Abstract
Most hematopoietic stem cells (HSCs) are assumed to reside in the so-called side population (SP) in adult mouse bone marrow (BM). We report the coexistence of non-SP HSCs that do not significantly differ from SP HSCs in numbers, capacities, and cell-cycle states. When stained with Hoechst 33342 dye, the CD34-/low c-Kit+Sca-1+lineage marker- (CD34-KSL) cell population, highly enriched in mouse HSCs, was almost equally divided into the SP and the main population (MP) that represents non-SP cells. Competitive repopulation assays with single or 30 SP- or MP-CD34-KSL cells found similar degrees of repopulating activity and frequencies of repopulating cells for these populations. Secondary transplantation detected self-renewal capacity in both populations. SP analysis of BM cells from primary recipient mice suggested that the SP and MP phenotypes are interconvertible. Cell-cycle analyses revealed that CD34-KSL cells were in a quiescent state and showed uniform cell-cycle kinetics, regardless of whether they were in the SP or MP. Bcrp-1 expression was similarly detected in SP- and MP-CD34-KSL cells, suggesting that the SP phenotype is regulated not only by Bcrp-1, but also by other factors. The SP phenotype does not specify all HSCs; its identity with stem cell function thus is unlikely.
Introduction
When stained with the DNA-binding supravital dye Hoechst 33342, bone marrow (BM) cells exhibit various intensities of fluorescence on flow cytometric analysis.1-4 Goodell et al5 were the first to report that a small but distinct population of cells capable of efficient Hoechst dye efflux, referred to as the side population (SP), is enriched in hematopoietic stem cells (HSCs). The SP has been shown to exist and to contain HSCs in mouse, pig, monkey, and human.5-13 Furthermore, the SP is reportedly present in a variety of tissues, containing tissue-specific stem cells,14-19 although in some cases more extensive studies are required to distinguish such cells from SP cells in the circulation.20,21 All these studies suggest a tight association between the SP phenotype and adult stem cell functions.12 More recently, several studies have shown that SP cells also exist in certain types of cancer, implying that the SP phenotype can define a class of cancer stem cells.22-25
One of the adenosine triphosphate (ATP)-binding cassette (ABC) transporters, ABCG2 or Bcrp-1, has been identified as a molecule responsible for the SP phenotype.26 While mice deficient in Bcrp-1 lack the SP in bone marrow, such mice do not show substantial hematopoietic abnormalities.27 The precise functional relationship between the SP phenotype and stem cell function thus remains elusive, and robust comparison of SP and non-SP cells with respect to their phenotypes, cell-cycle states, and stem cell functions is of critical importance.
We have established previously a method to enrich HSCs using a combination of monoclonal antibodies to cell surface markers with multiparameter flow cytometric analysis and cell sorting. CD34-/low c-kit+ Sca-1+, lineage-marker- (CD34-KSL) lineage marker cells are highly enriched in HSCs of mouse bone marrow. When we directly compared CD34-KSL cells and SP cells by 6-parameter flow cytometric analysis, we consistently observed SP and non-SP cells to be present in nearly equal numbers among CD34-KSL cells. Although the presence of HSC activity in the non-SP population recently has been noted,11-13,28 the number and activity of non-SP HSCs, as compared with those of SP HSCs, never have been evaluated quantitatively. We thus decided to compare functional aspects of SP- and non-SP-CD34-KSL cells by using in vivo HSC quantitative assays.29
Bone marrow HSCs were equally divided between the SP and non-SP fractions with no significant difference, on a clonal basis, in long-term repopulating activity; this surprised us. Furthermore, the SP and non-SP phenotypes were interconvertible after transplantation. Interestingly, similar levels of Bcrp-1 expression were detected in SP- and in non-SP-CD34-KSL cells; a fact with the possible implication that Brcp-1 function is regulated by other factors. Our data imply that while the SP phenotype is a convenient marker for stem cells, it is not directly associated with stem cell function.
Materials and methods
Mice
C57BL/6 mice congenic for the Ly5 locus (B6-Ly5.1 mice) were bred and maintained at Sankyo Labo Service Co (Tsukuba, Japan). B6-Ly5.2 mice were purchased from Japan SLC Inc (Hamamatsu, Japan). All procedures were approved by the Animal Care and Use Committee, Institute of Medical Science, University of Tokyo.
