Abstract
Pharmacologic infusion of activated protein C (APC) improves survival in severe sepsis, and platelet factor 4 (PF4) accelerates APC generation in a primate thrombin-infusion model. We now tested whether endogenous platelet PF4 content affects APC generation. Mice completely deficient in PF4 (mPF4−/−) had impaired APC generation and survival after thrombin infusion, similar to the impairment seen in heterozygote protein C–deficient (PC+/−) mice. Transgenic mice overexpressing human PF4 (hPF4+) had increased plasma APC generation. Overexpression of platelet PF4 compensated for the defect seen in PC+/− mice. In both a thrombin and a lipopolysaccharide (LPS) survival model, hPF4+ and PC+/−/hPF4+ mice had improved survival. Further, infusion of hPF4+ platelets improved survival of wild-type mice after an LPS challenge. These studies suggest that endogenous PF4 release may have biologic consequences for APC generation and survival in clinical sepsis. Infusions of PF4-rich platelets may be an effective strategy to improve outcome in this setting.
Introduction
In addition to its anticoagulant function, activated protein C (APC) has been shown to exert an antiinflammatory influence1,2 and inhibit apoptosis.3–5 All of these effects may contribute to the efficacy of APC infusions in improving outcome in various animal models of inflammation6–9 and in reducing mortality in patients with sepsis.10–12 In a lipopolysaccharide (LPS)–induced survival model of sepsis, heterozygous protein C–deficient (PC+/−) mice have enhanced vulnerability to death,6,8 reinforcing the crucial role played by endogenously generated APC in this setting. Recent clinical studies demonstrate that basal PC levels are an independent predictor of sepsis outcome13 and that endogenous vascular generation of APC is a crucial determinant of survival in sepsis.14
We have shown previously that platelet factor 4 (PF4, CXCL4) can enhance APC generation in vitro15 and in vivo16 ; however, the biologic impact of endogenous, platelet-derived PF4 in regulating systemic APC generation has not been tested. In this study, we examined whether endogenously released PF4 from activated platelets stimulates APC generation in response to thrombin infusion and improves outcome in thrombosis/inflammatory models. These studies were done in mice with targeted disruption of the murine Pf4 gene (mPf4−/−) and transgenic mice that overexpressed human PF4 (hPF4+).17 We found that platelet PF4 content correlated with APC generation after thrombin infusion and survival after thrombin or LPS challenge and that released PF4 can compensate for the lower PC levels in PC+/− mice.6,8 Further, infusion of platelets with high PF4 content is protective in the LPS challenge model, supporting endogenous PF4 as an important variable in outcome from sepsis and suggesting a novel therapy for sepsis.
Materials and methods
The mPf4−/− and hPF4+ mice have been previously described.17,18 PC+/− mice were supplied by Dr Frank Castellino.19 All mice were on a common C57Bl6 background, and littermate wild-type (WT) controls were used. Murine studies were approved by the Children's Hospital of Philadelphia institutional animal care and use committee.
Circulating plasma APC levels were measured by capture enzyme-linked immunosorbent analysis (ELISA)20 in blood drawn 10 minutes after injection of intravenous thrombin (80 U/kg; Sigma, St. Louis, MO) as described earlier.17 Platelet counts were measured in blood collected by retro-orbital puncture.17 PF4 levels were measured in plasma and the lungs homogenate supernatants21 using an Asserachrom PF4 kit (Diagnostica Stago, Parsippany, NJ). LPS, serotype O111:B4 (Sigma), was injected intraperitoneally at a dose of 25 or 40 mg/kg.6 Some animals were injected by tail vain with either hPF4 or with platelets prepared as previously described.22
Results and discussion
Because we have shown that PF4 elevates thrombin-dependent systemic vascular APC production in primates,16 we tested whether release of PF4 from activated platelets in vivo could have a similar effect in both thrombin and LPS injection murine models. We first asked whether platelets were activated in these models. Platelet counts decreased by 20% to 25% as compared with controls 10 minutes after thrombin and 2 hours after LPS injection (Table 1). In the same samples, plasma PF4 levels were increased 2-fold after thrombin and 1.2-fold after LPS injection (Table 1). Because released PF4 is rapidly bound to the surface of vascular cells,23 these plasma levels likely underestimate the PF4 release. We therefore measured PF4 accumulation in mice lungs. Total pulmonary PF4 levels increased significantly after both thrombin and LPS infusions (Table 1). Thus, both models are associated with platelet activation and PF4 release.
