 cells/mouse) than did 20-week-old B6 mice (1.02 ± 0.57 × 106 cells/mouse, n = 6) (P < .01). Figure 1E shows toluidine blue staining of the mesentery from 20-week-old NOG mice, and Figure 1F shows safranin-O staining of peritoneal cells, also from 20-week-old NOG mice.
In addition, we checked mast cells in lung and gastric tract, where MMCs were predominantly distributed. The lungs showed no mast cell at the alveoli (Figure 1G) but a few mast cells, which were safranin+ CTMCs, around major airways (Figure 1H). In the gastric stomach, mast cells were distributed in both submucosa and mucosa (Figure 1I-K). Mast cells in the submucosa were safranin+ CTMCs (arrowheads in Figure 1I-J), whereas unexpectedly safranin-negative, Alcian blue+ MMCs were identified in gastric mucosa (arrows in Figure 1I,K), even though NOG mice lack T cells because of
In NOG mice, lymph nodes could hardly be identified without human HSC transplantation: only 2 lymph nodes at the axillaries and one at the mesenteric region of 6 mice. Pathologic examination of these identified lymph nodes showed a small number of mast cells located in the connective tissue at the capsule and an even smaller number of mast cells at the trabecula of the lymph node, and none in the cortex or medulla (Figure 1L). They were all safranin+ CTMCs (data not shown). In spleen of NOG mice, mast cells were present in the connective tissue of the trabecula, as shown with arrows in Figure 1M. Again, they were all safranin+ CTMCs (data not shown). Except for the ease of finding lymph nodes from control B6 mice, the number and histologic distribution of mast cells in lymph nodes and spleen of 12-week-old NOG mice were not different from those of the control B6 mice and the older NOG mice.
Human mast cell development in mouse skin
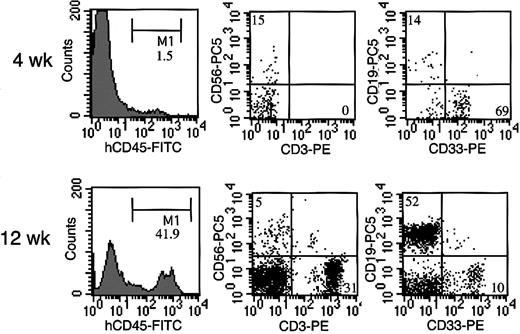
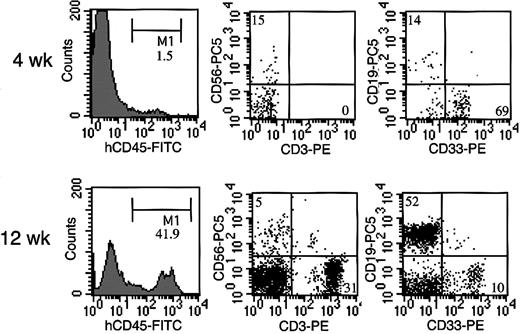
Four weeks after transplantation of cord blood CD34+ cells, human CD45+ cells were detected in NOG mouse peripheral blood, in which CD33+ myeloid cells were predominant, and CD19+ B cells and CD56+ NK cells were also present (Figure 2). Human CD45+ cells gradually increased, and 12 weeks after transplantation, we also observed abundant human CD3+ T cells in NOG mice, in agreement with previous study.18
Representative flow cytometric analysis of peripheral blood from NOG mice after HSC transplantation. Four weeks after the transplantation, less than 2% of the cells were human CD45, in which CD33+ myeloid cells were predominant, and CD19+ B cells and CD56+ NK cells were also present. Twelve weeks after the transplantation, more than 40% cells were replaced by human CD45+ cells, among which abundant human CD3+ T cells were identified.
Representative flow cytometric analysis of peripheral blood from NOG mice after HSC transplantation. Four weeks after the transplantation, less than 2% of the cells were human CD45, in which CD33+ myeloid cells were predominant, and CD19+ B cells and CD56+ NK cells were also present. Twelve weeks after the transplantation, more than 40% cells were replaced by human CD45+ cells, among which abundant human CD3+ T cells were identified.