Cells
Cells were prepared for flow cytometry sorting as previously described in detail.30 In brief, BM cells were obtained from 8- to 10-week-old B6-Ly5.1 mice and suspended in Hanks balanced salt solution (HBSS) supplemented with 2% fetal calf serum (FCS) and 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (staining medium). Low-density cells (< 1.077 g/mL) were isolated by density gradient centrifugation and were depleted of Lin-positive cells using magnetic beads. After adjusting the cell concentration to exactly 106 cells/mL, cells were stained with 5 μg/mL Hoechst 33342 (lot no. 31K4028; Sigma-Aldrich, St Louis, MO) at 37°C for 90 minutes as previously described by Goodell et al.5 After 2 washes, while being continuously maintained in staining medium at 4°C,30 cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD34, phycoerythrin (PE)-conjugated anti-Sca-1, allophycocyanin (APC)-conjugated anti-c-Kit, and biotinylated anti-Lin antibodies. Of note is that cells were incubated with 2.5 μg FITC-anti-CD34 antibody (RAM34) per 107 cells for 90 minutes. The biotinylated antibody was developed with streptavidin (SA)-Texas red. Eight-parameter 6-color analysis and sorting were performed on a FACS Vantage SE equipped with argon, dye, and ultraviolet (UV) lasers (BD Biosciences, San Jose, CA). The Hoechst dye was exited at 350 nm and detected with 450/BP20 and 660/BP20 optical filters. SP- or main population (MP)-CD34-KSL cells were sorted using the gates shown in Figure 1. A portion of sorted cells before transplantation was reanalyzed on a FACS with the same settings as those used in cell sorting, and more than 95% purity of sorted cells was confirmed (Figure S1, available at the Blood website; see the Supplemental Materials link at the top of the online article). Before single-cell sorting was performed, fluorescent beads were sorted at one bead per well of a 96-well microtiter plate, and more than 99% accuracy was obtained.
Embryonic stem (ES) cells
ES cells (EB3) were maintained in Glasgow minimum essential medium (Sigma-Aldrich) supplemented with 10% FCS, 0.1 mM nonessential amino acid (BD Biosciences), 0.1 mM β-mercaptoethanol, 1 mM sodium pyruvate, and 1000 U/mL mouse leukemia inhibitory factor (Chemicon, Temecula, CA).
Transplantation
Competitive repopulation with 2 × 105 competitor cells from B6-Ly5.1/Ly5.2-F1 mice was performed in B6-Ly5.2 mice lethally irradiated at a dose of 9.5 Gy as previously described.31,32 A single sorted test donor cell or 30 sorted test donor cells were transplanted per mouse. Peripheral blood cells of the recipient mice were analyzed 4 months after transplantation. After erythrocyte lysis, cells were stained with FITC-conjugated anti-Ly5.2, PE-conjugated anti-CD4 and -CD8, PE-Cy7-conjugated anti-B220, APC-conjugated anti-Gr-1 and -Mac-1, and biotinylated anti-Ly5.1 antibodies. The biotinylated antibody was developed with SA-Alexa 594. Mac-1/Gr-1+ cells were considered to be myeloid cells. B220+ cells were considered to be B-lymphoid cells. CD4/8+ cells were considered to be T-lymphoid cells. The percent chimerism was calculated by (percent Ly5.1+ test donor cells) ×100/(percent Ly5.1+ test donor cells + percent F1+ competitor cells) for total leukocytes, myeloid cells, B-lymphoid cells, or T-lymphoid cells.
BM cells (2 × 106) reconstituted with test donor cells were secondarily transplanted into a group of lethally irradiated mice without competitor cells. BM cells reconstituted with 30 SP- or MP-CD34-KSL cells were first stained with Hoechst 33342 and subsequently stained with FITC-anti-Ly5.2, PE-anti-Sca-1, APC-c-Kit, and biotinylated Lin antibodies, followed by staining with SA-Texas red. Cells were analyzed using FACSVantage (BD Biosciences).
Semiquantitative RT-PCR
Total RNA was extracted from SP- and MP-CD34-KSL cells and was reverse transcribed. cDNA was normalized with Gapdh copy numbers calculated based on quantitative PCR data using TaqMan rodent Gapdh control reagent (Perkin-Elmer Applied Biosystems, Foster City, CA) as previously described.26 One PCR cycle consisted of 20 seconds at 94°C, 20 seconds at 58°C, and 30 seconds at 72°C. To amplify Bcrp1, 5′-CCA TAG CCA CAG GCC AAA GT-3′ and 5′-GGG CCA CAT GAT TCT TCC AC-3′ primers were used. To amplify Gapdh, 5′-ATT GTC AGC AAT GCA TCC TGC-3′ and 5′-TCA TAC TTG GCA GGT TTC TCC-3′ were used.