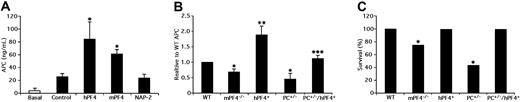
Before we addressed whether released PF4 is sufficient to affect APC generation in mice, we tested whether recombinant mPF4 was as effective as hPF4. Preinjection of either mPF4 or hPF4, but not the closely-related human chemokine NAP-2,24 increased APC generation significantly in WT mice after thrombin injection (Figure 1A).
The role of endogenously released PF4 in APC generation and survival after thrombin injection. (A) APC generation; basal (open bar) or 10 min after thrombin injection (black bars) in WT mice. Prior to thrombin, the noted recombinant chemokine or PBS only (control) was injected (IV) at 7.5 mg/kg for hPF4 and 10 mg/kg for mPF4 and NAP-2. The mean (± 1 SD) are shown. n = 5 to 6 per arm. *P < .001 relative to control. (B) Thrombin injections studies were done as in (B) in WT (n = 15), mPF4−/− (n = 16), hPF4+ (n = 22), PC+/− (n = 5) and PC+/−/hPF4+ (n = 7) mice. The means (± 1 SD) are shown. *P < .001 and **P < .003 relative to WT, and ***P < .003 comparing PC+/−/hPF4+ and PC+/− mice. (C) Survival after thrombin injection as in (B) of WT (n = 15), mPF4−/− (n = 21), hPF4+ (n = 22), PC+/− (n = 12) and PC+/−/hPF4+ (n = 7) mice. APC was assayed in surviving animals. P < .001. Statistical analysis was performed using Fisher exact test.
The role of endogenously released PF4 in APC generation and survival after thrombin injection. (A) APC generation; basal (open bar) or 10 min after thrombin injection (black bars) in WT mice. Prior to thrombin, the noted recombinant chemokine or PBS only (control) was injected (IV) at 7.5 mg/kg for hPF4 and 10 mg/kg for mPF4 and NAP-2. The mean (± 1 SD) are shown. n = 5 to 6 per arm. *P < .001 relative to control. (B) Thrombin injections studies were done as in (B) in WT (n = 15), mPF4−/− (n = 16), hPF4+ (n = 22), PC+/− (n = 5) and PC+/−/hPF4+ (n = 7) mice. The means (± 1 SD) are shown. *P < .001 and **P < .003 relative to WT, and ***P < .003 comparing PC+/−/hPF4+ and PC+/− mice. (C) Survival after thrombin injection as in (B) of WT (n = 15), mPF4−/− (n = 21), hPF4+ (n = 22), PC+/− (n = 12) and PC+/−/hPF4+ (n = 7) mice. APC was assayed in surviving animals. P < .001. Statistical analysis was performed using Fisher exact test.
Basal levels of APC in the plasma of mPf4−/− and hPF4+ mice under study did not differ from that of WT controls. On the other hand, basal APC levels of PC+/− mice were decreased to about 60% of WT (2.5 ± 1.3 and 1.5 ± 0.6 ng/mL, respectively) and were also lower (1.6 ± 0.4 ng/mL) in PC+/−/hPF4+ mice. However, after injection of thrombin, APC levels were 72% and 180% of WT in the mPf4−/− and hPF4+ mice, respectively (P < .001) (Figure 1B). As anticipated,6 PC+/− mice had 35% of WT APC generated (P < .003) (Figure 1B). In contrast, PC+/−/hPF4+ double transgenic mice had an APC level nearly identical to WT and significantly higher than PC+/− mice (P < .001) (Figure 1B). In addition, none of WT, hPF4+, or PC+/−/hPF4+ mice died during thrombin infusion whereas mPF4−/− and PC+/− mice significantly succumbed with rates of 24% and 58%, respectively (Figure 1C). These deaths may be consistent with these mice generating less protective APC levels.