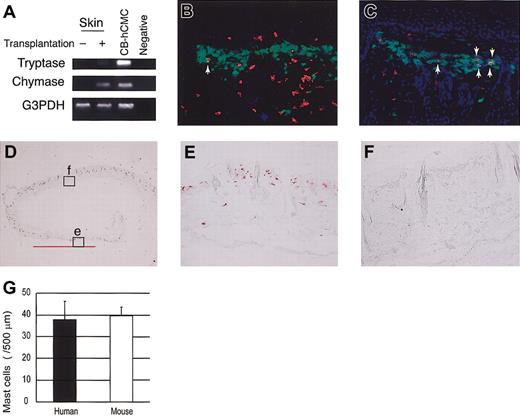
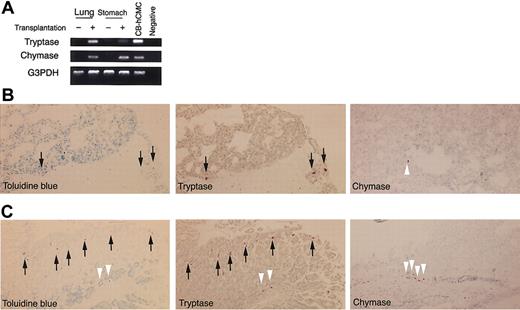
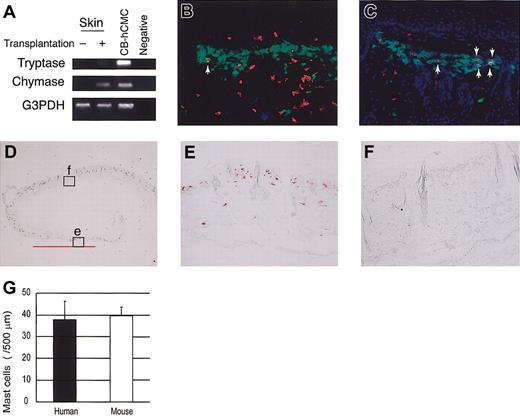
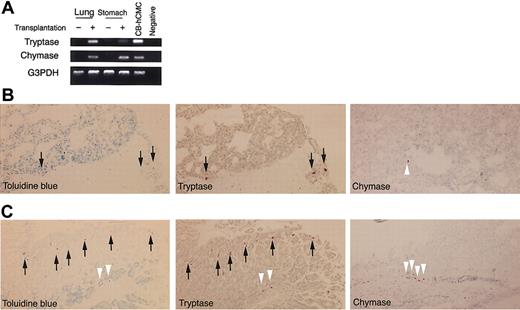
To identify mast cell development after human CD34+ cell transplantation into NOG mice, we first tried to detect tryptase and chymase messenger RNA (mRNA) expression in the skin. Four weeks or 8 weeks after xenotransplantation (n = 3), this expression was hardly detectable. Twelve weeks after the transplantation (n = 3), however, human mast cell-specific protease expression was identified in NOG mouse skin (Figure 3A). At this time, chymase mRNA was more prominent than tryptase mRNA.
Human mast cell development in the mouse skin. (A) RT-PCR analysis for tryptase and chymase mRNA expression. The skin of NOG mice 12 weeks after the transplantation of human CD34+ cells expressed human mast cell-specific tryptase and chymase mRNA. CB-hCMC indicates cord blood-derived human cultured mast cells. (B-C) Acetone-fixed frozen sections of NOG mouse skin 12 weeks (B) and 20 weeks (C) after the transplantation of human cord blood CD34+ cells were stained for human CD45 (red fluorescent with Cy3), mast cells (yellowish green with FITC-avidin), and nuclei (blue with Hoechst 33342). Arrows indicate human CD45+ mast cells, which are stained orange. Magnification, × 200. (D-F) Human MC specific chymase+ cells in the mouse skin. Acetone-fixed frozen sections of NOG mouse skin 24 weeks after the transplantation were stained with antihuman chymase mAb. Human chymase+ cells proliferated focally in the upper dermis (e), represented by the bar bellows, whereas in other lesions on the same samples nonstained granulated cells were located in the upper dermis (f). Magnification, × 12.5 (D) and × 200 (E-F). (G) The number of chymase+ human mast cells and nonstained granulated mouse mast cells in NOG mouse skin 24 weeks after the transplantation. The number of human and mouse mast cells supported by mouse dermis was almost identical. Bar graphs display mean ± SD values from 5 different preparations.
Human mast cell development in the mouse skin. (A) RT-PCR analysis for tryptase and chymase mRNA expression. The skin of NOG mice 12 weeks after the transplantation of human CD34+ cells expressed human mast cell-specific tryptase and chymase mRNA. CB-hCMC indicates cord blood-derived human cultured mast cells. (B-C) Acetone-fixed frozen sections of NOG mouse skin 12 weeks (B) and 20 weeks (C) after the transplantation of human cord blood CD34+ cells were stained for human CD45 (red fluorescent with Cy3), mast cells (yellowish green with FITC-avidin), and nuclei (blue with Hoechst 33342). Arrows indicate human CD45+ mast cells, which are stained orange. Magnification, × 200. (D-F) Human MC specific chymase+ cells in the mouse skin. Acetone-fixed frozen sections of NOG mouse skin 24 weeks after the transplantation were stained with antihuman chymase mAb. Human chymase+ cells proliferated focally in the upper dermis (e), represented by the bar bellows, whereas in other lesions on the same samples nonstained granulated cells were located in the upper dermis (f). Magnification, × 12.5 (D) and × 200 (E-F). (G) The number of chymase+ human mast cells and nonstained granulated mouse mast cells in NOG mouse skin 24 weeks after the transplantation. The number of human and mouse mast cells supported by mouse dermis was almost identical. Bar graphs display mean ± SD values from 5 different preparations.
