Abstract
Imatinib mesylate, a targeted inhibitor of BCR-ABL tyrosine kinase, is the standard of care for chronic myeloid leukemia (CML). A phase 2 trial of imatinib in late chronic-phase (CP) CML after interferon-α (IFNα) failure enrolled 532 patients, 454 with a confirmed diagnosis of CP CML. Median time from diagnosis was 34 months; median duration of imatinib treatment was 65 months. Cumulative best rates of major cytogenetic response (MCyR) and complete cytogenetic response (CCyR) were 67% and 57%, respectively. At the 5-year landmark, 184 (41%) of the 454 patients are in CCyR. At more than 6 years, 199 (44%) of the 454 patients remain on imatinib. Most responses occurred within 12 months of starting imatinib; however, some patients achieved initial MCyR and CCyR more than 5 years after imatinib initiation. Estimated rates of freedom from progression to accelerated phase (AP) and blastic phase (BP) and overall survival at 6 years were 61% and 76%, respectively. Both freedom from progression to AP/BP and overall survival (OS) were associated with cytogenetic response level at 12 months. No increase in rates of serious adverse events was observed with continuous use of imatinib for up to 6.5 years, compared with earlier time points. Imatinib continues to be an effective and safe therapy for patients with CP CML after failure of IFN.
Introduction
Chronic myeloid leukemia (CML) is characterized by the presence of the Philadelphia chromosome (Ph), a consequence of an aberrant reciprocal translocation of genetic material between chromosomes 9 and 22, resulting in the fusion of portions of the genes encoding BCR and ABL.1 The BCR-ABL fusion gene encodes a constitutively active leukemogenic protein–tyrosine kinase.2 BCR-ABL kinase activity is inhibited by the selective activity of imatinib, a small molecule targeted agent3 that has demonstrated remarkable efficacy and tolerability. Based on clinical results from randomized trials, imatinib is approved for first-line treatment of all phases of CML.4-9
Imatinib was evaluated in newly diagnosed chronic-phase (CP) CML in a randomized phase 3 clinical trial named IRIS (International Randomized Study of Interferon and STI571). In IRIS, patients with newly diagnosed CP CML were randomized to treatment with either imatinib 400 mg/d or interferon-α (IFNα) plus cytarabine (ara-C).8-10 When the IRIS data were analyzed 5 years after the last patient was enrolled, the estimated 5-year overall survival (OS) rate with imatinib was 89%, and the event-free survival rate was 83%.9 The IRIS study design allowed for crossover between treatment arms for either disease progression or intolerance to treatment. Because 359 patients (65%) crossed over from the IFN to the imatinib arm, a reliable comparison of survival between the 2 arms was not possible. Compared with historical data of patients receiving IFN plus ara-C, complete cytogenetic response (CCyR) rates, survival free of transformation, and overall survival were significantly higher with imatinib compared with IFN plus ara-C.11,12 Survival data from the IRIS study showed that long-term OS correlated with response, as 97% of patients who achieved a cytogenetic response at 12 months were likely to be alive 5 years after starting treatment.9,13
The study that forms the basis for this analysis, STIA 0110, was a phase 2 single-arm trial that enrolled patients with CP CML who either progressed while on treatment with IFN-based regimens or who were intolerant to IFN. Patients received imatinib 400 mg/d. The purpose of this analysis is to evaluate cytogenetic response, progression to accelerated phase (AP) and blastic phase (BP), OS, as well as major safety events in this population. Patients have now been followed for at least 6 years since the final patient was enrolled.
Methods
The Institutional Review Board of each participating site has approved the study being reported.
A description of this phase 2, open-label, multicenter, nonrandomized study that investigated the efficacy and safety of imatinib in patients with CP CML who received prior IFN-based therapy has been published.7 CP CML was defined by the presence of less than 15% blasts, and less than 30% blasts plus promyelocytes in the peripheral blood and bone marrow, less than 20% peripheral basophils, and a platelet count of at least 100 × 109/L. Hematologic failure was defined as either hematologic resistance (failure to achieve a complete hematologic response [CHR] after at least 6 months of IFN) or a relapse after a CHR had been achieved, with white cell counts increased to at least 20 ×109/L during IFN therapy. Cytogenetic failure was defined as either cytogenetic resistance (at least 65% of metaphase cells being Ph+ after at least one year on IFN) or relapse after achievement of major cytogenetic response (MCyR). A relapse was considered to have occurred if the proportion of Ph+ cells increased by 30% or more or to at least 65%. Intolerance of IFN was defined by the presence of any grade 3 or 4 nonhematologic toxicity persisting for one month or more during therapy with recombinant IFN at a dose of at least 25 million international units per week.