Immunostaining
By using a flow cytometer, SP- or MP-CD34-KSL cells were directly sorted into a medium drop. After fixation with 2% paraformaldehyde for 30 minutes and blocking in 10% goat serum for 30 minutes, at room temperature, cells were incubated with rabbit anti-mouse Bcrp-1 polyclonal antibody (Kamiya Biomedical, Seattle, WA) for 12 hours at 4°C. The cells were then washed and incubated with Alexa-647-conjugated goat anti-rabbit IgG (Molecular Probes, Carlsbad, CA) for 30 minutes at room temperature. After staining with 4,6-diamidino-2-phenylindole (DAPI), cells were analyzed with a Leica TCS SP2 AOBS confocal microscope (Leica Microsystems, Wetzlar, Germany) equipped with a Leica 63×/1.4 numeric aperture oil objective lens. Data were collected and analyzed using Leica confocal software version 2.61, and images were processed using Adobe Photoshop CS (Adobe Systems, San Jose, CA).
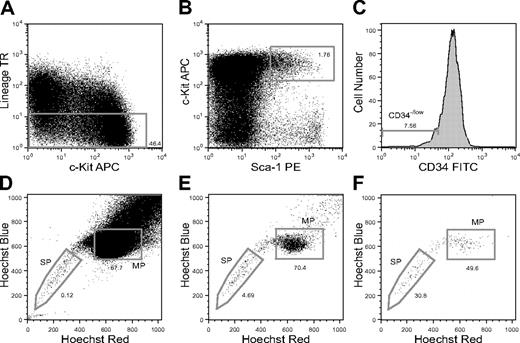
SP analysis of CD34-KSL cells in mouse BM. Flow cytometric analysis of CD34-KSL cells stained with Hoechst 33342 is shown. (A) Lin+ cell-depleted mouse BM cells are displayed for c-Kit and Lin expression with the sorting gate for Lin- cells. (B) Lin- cells are displayed for Sca-1 and c-Kit expression with the sorting gate for Sca-1+c-Kit+ cells. TR indicates Texas red. (C) The histogram shows the CD34 expression of KSL cells with the sorting gate for CD34- cells. (D) Lin- cells are displayed for Hoechst red and blue. (E) KSL cells are displayed for Hoechst red and blue. (F) CD34-KSL cells are displayed for Hoechst red and blue. The sorting gates for the SP and MP phenotypes among CD34-KSL cells are presented.
SP analysis of CD34-KSL cells in mouse BM. Flow cytometric analysis of CD34-KSL cells stained with Hoechst 33342 is shown. (A) Lin+ cell-depleted mouse BM cells are displayed for c-Kit and Lin expression with the sorting gate for Lin- cells. (B) Lin- cells are displayed for Sca-1 and c-Kit expression with the sorting gate for Sca-1+c-Kit+ cells. TR indicates Texas red. (C) The histogram shows the CD34 expression of KSL cells with the sorting gate for CD34- cells. (D) Lin- cells are displayed for Hoechst red and blue. (E) KSL cells are displayed for Hoechst red and blue. (F) CD34-KSL cells are displayed for Hoechst red and blue. The sorting gates for the SP and MP phenotypes among CD34-KSL cells are presented.
Cell-cycle analysis
Cell-cycle analysis was performed on 20 000 or more SP and MP cells isolated from mouse BM using a MoFlo (Dako, Glostrup, Denmark). Sorted cells were fixed with 70% ethanol in phosphate-buffered saline (PBS), stained with 50 μg/mL propidium iodide (PI), and analyzed on a FACS Vantage SE as previously described.32
For simultaneous analysis of Hoechst 33342 and Pyronin Y (Sigma-Aldrich), Pyronin Y was added to cells during Hoechst staining. Cells were incubated with 5 μg/mL Hoechst dye for 45 minutes and then further incubated with Hoechst dye and 1 μg/mL Pyronin Y for 45 minutes.33 Cells were subjected to antibody staining procedures as described in “Cells” except that PE-anti-Sca-1 antibody was replaced by PE-Cy5.5-anti-Sca-1 antibody, and biotinylated Lin antibodies were reacted with SA-APC-Cy7. Cells were analyzed by MoFlo.
To study the cell-cycle kinetics of SP- or MP-CD34-KSL cells, bromodeoxyuridine (BrdU; Sigma-Aldrich) was administered continuously to mice via drinking water for 1, 2, or 3 weeks. A bottle of water containing 0.5 mg/mL BrdU was changed once a week. After BrdU incorporation in vivo, cells were isolated from the bone marrow of mice and stained with Alexa 488-conjugated anti-BrdU antibody (Invitrogen Japan, Tokyo, Japan) and PI. Cells were analyzed on a FACSCalibur (BD Biosciences).