In addition, we stained skin sections with antihuman CD45 mAb, which did not react to NOG mice not receiving transplants (data not shown). Four weeks or 8 weeks after xenotransplantation (n = 3), unexpectedly we observed human CD45+ cells mainly in the upper dermis and some in the basal layer of the epidermis. However, none of these human CD45+ cells were positive for FITC-avidin, indicating they were not mast cells. Twelve weeks after the transplantation (n = 8), finally we could detect a small number of avidin-FITC and human CD45 double-positive mast cells in the dermis (Figure 3B). Then, the number of double-positive human mast cells gradually but focally increased to produce clusters in the dermis of NOG mice (Figure 3C).
Next, we checked the presence of human mast cell-specific tryptase and chymase by immunochemical staining. Staining the skin of NOG mice not receiving transplants with antihuman chymase mAb showed no positive cells, confirming that there was no cross-reaction to mouse mast cells (data not shown). More than 12 weeks after xenotransplantation into NOG mice, we recognized human chymase+ cells focally in the dermis, and after 24 weeks (n = 6), some mast cell clusters consisted of more than 100 human chymase+ cells (Figure 3D-E). The number and size of the clusters consisting of chymase+ cells in NOG mouse skin differed even among the specimens obtained from the same mouse. As shown in Figure 3E, in some areas of NOG mouse skin, proliferated mast cells consisted of only chymase+ human mast cells but not murine mast cells. We counted the mast cells in the area under 500 μm of skin surface and above the sebaceous gland of the dermis and found that the number of human mast cells in the area consisting of only chymase+ cells (38.0 ± 8.4 cells/500 μm, mean ± SD values from 5 different preparations) and that of mouse mast cells in the area consisting of only nonstained granulated cells (39.8 ± 3.9) was almost the same 24 weeks after the transplantation (Figure 3G).
At the same time, we recognized cells showing strong positive reaction for human tryptase in the upper dermis of NOG mice on the formalin-fixed preparations. These cells were recognized only more than 12 weeks after xenotransplantation (data not shown).
Differentiation into mast cells from HSCs
To confirm de novo generation of human mast cells from HSCs, we transplanted lineage-depleted cells into NOG mice and analyzed the skin, lung, and stomach 4, 8, 12, and 20 weeks after the transplantation (n = 3 for each time point). But the time course of mast cell development in those tissues after transplantation with lin–/CD34+ cells was the same as that with whole CD34+ cells.
Human mast cell development supported by mouse SCF
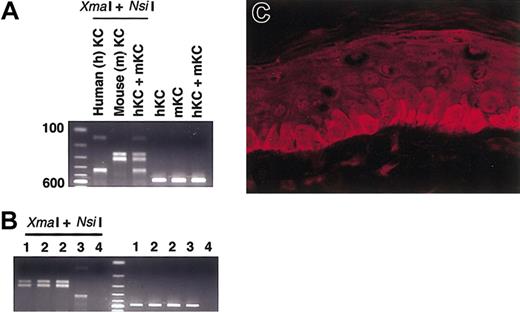
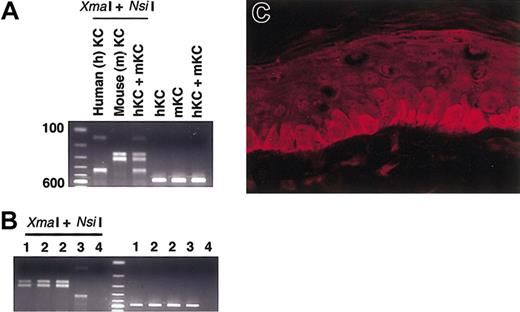
To identify what kind of environment that supports human mast cell development in NOG mouse skin, we examined SCF production. Restriction enzymes could identify the species origin of amplified PCR-products: XmaI cut human products to 137 and 425 base pairs (bp), whereas NsiI cut murine products to 257 and 305 bp (Figure 4A). Although mast cells themselves can produce SCF,32 and we actually detected SCF derived from in vitro–cultured human mast cells, digestion of PCR products suggested that murine SCF was dominant in the skin of NOG mice even when human mast cells had been reconstituted after the successful transplantation of human CD34+ cells (Figure 4B). We got the same results using any of the cDNA samples prepared from the NOG mice 8 weeks, 12 weeks, and 20 weeks after the xenotransplantation.