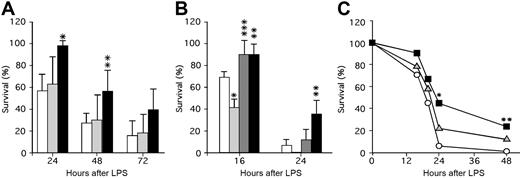
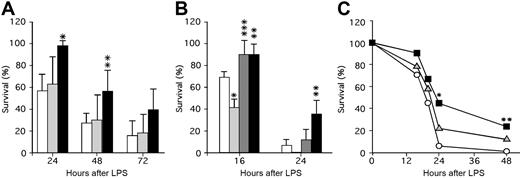
These studies suggest that APC generation and survival following thrombin infusion is promoted by released endogenous PF4. It is not observed for basal levels of APC that reflect the steady-state situation in which APC levels are not elevated and platelets are not activated. To further assess the role of platelet PF4 content in a model of thrombosis/inflammation, we tested murine lines with different PF4 levels for survival after an LPS challenge, a setting in which APC infusion is efficacious.8 Following LPS injection, mPf4−/− and WT mice had comparable 24-hour survival rates (Figure 2A), while hPf4+ mice had markedly improved survival (P < .001 compared with WT). This survival advantage for the hPF4+ mice continued into the second day, though by the next day improved survival was no longer observed (Figure 2A). It is possible that the absence of PF4 dose effect is due to relatively small differences in the PF4 levels between mPf4−/− and WT and relatively large differences between WT and hPF4+ mice, because these mice express 5 to 6 times more PF4 than humans on the top of endogenous mouse PF4.
The role of PF4 in survival in a murine model of LPS-induced endotoxemic shock. (A) LPS (25 mg/kg intraperitoneally) was injected and survival monitored for 24 hours, 48 hours, and 72 hours after LPS. WT (white bars; n = 7 experiments with a total of 49 animals), mPF4−/− (gray bars; n = 5 experiments with a total of 26 animals), and hPF4+ (black bars; n = 4 experiments with a total of 29 animals) mice were studied. The mean (± 1 SD) is shown with statistical analysis performed using the Student t test. *P < .001 and **P < .01 compared with WT littermate controls at the same time point. (B) LPS (40 mg/kg) was injected intraperitoneally and survival monitored at 16 hours and 24 hours after LPS. WT (white bars; n = 3 experiments with a total of 17 animals), PC+/− (light gray bars; n = 3 experiments with a total of 17 animals), PC+/−/hPF4+ (dark gray bars; n = 3 experiments with a total of 14 animals), and hPF4+ (black bars; n = 2 experiments with a total of 10 animals) mice were studied. Statistical analysis was performed as in panel A. *P < .005 and **P < .05 relative to WT mice at the same time point, and ***P < .01 relative to PC+/− mice at the same time point. (C) Either about 2 × 108 platelets or buffer (Tyrode Hepes buffer, pH 7.2, containing 2 mg/mL BSA17 ) in 200 μL per mouse were infused intravenously immediately prior to injection of LPS (40 mg/kg intraperitoneally), and survival was noted at 16, 20, 24, and 48 hours. WT mice were infused with either buffer only (white circles; n = 29), mPF4−/− platelets (gray triangles; n = 35), or hPF4+ platelets (black squares; n = 30). The mean at each time point is shown. *P < .01 and **P < .05 compared with buffer by the Fisher exact test.
The role of PF4 in survival in a murine model of LPS-induced endotoxemic shock. (A) LPS (25 mg/kg intraperitoneally) was injected and survival monitored for 24 hours, 48 hours, and 72 hours after LPS. WT (white bars; n = 7 experiments with a total of 49 animals), mPF4−/− (gray bars; n = 5 experiments with a total of 26 animals), and hPF4+ (black bars; n = 4 experiments with a total of 29 animals) mice were studied. The mean (± 1 SD) is shown with statistical analysis performed using the Student t test. *P < .001 and **P < .01 compared with WT littermate controls at the same time point. (B) LPS (40 mg/kg) was injected intraperitoneally and survival monitored at 16 hours and 24 hours after LPS. WT (white bars; n = 3 experiments with a total of 17 animals), PC+/− (light gray bars; n = 3 experiments with a total of 17 animals), PC+/−/hPF4+ (dark gray bars; n = 3 experiments with a total of 14 animals), and hPF4+ (black bars; n = 2 experiments with a total of 10 animals) mice were studied. Statistical analysis was performed as in panel A. *P < .005 and **P < .05 relative to WT mice at the same time point, and ***P < .01 relative to PC+/− mice at the same time point. (C) Either about 2 × 108 platelets or buffer (Tyrode Hepes buffer, pH 7.2, containing 2 mg/mL BSA17 ) in 200 μL per mouse were infused intravenously immediately prior to injection of LPS (40 mg/kg intraperitoneally), and survival was noted at 16, 20, 24, and 48 hours. WT mice were infused with either buffer only (white circles; n = 29), mPF4−/− platelets (gray triangles; n = 35), or hPF4+ platelets (black squares; n = 30). The mean at each time point is shown. *P < .01 and **P < .05 compared with buffer by the Fisher exact test.