Results
SP analysis of CD34-KSL cells
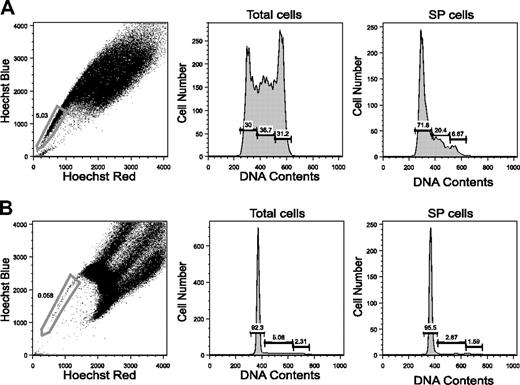
As representatively shown in Figure 1, lineage marker- (Lin-) cells (Figure 1A), KSL cells (Figure 1B), and CD34-KSL cells (Figure 1C) were analyzed for Hoechst dye efflux activity by flow cytometry using a 3-laser system. The staining pattern of Lin- cells was similar to the previously described pattern of unfractionated cells.5 SP cells accounted for 0.23% ± 0.17% (mean ± SD, n = 7) of Lin- cells (Figure 1D), 2.5% ± 1.8% of KSL cells (Figure 1E), and 39% ± 15% of CD34-KSL cells (Figure 1F) in 7 independent analyses. These data show that the proportion of SP cells significantly increases as step-by-step gating proceeds (Table S1). Interestingly, 41% ± 17% (mean ± SD, n = 7) of the CD34-KSL cells were found in a limited area of the non-SP fraction, designated here as the MP (Figure 1F).
CD34-KSL cells composed only 0.13% of Lin- BM cells (Table S2). They composed 26% of SP-Lin- cells and 0.065% of MP-Lin- cells. All these data, consistent with previous reports,6,10,12 verified that our SP analyses were appropriately carried out and also showed that SP cells are remarkably enriched in CD34-KSL cells (Table S1). Conversely, CD34-KSL cells are remarkably enriched in SP cells (Table S2). SP-CD34-KSL cells and MP-CD34-KSL cells accounted for 0.052% ± 0.034% and 0.047% ± 0.015% of Lin- cells, respectively (Table S3). These data indicate that equivalent numbers of CD34-KSL cells exist in SP BM cells and in MP BM cells.
Equivalent HSC activity in SP- and MP-CD34-KSL cells
Thirty SP- or MP-CD34-KSL cells were sorted on a flow cytometer and transplanted into lethally irradiated mice with 2 × 105 BM competitor cells. Peripheral blood cells of the recipient mice were analyzed for myeloid, B-lymphoid, and T-lymphoid lineage reconstitution 4 months after transplantation by flow cytometry. As shown in Table 1, 9 of 9 recipient mice showed multilineage reconstitution with test donor cells to similar degrees, regardless of whether SP- or MP-CD34-KSL cells were transplanted.
To examine self-renewal activity among these cells, secondary transplantation was performed. Two pairs of mice with similar levels of chimerism were selected from mice that had been reconstituted with SP- or MP-CD34-KSL cells (Table 1), and 2 × 106 BM cells from each mouse were transplanted into a group of lethally irradiated mice. Results are shown in Table 2. All surviving recipient mice again showed multilineage reconstitution 4 months after transplantation. When either SP- or MP-CD34-KSL cell-derived BM cells were transplanted, 1 group of secondary recipient mice showed relatively higher chimerism than the other. These data likely reflect heterogeneity among SP and MP HSCs. Nonetheless, they indicate that long-term multilineage repopulating cells with self-renewal capacity are present not only in the SP, but also in the MP.
The frequency of HSCs among SP- or MP-CD34-KSL cells
While HSC activity was detected in both SP- and MP-CD34-KSL cells, the frequency of HSCs among these cells remained unclear. To address this issue, we performed single-cell reconstitution assays. Single SP- or MP-CD34-KSL cells were transplanted into lethally irradiated mice together with 2 × 105 BM competitor cells. The recipient mice were analyzed 4 months after transplantation. Data are shown in Table 3. Successful multilineage reconstitution was observed in 10 of 44 (22.7%) recipient mice when single CD34-KSL cells were transplanted, consistent with our previous observations.29 Transplantation of single SP-CD34-KSL cells resulted in multilineage reconstitution in 11 of 52 (21.2%) recipient mice. Transplantation of single MP-CD34-KSL cells did so in 7 of 41 (17.1%) recipient mice. These frequencies did not differ significantly. There was no difference in degree of chimerism among the groups of mice reconstituted with 3 different purified cells. With respect to the percentages of SP- and MP-CD34-KSL cells among Lin- BM cells (Table S3), 0.011% and 0.008% of the Lin- BM cells were SP- and MP-CD34-KSL cells with multilineage reconstitution activity, indicating that almost equal numbers of HSCs were present in the SP and the MP.