SCF expression in the mouse skin. (A) Control study for PCR and restriction enzymes. Templates from human DJM-1 and mouse Pam212 keratinocytes were amplified after PCR and then digested with XmaI and NsiI. Human SCF products were cut to 137 and 425 bp and mouse SCF products to 257 and 305 bp. KC indicates keratinocytes. (B) Human mast cell development in NOG mouse skin was predominantly supported by mouse SCF. Expected 562-bp bands were observed after PCR reaction. The digestion patterns with XmaI and NsiI suggested the main source of SCF in the skin was mouse-derived SCF even after the xenotransplantation. 1 indicates NOG mouse not receiving transplant; 2, NOG mouse 12 weeks after xenotransplantation; 3, in vitro–derived cultured human mast cells from cord blood; and 4, negative control. The findings were similar when cDNA template from NOG mouse skin was used 8 weeks and 20 weeks after the transplantation. (C) Mouse SCF protein distribution in the NOG mouse skin. SCF localized in a physiologically cytoplasmic pattern in the epidermis. Magnification × 400.
SCF expression in the mouse skin. (A) Control study for PCR and restriction enzymes. Templates from human DJM-1 and mouse Pam212 keratinocytes were amplified after PCR and then digested with XmaI and NsiI. Human SCF products were cut to 137 and 425 bp and mouse SCF products to 257 and 305 bp. KC indicates keratinocytes. (B) Human mast cell development in NOG mouse skin was predominantly supported by mouse SCF. Expected 562-bp bands were observed after PCR reaction. The digestion patterns with XmaI and NsiI suggested the main source of SCF in the skin was mouse-derived SCF even after the xenotransplantation. 1 indicates NOG mouse not receiving transplant; 2, NOG mouse 12 weeks after xenotransplantation; 3, in vitro–derived cultured human mast cells from cord blood; and 4, negative control. The findings were similar when cDNA template from NOG mouse skin was used 8 weeks and 20 weeks after the transplantation. (C) Mouse SCF protein distribution in the NOG mouse skin. SCF localized in a physiologically cytoplasmic pattern in the epidermis. Magnification × 400.
SCF has 2 phenotypes depending on its distribution pattern. It has been reported that in patients with cutaneous mastocytosis, SCF protein shifts from a cytoplasmic pattern to an intercellular pattern and represents a secretion phenotype.33 In NOG mice, SCF was mainly produced at the epidermis where SCF localized in cytoplasm represents a physiologic phenotype (Figure 4C). This finding was similar to the findings observed before or after the transplantation of human CD34+ cells into the mice.
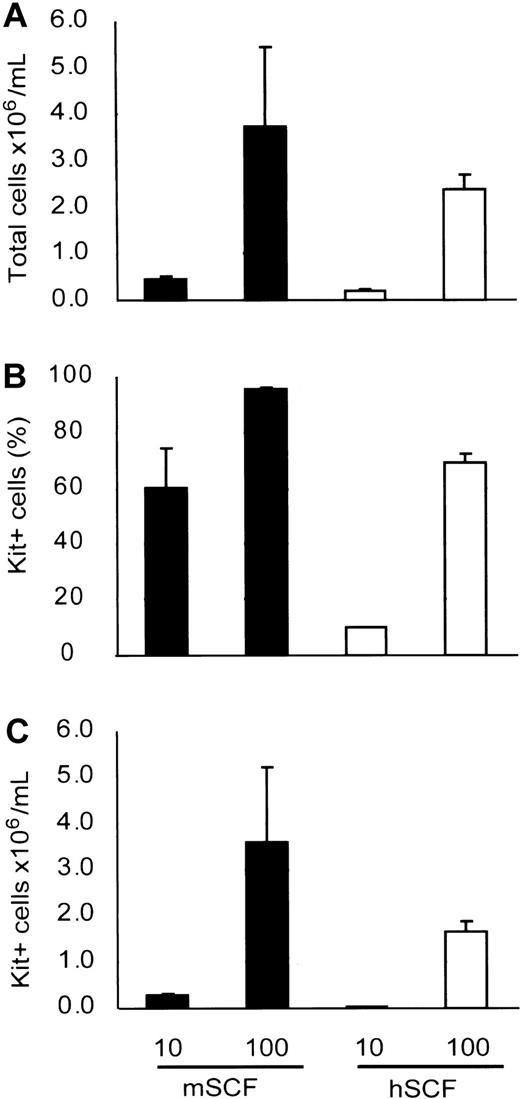
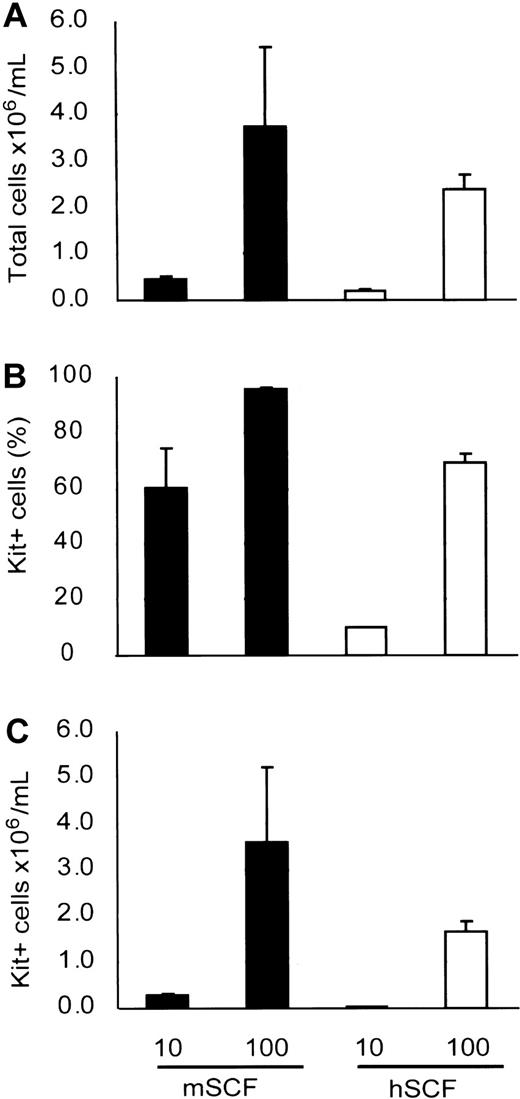