Five-hundred thirty-two patients were enrolled into STIA 0110 between December 1999 and May 2000; 454 patients whose diagnosis was confirmed as CP CML form the basis for this analysis. Of these, 133 had hematologic failure, 160 had cytogenetic failure, and 161 had intolerance to prior IFN therapy. Dose escalation of imatinib to 600 mg/d and then 800 mg/d was permitted in patients who did not achieve CHR after 3 months of treatment, whose disease relapsed within 3 months after achievement of CHR, or in whom MCyR had not been achieved after 12 months. The primary end point was the rate of MCyR. Secondary end points included time to progression to AP and BP, OS, and safety events. After July 31, 2002, the amount of data collected was reduced to include drug administration, reason for discontinuing imatinib, serious adverse events (SAEs), and OS. In addition, the frequency of bone marrow assessments was reduced to every 6 to 12 months, with a further reduction in the frequency of assessments in patients in complete cytogenetic response (CCyR) to every 12 months after July 31, 2004. This analysis is based on a data obtained with a cutoff date of July 31, 2006.
Response criteria were as previously described. Cytogenetic response refers to the best response achieved and is categorized as follows: complete (CCyR) if Ph-positive metaphases were 0%, partial (PCyR) if they were 1% to 35%, minor if they were 36% to 65%, and minimal if they were 66% to 95%. A major cytogenetic response (MCyR) included CCyR and PCyR.
Time-to-event analyses were performed using the Kaplan-Meier method, with statistical significance (P values) assessed using the log-rank test. Overall survival was censored at the last contact date for patients still alive, or at the date of allogeneic stem cell transplantation (SCT) for patients who discontinued to undergo this procedure. However, for patients who discontinued imatinib study treatment, no further information on subsequent treatment or SCT was collected. Time to progression to AP or BP (progression-free survival, PFS) was evaluated until the patient's last day of study treatment. For patients without progression to AP or BP, or CML-unrelated death during treatment, time to progression to AP/BP was censored at the last known assessment. Any discontinuation due to “unsatisfactory therapeutic effect” was considered progression, although this reason was also used in cases where loss or lack of response was the reason, which did not necessarily indicate a progression to a more advanced phase of CML.
Results
Patient disposition
The median time from diagnosis of CML for the 454 patients who initiated the study treatment was 34 months. Other baseline characteristics are listed in Table 1. A total of 255 patients (56%) discontinued the study for reasons listed in Table 2. Of these discontinuations, 132 patients (29%) listed unsatisfactory therapeutic effect as the reason, 35 (8%) were due to adverse events or abnormal laboratory values (16 cases considered drug-related and 4 with unknown relation to study drug). An additional 37 patients (8%) were characterized as having withdrawn consent, 8 (2%) were lost to follow up, and 6 (1%) discontinued to undergo SCT. A total of 19 patients (4%) died during treatment, 5 of these had been reported in a previous publication7 based on July 31, 2002, data cutoff. The following reasons were listed to account for the 14 deaths documented after July 2002: progressive disease (4 cases); cardiac disorders (3 cases); lung cancer, cerebral hemorrhage, renal failure secondary to amyloidosis, Candida pneumonia, and small intestine obstruction in one case each; and undefined reasons in 2 cases. For the 199 patients (44%) still on imatinib therapy at data cutoff, the median follow up was 76 months.
The median duration of exposure to imatinib was 65 months. A total of 253 patients (56%) had imatinib dose escalation to a maximum of either 600 mg/d (n = 57; 13%) or 800 mg/d (n = 196; 43%). The 8 patients who erroneously started at 600 mg were not counted among those who had dose escalations to 600 mg; 3 of them had the imatinib dose increased to 800 mg later in the trial, and were included in that group. Dose escalation occurred after a median of 16.5 months. The median dose for the entire study group was 400 mg/d (mean, 449 mg/d).
Efficacy
The efficacy results summarized are for the 454 patients with a confirmed diagnosis of CP CML.
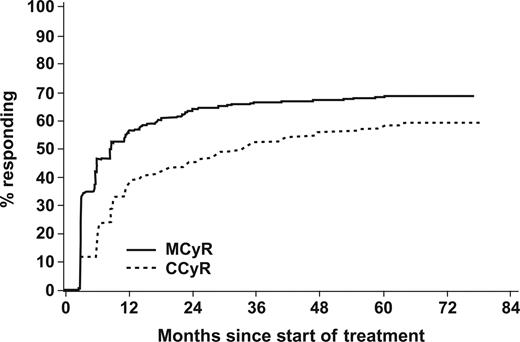
A CHR was achieved in 96% of patients at some point during the study. The cumulative rate of MCyR was 67% and of CCyR, 57% (Table 3; Figure 1). For the entire group, the median time to MCyR was 3.4 months. The MCyR rate was 72% with intolerance to prior IFN, 71% in cytogenetic failures, and 57% in hematologic failures to prior IFN (Table 4). In 53 patients (12%), the first MCyR was observed after being on imatinib therapy for more than 12 months; in 31 (58%) of these patients, MCyR was achieved after imatinib dose escalation to 600 mg/d or 800 mg/d. Of the 454 patients, 10 (2%) achieved an MCyR after being on imatinib for more than 36 months; 9 of them required imatinib dose escalation. The estimated rate of MCyR patients without confirmed loss of MCyR and without discontinuation due to unsatisfactory therapeutic effect was 74% at 5 years after achievement of MCyR. Of the 305 patients with MCyR, 192 (63%) are still on imatinib study treatment, of whom only 7 had a last available cytogenetic assessment not indicating MCyR.