Secondary transplantation was performed using BM cells reconstituted with single SP- or MP-CD34-KSL cells (Table 4). Regardless of whether an SP-CD34-KSL cell or an MP-CD34-KSL cell had primarily been transplanted, groups of mice that had received BM cells from mice with high chimerism showed high levels of multilineage reconstitution. The other groups of mice that had received BM cells from mice with lower chimerism showed low levels of chimerism. These results indicate that self-renewing HSCs are present in both the SP and the MP at the clonal level, although their self-renewal capacities vary.
Bcrp-1 expression in SP- or MP-CD34-KSL cells
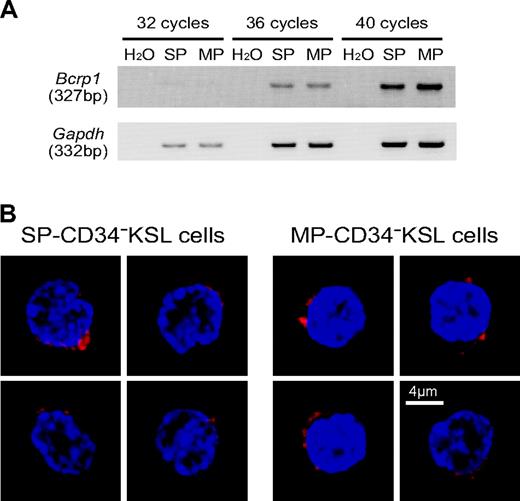
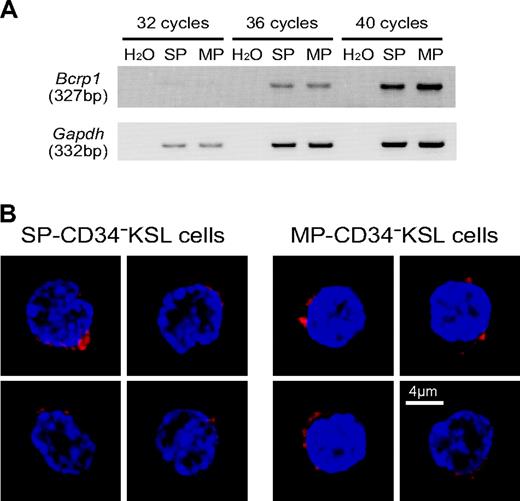
We examined expression levels of Bcrp1 by semiquantitative RT-PCR. As shown in Figure 2A, the transcript of Bcrp1 was detected in SP-CD34-KSL cells as expected. Interestingly, it also was found at similar levels in MP-CD34-KSL cells. We then examined the expression of Bcrp-1 protein by immunostaining. As shown in Figure 2B, Bcrp-1 protein was similarly detected in the cytoplasm and membrane of SP-CD34-KSL cells as well as in those of MP-CD34-KSL cells. These data clearly demonstrate dissociation between Bcrp-1 expression and dye efflux function of Bcrp-1. Dye efflux function of Bcrp-1 appears to be modulated by other factors. This interpretation raises the possibility that the SP and MP phenotypes are interchangeable, depending on the functional state of Bcrp-1.
Unstable SP phenotype in HSCs
We assumed that the Hoechst 33342 staining profile of HSCs can change depending on functional levels of Bcrp-1. To test this hypothesis, SP analysis was performed on BM cells reconstituted with SP or MP HSCs 4 months after transplantation (Table 1). As demonstrated in Figure 3, both SP- and MP-KSL cells were generated from 30 SP-CD34-KSL cells. Both SP- and MP-KSL cells were generated from 30 MP-CD34-KSL cells as well. These data indicate that after transplantation the SP phenotype switched to the MP phenotype and vice versa.