Our result suggests that murine SCF under normal conditions supported human mast cell development in NOG mouse. We also checked whether murine SCF could support human mast cell development from cord blood by using an in vitro culture system. Six weeks after the cultivation, more mast cells had developed with murine SCF than human SCF (Figure 5), indicating that murine SCF can support human mast cell development from HSCs even more efficiently than human SCF.
Mouse SCF effectively supports human mast cell development in vitro. Human cord blood CD34+ cells were cultured with suboptimal (10 ng/mL) and optimal (100 ng/mL) doses of recombinant SCF. The number of mast cells (C) after 6 weeks of culture was assessed in terms of the total number of the viable cells (A) and the Kit+ percentage (B) determined by flow cytometric analysis. Bar graphs display mean ± SD values from 3 independent experiments.
Mouse SCF effectively supports human mast cell development in vitro. Human cord blood CD34+ cells were cultured with suboptimal (10 ng/mL) and optimal (100 ng/mL) doses of recombinant SCF. The number of mast cells (C) after 6 weeks of culture was assessed in terms of the total number of the viable cells (A) and the Kit+ percentage (B) determined by flow cytometric analysis. Bar graphs display mean ± SD values from 3 independent experiments.
Lung and gastric stomach
To determine human mast cell development in organs other than skin, we examined lung and gastric stomach, where MCT was dominant in humans. Twelve weeks after transplantation of human cord blood CD34+ cells, we could detect both tryptase and chymase mRNA in lung and gastric stomach of NOG mice (Figure 6A). In contrast to the skin, the intensity of tryptase mRNA was stronger than that of chymase mRNA in lung.
Human mast cell development in the mouse lung and gastric stomach. (A) RT-PCR analysis for tryptase and chymase mRNA expression. The lung and gastric stomach of NOG mice after the transplantation of human CD34+ cells expressed human mast cell-specific tryptase and chymase mRNA. CB-hCMC indicates cord blood-derived human cultured mast cells. (B-C) Histologic findings for lung (B) and gastric stomach (C). Very small numbers of formalin-resistant toluidine blue+ cells appeared in the lung 20 weeks after the transplantation and, in sequential sections, were almost identical to tryptase+ cells (arrows). In gastric stomach, formalinresistant toluidine blue+ cells were identified in both the mucosa and submucosa. In the acetone-fixed frozen thin sections stained with antichymase mAb, chymase+ cells (white arrowheads) were located only in submucosal lesions. Magnification, × 200 (toluidine blue and tryptase) and × 100 (chymase).