Time to best cytogenetic response. A total of 305 (67%) achieved a major cytogenetic response (MCyR), 53 of these responses were achieved after more than 1 year of imatinib treatment (10 of them only after 3 years). A complete cytogenetic response (CCyR) was achieved in 259 patients (57%), 88 of these responses were achieved after more than 1 year of imatinib treatment (28 of them only after 3 years).
Time to best cytogenetic response. A total of 305 (67%) achieved a major cytogenetic response (MCyR), 53 of these responses were achieved after more than 1 year of imatinib treatment (10 of them only after 3 years). A complete cytogenetic response (CCyR) was achieved in 259 patients (57%), 88 of these responses were achieved after more than 1 year of imatinib treatment (28 of them only after 3 years).
The cumulative rate of CCyR was 57% (Figure 1). The median time to CCyR was 8.3 months. The CCyR rate was 61% with intolerance to prior IFN, 60% in cytogenetic failures, and 48% in hematologic failures to prior IFN (Table 4). A total of 88 patients (19%) achieved a CCyR after being on imatinib therapy for more than 12 months; in 48 (55%) of them CCyR was achieved after dose escalation to 600 mg/d or 800 mg/d. Twenty-eight patients (6%) achieved CCyR after being on imatinib for more than 36 months; 22 of them were dose escalated. At the 5-year landmark, 184 (41%) of the 454 patients are in CCyR.
Progression-free survival
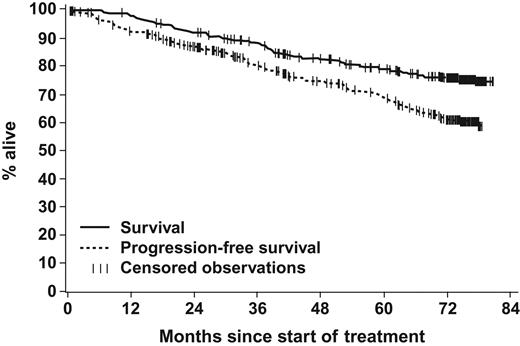
The estimated PFS rate at 6 years was 61% (Figure 2). The estimated PFS rate was 68% with cytogenetic relapse on IFN, 62% with intolerance to IFN, and 51% with hematologic relapse or resistance to prior IFN (P = .018). For this analysis, a conservative approach was used, in the sense that patients who discontinued imatinib due to an unsatisfactory therapeutic effect were categorized as progression to AP or BP. In the later years of this study, these patients may also have discontinued due to this reason to receive other targeted systemic agents outside the study.
Overall survival and progression-free survival. Of the 454 patients, 109 (24%) died during imatinib treatment or during long-term follow-up after discontinuation of imatinib. The estimated 6-year overall survival (considering all deaths) was 76% (95% confidence interval, 71% to 80%). During study treatment, 157 patients (35%) either had values indicative of accelerated phase or blasts crisis or discontinued imatinib due to unsatisfactory therapeutic effect or death. The estimated 6-year PFS rate was 61% (95% confidence interval, 56% to 66%).
Overall survival and progression-free survival. Of the 454 patients, 109 (24%) died during imatinib treatment or during long-term follow-up after discontinuation of imatinib. The estimated 6-year overall survival (considering all deaths) was 76% (95% confidence interval, 71% to 80%). During study treatment, 157 patients (35%) either had values indicative of accelerated phase or blasts crisis or discontinued imatinib due to unsatisfactory therapeutic effect or death. The estimated 6-year PFS rate was 61% (95% confidence interval, 56% to 66%).
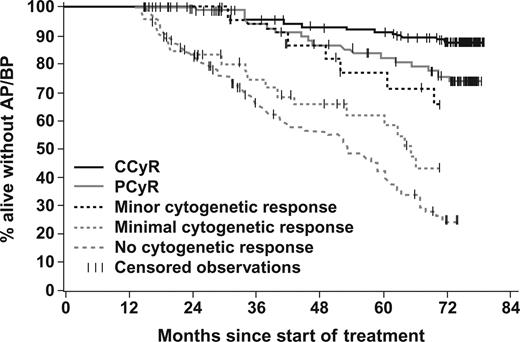
Achievement of cytogenetic response by 12 months significantly correlated with PFS. The estimated 6-year PFS rate was 84% with MCyR and 36% without MCyR by 12 months (P < .001); 88% of patients in CCyR versus 76% of those in PCyR had PFS (P = .016; Figure 3). Among patients without a MCyR, patients with a minor cytogenetic response did remarkably well with an estimated 6-year PFS rate of 66%.