Cell-cycle analyses of SP- or MP-CD34-KSL cells
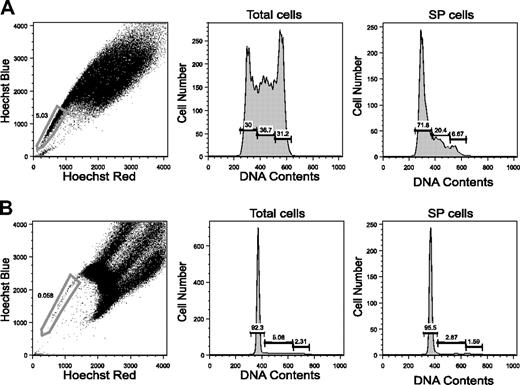
Are the SP phenotype and the cell-cycle state linked?34 To address this issue, cell-cycle analysis was performed on isolated SP cells. We first examined mouse embryonic stem (ES) cells because a large proportion of these cells is known to have the SP phenotype and to be in the cell cycle.26 As shown in Figure 4A, only 30% of the ES cells were in the G0/G1 phases, and the remaining cells were in the S phase or the G2/M phases. When SP ES cells were separated and analyzed for cell-cycle status, about 70% of such cells were in the G0/G1 phases. The remaining cells were in the S/G2/M phases. Thus, the SP phenotype does not always indicate that the cells that manifest it remain in the G0/G1 phases.35
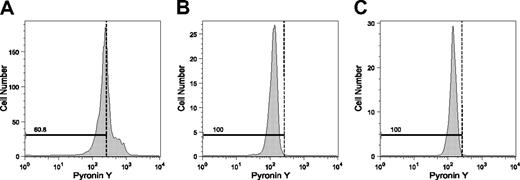
We next examined SP BM cells to see if this was also the case for primarily isolated cells. As shown in Figure 4B, 3.5% ± 1.1% (mean ± SD, n = 4) of the SP cells were indeed in the S/G2/M phases. These data, together with previous observations,5 indicate that cycling cells can have the SP phenotype. To distinguish cells in the G0 phase from those in the G1 phase among SP- or MP-CD34-KSL cells, we tried Pyronin Y staining; this detects RNA in these cells.36 BM cells simultaneously stained with the Hoechst and Pyronin Y dyes were antibody marked and were analyzed by flow cytometry. As shown in Figure 5, about 40% of the CD34+KSL cells were stained by Pyronin Y. In sharp contrast, SP- and MP-CD34-KSL cells were stained only scantily by Pyronin Y. These data suggest that BM HSCs are likely to be in the G0 phase, regardless of their capacity for Hoechst dye efflux.
Bcrp-1 expression in SP- or MP-CD34-KSL cells. (A) The expression of Bcrp1 and Gapdh was examined by RT-PCR. cDNA was synthesized from total RNA extracted from SP- or MP-CD34-KSL cells. Normalized amounts of cDNA templates were amplified by 32, 36, or 40 cycles. (B) The expression of Bcrp-1 was examined by immunostaining with anti-Bcrp-1 antibody. SP-CD34-KSL cells and MP-CD34-KSL cells were stained with anti-Bcrp-1 antibody, followed by incubation with Alexa-647-conjugated anti-rabbit IgG and DAPI. At least 50 cells were analyzed with a confocal microscope. Two cells are representatively shown for each cell type. Bcrp-1 seems unevenly distributed in the cytoplasm and/or membrane. Scale bar is 4 μm.
Bcrp-1 expression in SP- or MP-CD34-KSL cells. (A) The expression of Bcrp1 and Gapdh was examined by RT-PCR. cDNA was synthesized from total RNA extracted from SP- or MP-CD34-KSL cells. Normalized amounts of cDNA templates were amplified by 32, 36, or 40 cycles. (B) The expression of Bcrp-1 was examined by immunostaining with anti-Bcrp-1 antibody. SP-CD34-KSL cells and MP-CD34-KSL cells were stained with anti-Bcrp-1 antibody, followed by incubation with Alexa-647-conjugated anti-rabbit IgG and DAPI. At least 50 cells were analyzed with a confocal microscope. Two cells are representatively shown for each cell type. Bcrp-1 seems unevenly distributed in the cytoplasm and/or membrane. Scale bar is 4 μm.
We then analyzed the in vivo cell-cycle kinetics of SP- and MP-CD34-KSL cells. BrdU was administered to mice for 1, 2, or 3 weeks. The mice were then killed and SP- and MP-CD34-KSL cells were isolated from the bone marrow. BrdU uptake by SP- and MP-CD34-KSL cells was assessed by flow cytometry. As representatively shown in Figure S2, these cells were similarly labeled with BrdU after 1, 2, or 3 weeks of administration. These cells thus are similar in not only their cell-cycle states but also their turnover rates.