Human mast cell development in the mouse lung and gastric stomach. (A) RT-PCR analysis for tryptase and chymase mRNA expression. The lung and gastric stomach of NOG mice after the transplantation of human CD34+ cells expressed human mast cell-specific tryptase and chymase mRNA. CB-hCMC indicates cord blood-derived human cultured mast cells. (B-C) Histologic findings for lung (B) and gastric stomach (C). Very small numbers of formalin-resistant toluidine blue+ cells appeared in the lung 20 weeks after the transplantation and, in sequential sections, were almost identical to tryptase+ cells (arrows). In gastric stomach, formalinresistant toluidine blue+ cells were identified in both the mucosa and submucosa. In the acetone-fixed frozen thin sections stained with antichymase mAb, chymase+ cells (white arrowheads) were located only in submucosal lesions. Magnification, × 200 (toluidine blue and tryptase) and × 100 (chymase).
Although murine MMCs were sensitive to formalin and invisible on formalin-fixed preparations before the transplantation (data not shown), in the formalin-fixed specimens from lung 20 weeks after xenotransplantation, a small number of toluidine blue+ cells, 3 to 12 cells, was observed in the frontal section of the unilateral lung (n = 4). In sequential sections, we confirmed most of the toluidine blue+ cells were strongly positive for human tryptase. Human chymase+ cells were seen only in the connective tissue around major airways (Figure 6B) and in the submucosal tissue under the esophagus (data not shown). In gastric stomach, we could detect formalin-resistant toluidine blue+ cells in both gastric mucosa and submucosa, which were identically stained by human tryptase in sequential sections (Figure 6C). However, chymase+ cells were located only in submucosa where they showed focal proliferation in clusters.
Bone marrow and peripheral blood
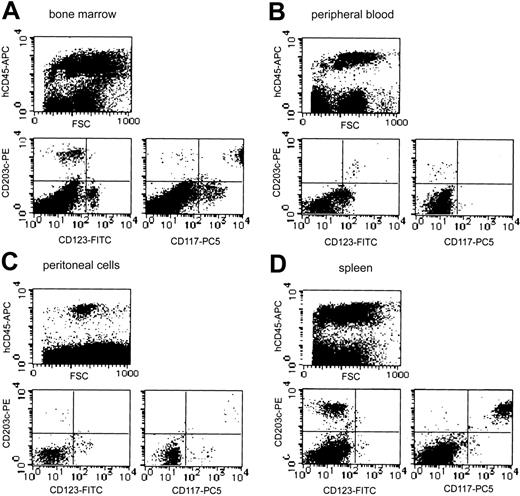
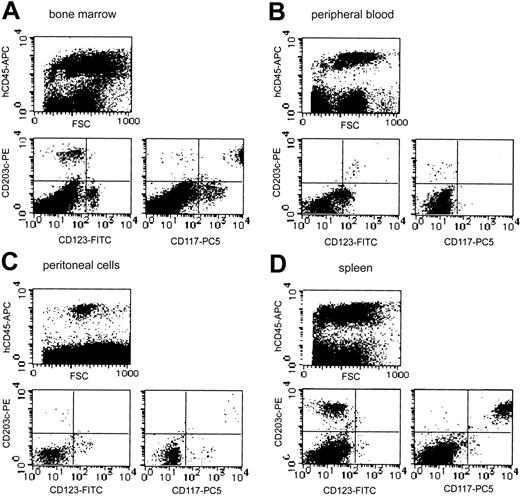
Although neither tryptase+ cells nor toluidine blue+ cells could be detected on smear preparations periodically obtained from bone marrow and peripheral blood (data not shown), analysis of more than 20 000 cells by flow cytometry identified a small number of CD203c+ cells in human CD45+ cells from the bone marrow (3.4% in human CD45+ cells) and peripheral blood (1.2% in human CD45+ cells) 20 weeks after the xenotransplantation (Figure 7A). In bone marrow, most of these CD203c+ cells also strongly expressed Kit (CD117), suggesting they were human mast cells. A small portion of Kit-negative and IL-3 receptor α-chain (CD123) weakly positive cells were also observed in CD203c+ cells, suggesting the presence of human basophils. However, all CD203c+ cells in the peripheral blood were negative for Kit but expressed IL-3 receptor, indicating that human basophils were reconstituted from the transplanted human CD34+ cells and circulated in NOG mice (n = 4).