The above data suggest a protective role of PF4 in LPS-induced toxicity. We then asked whether the improved survival could be due to PF4's interaction with the APC pathway by testing the effect of the endogenous PF4 level on the survival of PC+/− mice. Again as anticipated,6 by 16 hours after LPS, PC+/− mice had poor survival compared with WT mice (P < .005), while PC+/−/hPF4+ mice were protected with improved survival (P < .01 compared with the PC+/− mice) (Figure 2B). By 24 hours, the survival advantage of the PC+/−/hPF4+ mice was no longer significant, although hPF4+ mice continued to do better than WT mice (P < .05) (Figure 2B).
Preinjection of hPF4 markedly increased generation of APC in plasma 10 minutes after injection of thrombin (Figure 1A). To see if infused recombinant hPF4 improved survival of WT mice after LPS challenge, we injected mice with hPF4 followed immediately with LPS (40 mg/kg) and observed them for 48 hours. Injection of hPF4, up to 10 mg/kg, did not increase the survival of WT mice (data not shown).
It is possible that to improve outcome after LPS challenge, PF4 needs to be given at higher doses and/or as a continuous infusion, because the initial circulating half-life of PF4 in WT mice is 20 minutes.23 An alternative mechanism to effectively deliver PF4 over a prolonged time may be to infuse platelets enriched in PF4. To test this, we injected platelets from mPF4−/− and hPF4+ mice into WT recipient undergoing an LPS challenge. Injection of hPF4+ platelets significantly, as measured by Kaplan-Meier analysis, improved survival of WT mice (P < .004), while mPF4−/− platelets did not (P > 0.2) (Figure 2C). These results suggest that PF4 released from platelets may be more effective than bolus injection to prevent death in an LPS endotoxic shock model.
These studies show that endogenous PF4 release affects the level of APC generation in response to thrombin and enhances survival after LPS challenge, a model of sepsis that is strongly influenced by endogenous APC generation. We propose that like factor VLeiden, which is a polymorphism that affects APC generation and appears to influence survival after sepsis, platelet PF4 levels may also have a positive correlation with outcome in sepsis and related clinical disorders. Our LPS challenge data (Figure 2) do not rule out other, APC-independent, beneficial effects of platelet PF4 on survival. Further clinical studies to confirm a correlation between platelet PF4 content and survival in systemic thrombotic/inflammatory disorders are now needed, as are studies to examine whether infusions of PF4 or platelets with high PF4 content may be efficacious in such settings.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants HL084006 and HL40387 from the National Institutes of Health.
We thank Dr Francis J. Castellino from Notre Dame University for the PC+/− mice, Dr Charles T. Esmon for providing the monoclonal antibody (AMGCPC1587) for the mouse APC capture ELISA assay, Mrs Li Zhai from The Children's Hospital of Philadelphia for purification of the chemokines, Mr William J. Smith from University of Pennsylvania for help with lung study, and Mr Tyson Rogers, biostatistician from University of Minnesota, for performing the survival Kaplan-Meier analysis.
National Institutes of Health
Authorship
Contribution: M.A.K. designed and performed research, analyzed data, and prepared the manuscript; S.A.M. and M.P.L. performed research and analyzed data; and M.P. and A.S. helped in designing the research, interpretation of the data and in writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: M. Anna Kowalska, The Children's Hospital of Philadelphia, 3615 Civic Center Blvd, ARC Rm 316I, Philadelphia, PA 19104; e-mail: kowalska@email.chop.edu.