Discussion
The phenotype of SP cells is strongly influenced by the cell concentration, the dye concentration, and by the incubation time and temperature present during staining.12 We therefore paid special attention to exact adherence to the original protocols for SP cell identification,5 with the use of the same lot of Hoechst 33342 throughout this study. Analyses of BM cells stained with the Hoechst dye consistently yielded similar SP patterns. It was possible, however, that SP cells that were somewhat overstained might have been shifted toward the MP phenotype. If this were the case, only HSCs with the greatest dye efflux capacity should have remained in the SP, resulting in more enrichment for HSCs. However, even when cells were stained with a 1.5-fold concentration of the Hoechst dye, we observed neither an increase in the frequency of HSCs nor an increase in repopulating activity among isolated SP-CD34-KSL cells (data not shown).
HSC activity is remarkably enriched in the SP of adult mouse bone marrow.5,6,9-13 This is most likely due to the significantly greater frequency of HSCs in the SP than in the non-SP.11 Among the SP, cells with the highest dye efflux activity (lower SP cells) are reported to contain more HSCs than do cells with less dye efflux activity (upper SP cells).6,10,12 These data supported an idea that the SP phenotype is directly correlated with the primitive state of HSCs. Despite a number of studies on SP cells, little attention has been paid to non-SP HSCs. No study has formally compared HSC numbers or HSC expression of Bcrp-1 between the SP and the non-SP cohorts. More importantly, no study has asked whether SP HSCs have greater repopulating and self-renewal activities than do non-SP HSCs. This is because detection of HSCs in the non-SP was not so simple as detection of HSCs in the SP. Indeed, the frequency of CD34-KSL cells differed 400-fold between SP-Lin- and MP-Lin- populations (Table S2). To compare non-SP HSCs with SP HSCs in a quantitative manner, particularly on a clonal basis, HSCs needed to be highly purified from among non-SP cells by combining other isolation protocols.
SP analysis of KSL cells derived from SP- or MP-CD34-KSL cells. (A) BM cells from mice reconstituted with 30 SP-CD34-KSL cells were analyzed 4 months after transplantation. (B) BM cells from mice reconstituted with 30 MP-CD34-KSL cells are analyzed simultaneously. Test donor cells are identified as Ly5.2 cells. Sequential gating is shown from the left to the right panels, with Ly5.2-KSL cells (staining with both Hoechst red and Hoechst blue) in the panel at far right.
SP analysis of KSL cells derived from SP- or MP-CD34-KSL cells. (A) BM cells from mice reconstituted with 30 SP-CD34-KSL cells were analyzed 4 months after transplantation. (B) BM cells from mice reconstituted with 30 MP-CD34-KSL cells are analyzed simultaneously. Test donor cells are identified as Ly5.2 cells. Sequential gating is shown from the left to the right panels, with Ly5.2-KSL cells (staining with both Hoechst red and Hoechst blue) in the panel at far right.
Cell-cycle analysis for SP and MP cells. (A) SP analysis for mouse ES cells is shown with the sorting gate. (B) SP analysis for mouse BM cells is shown with the sorting gate. The G0/G0, S, and G2/M phases were analyzed for total cells and for SP cells.
Cell-cycle analysis for SP and MP cells. (A) SP analysis for mouse ES cells is shown with the sorting gate. (B) SP analysis for mouse BM cells is shown with the sorting gate. The G0/G0, S, and G2/M phases were analyzed for total cells and for SP cells.
In the present study, comparable percentages of SP cells and MP cells among CD34-KSL cells (Figure 1) enabled us to compare their stem cell activity in detail. A series of transplantation experiments with purified cells provided evidence for the presence of HSCs in not only the SP, but also the MP (Tables 1, 2, 3, 4). The frequency of SP-CD34-KSL cells did not differ from that of MP-CD34-KSL cells among Lin- BM cells (Table S3). The frequency of long-term repopulating cells among SP-CD34-KSL cells did not differ from that among MP-CD34-KSL cells (Table 3). By calculation, among 105 Lin- BM cells 11 HSCs should exist as SP-CD34-KSL cells, and 8 HSCs should exist as MP-CD34-KSL cells. Repopulating activity among SP-CD34-KSL cells was equivalent to that among MP-CD34-KSL cells (Tables 1, 3), and self-renewal activity was similarly detected in both SP- and MP-CD34-KSL cells (Tables 2, 4). These data clearly indicate that as many non-SP-CD34-KSL HSCs as SP-CD34-KSL HSCs exist in mouse BM and that their capacities are indistinguishable.