Representative flow cytometric analysis of bone marrow, peripheral blood, peritoneal cells, and spleen from NOG mice after HSC transplantation. Human mast cell and basophil development from the transplanted human CD34+ cells were identified as CD203c/Kit (CD117) double-positive cells and CD203c/IL-3 receptor α-chain (CD123) double-positive cells, respectively, in human CD45+ cells. The percentage of human CD45+ cells differed depending on the transplanted cord blood-derived cells, but the proportion of CD203c/Kit double-positive cells and CD203c/CD123 double-positive cells in reconstituted human CD45+ cells was similar in 4 independent experiments.
Representative flow cytometric analysis of bone marrow, peripheral blood, peritoneal cells, and spleen from NOG mice after HSC transplantation. Human mast cell and basophil development from the transplanted human CD34+ cells were identified as CD203c/Kit (CD117) double-positive cells and CD203c/IL-3 receptor α-chain (CD123) double-positive cells, respectively, in human CD45+ cells. The percentage of human CD45+ cells differed depending on the transplanted cord blood-derived cells, but the proportion of CD203c/Kit double-positive cells and CD203c/CD123 double-positive cells in reconstituted human CD45+ cells was similar in 4 independent experiments.
Peritoneal cavity and mesentery
The 2.1 ± 0.5 × 106 cells collected from the NOG mouse peritoneal cavity 20 weeks after the transplantation contained 3.9% ± 1.2% safranin+ CTMCs (n = 4). On the cytospin preparation from the collected peritoneal cells, we failed to detect chymase+ human mast cells (data not shown). Flow cytometry showed that less than 0.9% of the collected peritoneal cells expressed human CD45 (Figure 7C). Among these human CD45+ cells, 2.1% expressed CD203c (0.02% of all peritoneal cells), which were human Kit+ mast cells.
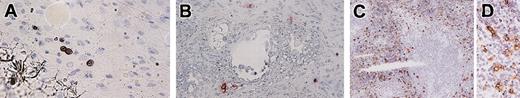
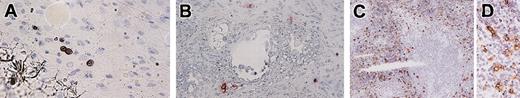
In the mesentery of NOG mice 20 weeks after the transplantation, we recognized a small number of human mast cell clusters consisting of 2 to 6 chymase+ cells (Figure 8A).
Immunochemical staining for human mast cell-specific chymase in mesentery, lymph nodes, and spleen. (A) In the mesentery, human mast cells formed clusters consisting of 2 to 6 chymase+ cells. (B) In the lymph nodes, chymase+ cells localized around vessels in the trabecula. (C-D) Abundant chymase+ cells were extensively distributed in the red pulp but not in the white pulp of the spleen. Magnification, × 400 (A-B,D) and × 100 (C).
Immunochemical staining for human mast cell-specific chymase in mesentery, lymph nodes, and spleen. (A) In the mesentery, human mast cells formed clusters consisting of 2 to 6 chymase+ cells. (B) In the lymph nodes, chymase+ cells localized around vessels in the trabecula. (C-D) Abundant chymase+ cells were extensively distributed in the red pulp but not in the white pulp of the spleen. Magnification, × 400 (A-B,D) and × 100 (C).
Lymph nodes and spleen
Twenty weeks after the transplantation, when human lymphocytes had already been reconstituted, only a small number of human mast cells (0 to 4 cells in the frontal section, n = 4) was identified in the lymph nodes. They were located at the trabecula and comprised human chymase+ connective tissue type mast cells (Figure 8B).
Abundant human chymase+ cells could be identified extensively in the red pulp but not in the white pulp of the spleen of NOG mice (n = 4) by immunochemistry (Figure 8C). These cells did not form clusters in the spleen. As shown in Figure 7D, flow cytometry confirmed the abundant presence of human mast cells as CD203c and Kit double-positive cells (9.8% in human CD45+ cells and 3.3% in all spleen cells).
Discussion
Most of our studies concerning mast cells have used rodent cells. However, rodent and human mast cells show lots of heterogeneities.34 Their dependence on growth factors is different, and the contents of secretory granules are also different. In particular, both types of human mast cells located in the mucosa and submucosa contain heparin, whereas murine MMCs lack heparin, resulting in their sensitivity to formalin. Thus, rodent mast cells are not always a suitable model for studying mast cells under the physiologic and pathologic condition in humans. The established SCF-dependent human mast cell cultures from HSCs in cord blood,29,30,35 fetal liver,31,36 peripheral blood, and bone marrow37 provide new opportunities for mast cell research. By using in vitro–cultured human mast cells, we have been able to identify several new aspects of human mast cells, for example, the effect of IL-4 on human mast cells.38-40 We also hypothesized that human mast cells participate in tissue remodeling by releasing fibrosis-induced mediators and cytokines41 as well as produce enzymes which degenerate the extracellular matrix, known as metalloproteases.42 However, to study the functional roles and developmental mechanism of mast cells in humans, the establishment of an appropriate in vivo model was needed.
In the study presented here we show for the first time human mast cell development in mice after xenotransplantation. Twenty weeks after xenotransplantation, we noticed the cluster formation of human mast cells, in which sometimes more than 100 human chymase+ mast cells were reconstituted in the dermis of NOG mice. The question arises why human mast cells developed in NOG mice, although those derived from transplanted human HSCs have never been found in other mice. In our opinion, NOG mice have, first of all, a strong capacity for human cell engraftment, as various kinds of blood cells, including T cells, were reconstituted after xenotransplantaion.21 Second, NOG mice may provide a suitable environment for mast cells, as dramatic mast cell proliferation was observed in the dermis of 20-week-old mice. Mast cell hyperplasia was recently reported in γδ T-cell–deficient mice with NOD background,43 which means that mast cells in NOG mice may proliferate by being liberated from γδ T-cell regulation. The importance of the environment, which tightly regulates the number of proliferated mast cells, may also be supported by the observation that the number of mast cells in the same size area in NOG mouse skin was almost identical regardless of the origin of the proliferated mast cells.
RT-PCR analysis revealed that murine SCF made a major contribution to human mast cell development in NOG mice. Because we did not administer any human cytokines, the development of human mast cells in NOG mice indicates that SCF alone may be sufficient for human MCTC development. It is also possible that some factors produced from human cells developed in mice from transplanted HSCs are synergistically involved with SCF, because human blood cells, including T cells, were reconstituted in NOG mice. Yet another possibility is that recipient mice supply some factors favorable to human mast cell development, which seems to be supported by the previous finding of an increase of human chymase+ dermal mast cells after healthy human skin plantation onto SCID mice.44
In the spleen of NOG mice, we unexpectedly identified abundant human chymase+ mast cell development, indicating that mouse spleen may provide a suitable condition for the development of human connective-tissue-type mast cells from the HSCs or committed precursors. A detailed analysis of the molecular-based mechanisms of the environment provided in the spleen should be very helpful for a better understanding of the requirement for human connective-tissue-type mast cell development.
The development of MMCs in NOG mice is of potential interest, because T-cell–derived cytokines are reportedly important for murine MMC development. Although NOG mice lack murine T cells, we found safranin-negative, Alcian blue+ murine MMCs at almost normal concentrations in gastric mucosa, suggesting that factors supporting murine MMC development are produced not only by T cells.45 Similarly, the factors required for human MCT development remain unclear. Although some factors produced by reconstituted human T cells or other human progenies may support MCT development in NOG mice, another possibility is that SCF is sufficient for mucosal-type mast cell development in humans. In mice, parasite infection induces reactive MMC proliferation depending on the action of IL-3,46,47 so that parasite infection of NOG mice which have undergone xenotransplantation may provide more information about human MCT development.
The absence of phenotypically identified mast cells on the smear preparations and CD203c/Kit double-positive cells analyzed by flow cytometry in peripheral blood indicates that human mast cells develop from immature cells without a characteristic phenotype and that their complete differentiation into mature mast cells takes place only after they have migrated to peripheral tissues. This hypothesis seems to be supported by cluster formation of human mast cells in NOG mice. Human mast cell development from contaminated precursors is unlikely, because the time course of human mast cell appearance in NOG mice that received transplants of lin–/CD34+ cells was not different from mice that received transplants of whole CD34+ cells. Whether immature cells circulating in the peripheral blood are either already committed to becoming mast cells or maintain their capability to differentiate into other lineages than mast cells is an interesting subject for further studies.
In this study, we established for the first time an in vivo model for human mast cell development. Not only human connective tissue-type MCTC but also human mucosal-type MCT can develop in NOG mice. Our model, thus, provides useful information about human mast cell development from HSCs in vivo. In addition, this model may also pave the way to a potential tool for the in vivo investigation of human mast cell functions.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-04-1160.
Supported by grant 14770405 (N.K.) and Grant-in-Aid for Creative Scientific Research (S) 13GS0009 (T.N.) from the Ministry of Education, Science, Sports, and Culture, Japan, as well as the Long-Range Research Initiative grant CS05-07 (Y.M.) from the Japan Industry Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Atsushi Otsuka and Yukiko Tamaki (Department of Dermatology, Kyoto University) for their technical assistance and Dr Akane Tanaka (Department of Veterinary Clinic, Tokyo University of Agriculture and Technology) and Dr Michiyo Kambe (Department of Clinical Pathology, Kyoto University) for helpful discussion and advice. Language assistance was provided by Jan K. Visscher.