Consistent with previous observations,10,12 most Lin-SP BM cells exhibited the KSL phenotype (Figure S3). CD34 expression was not detected or was detected at a low level in approximately one third of these cells. The remaining cells thus were considered to be CD34+KSL cells. These data are consistent with observations by Matsuzaki et al10 and by Pearce et al,11 but are inconsistent with observations by Goodell et al.6,12 It was not easy to distinguish CD34-positive cells among SP-KSL cells because of continual low-level expression of CD34. We ascribe this as well to a low affinity for CD34 of the anti-CD34 antibody that we used. We thus chose to use a relatively large amount of the antibody and to prolong incubation with it. When relatively large amounts of anti-CD34 antibody were used, nonspecific binding was possible, as recently pointed out.12 However, our dose of the antibody (0.25 μg/106 cells) seemed not to cause significant nonspecific binding. Moreover, as shown in Table S4, SP-CD34+KSL cells, when separately transplanted, did not show repopulating activity, whereas SP-CD34-KSL cells did. These data are consistent with our initial observations on transplantation with CD34-KSL cells and CD34+KSL cells.37 Therefore, functionally distinct populations can be separated using a proper staining protocol that employs anti-CD34 antibody. If cells are stained with much smaller amounts of this antibody or stained for a much shorter incubation time, most KSL cells may be assessed as “negative” for CD34 antigen. Addition of Flt-3 staining to this staining protocol provides a better indication of proper CD34 staining and gating because all Flt-3+KSL cells are CD34+.38
A few of the SP cells were in the S/G2/M phases (Figure 4). These cycling SP cells were inferred to be CD34+KSL cells because all CD34-KSL cells were in the G0 phase (Figure 5). Of note is that all MP-CD34-KSL cells were also in the G0 phase (Figure 5). Because SP- and MP-CD34-KSL cells entered the cell cycle at similar rates (Figure S2), cell cycles of these cells seemed regulated in a similar fashion. We detected both Bcrp1 transcript and its protein in comparable quantities in SP- and MP-CD34-KSL cells (Figure 2). Bcrp-1-GFP knock-in mice, in which a GFP reporter gene was inserted into the Bcrp1 locus, were recently generated.39 Consistent with our observations, Bcrp-1-GFP was detected in these mice in not only SP cells, but also non-SP cells. Other factors, in addition to Bcrp-1 expression, are likely to be required for efficient efflux of the Hoechst dye. Such factors may be available in SP HSCs, but not in non-SP HSCs,39 and may be related with Akt signaling as suggested.40 Nevertheless, considering that the SP and MP phenotypes are reversible and interconvertible one into the other, the function of Bcrp-1 may not be directly related to HSC function.
Pyronin Y staining for SP- and MP-CD34-KSL cells. Pyronin Y staining was performed to detect cells in the G0 phase. BM cells were immunostained with a combination of antibodies as well as stained with Hoechst 33342 and Pyronin Y, and then were analyzed by flow cytometry. (A) CD34+KSL cells are shown. (B) SP-CD34-KSL cells are shown. (C) MP-CD34-KSL cells are shown. The dotted lines separating the G0 phase from the G1 phase were tentatively set according to intensities of Pyronin Y fluorescence in cells in the S/G2/M phases.
Pyronin Y staining for SP- and MP-CD34-KSL cells. Pyronin Y staining was performed to detect cells in the G0 phase. BM cells were immunostained with a combination of antibodies as well as stained with Hoechst 33342 and Pyronin Y, and then were analyzed by flow cytometry. (A) CD34+KSL cells are shown. (B) SP-CD34-KSL cells are shown. (C) MP-CD34-KSL cells are shown. The dotted lines separating the G0 phase from the G1 phase were tentatively set according to intensities of Pyronin Y fluorescence in cells in the S/G2/M phases.
The SP phenotype is a simple and convenient marker when one searches for HSCs and is extremely useful in animal species where antibodies to permit enrichment of HSCs are not available. However, as mentioned earlier, Hoechst staining is influenced by a number of factors and can vary. In addition, our results clearly indicate that the SP phenotype does not mark all HSCs at any one time. Use of the SP phenotype when searching for other somatic stem cells or for cancer stem cells requires an understanding of these specific features.
Prepublished online as Blood First Edition Paper, June 27, 2006; DOI 10.1182/blood-2006-03-010207.
Supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Y.M. and S.Y. performed research; H.E. and H.N. designed research and wrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Drs A. S. Knisely and H. Miyoshi for critical reading of the manuscript.