Landmark analysis of progression-free survival according to the cytogenetic response at 12 months. A total of 408 patients who were remaining progression free on study treatment at 12 months were categorized according to their degree of cytogenetic response by 12 months. The estimated 6-year PFS rate was 88% in patients with CCyR, 76% for PCyR, 66% for minor CyR, 43% for minimal CyR, and only 25% for patients who had not achieved any cytogenetic response on the first 12 months of imatinib treatment.
Landmark analysis of progression-free survival according to the cytogenetic response at 12 months. A total of 408 patients who were remaining progression free on study treatment at 12 months were categorized according to their degree of cytogenetic response by 12 months. The estimated 6-year PFS rate was 88% in patients with CCyR, 76% for PCyR, 66% for minor CyR, 43% for minimal CyR, and only 25% for patients who had not achieved any cytogenetic response on the first 12 months of imatinib treatment.
Survival
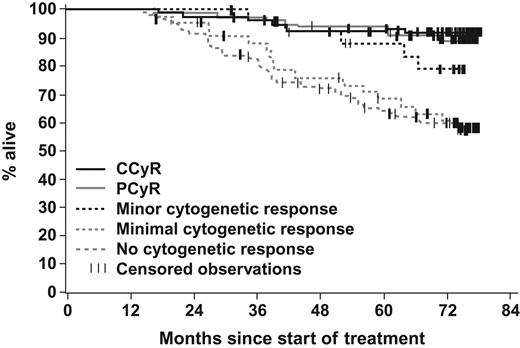
The estimated 6-year OS was 76% (Figure 2). The estimated 6-year survival rates by cytogenetic response at the 12-month landmark were 92% for CCyR, 89% for PCyR, and 63% for no MCyR (P < .001 for MCyR vs no MCyR; Figure 4). No significant differences in OS were observed between patients achieving CCyR and PCyR (P = .520). The annual mortality rate remained stable over time.
Landmark analysis of overall survival according to the cytogenetic response at 12 months. A total of 421 patients who were on study treatment at 12 months were categorized according to their degree of cytogenetic response by 12 months. The estimated 6-year OS rate was 92% in patients with CCyR, 89% for PCyR, 79% for minor CyR, and 60% for minimal CyR as well as for patients who had not achieved any cytogenetic response on the first 12 months of imatinib treatment.
Landmark analysis of overall survival according to the cytogenetic response at 12 months. A total of 421 patients who were on study treatment at 12 months were categorized according to their degree of cytogenetic response by 12 months. The estimated 6-year OS rate was 92% in patients with CCyR, 89% for PCyR, 79% for minor CyR, and 60% for minimal CyR as well as for patients who had not achieved any cytogenetic response on the first 12 months of imatinib treatment.
Safety
The routine collection of adverse events (AEs) was stopped after the July 31, 2002, cutoff; only serious adverse event (SAE) data continued to be gathered. At the 18-month follow-up,7 the incidence of individual grade 3 or 4 nonhematologic AEs was less than 5% in the overall study population of 532 patients. A total of 15 cases (2.8%) of cardiac failure conditions or left ventricular dysfunction were documented up to July 31, 2002, including one patient who had both heart failure and cardiomegaly but was counted as one case only. Among these 15 cases, 12 were classified as congestive heart failure and 9 of these had no preexisting congestive heart failure. Of these 9 cases (1.7%, 0.8 cases per 100 patient-years of exposure), 5 were grade 3 or 4, and 4 were considered to be drug related (0.8%). Table 5 lists the most frequent AEs and the severe (grade 3 or 4) adverse events as of the July 31, 2002, cutoff based on all 532 patients enrolled into the study, as well as SAEs in the 395 patients who were ongoing after July 31, 2002. No additional drug-related cardiac SAEs were reported between July 2002 and July 2006, indicating that imatinib-related cardiac events remain uncommon. There has been no change in the incidence of nonhematologic AEs over time.
Table 6 lists the percentage of patients who were reported to experience new or worsening grade 3 or 4 biochemical and laboratory abnormalities. Data are listed for the first 12 months of the study and then for the time period after 12 months. The data in Table 6 are based on data collected on 532 patients up to the July 31, 2002, data cutoff. Myelosuppression was more common earlier in the course of imatinib therapy. The incidence of grade 3 or 4 laboratory abnormalities was low throughout the study. After July 31, 2002, hematologic SAEs were reported in less than 1% of patients for neutropenia and thrombocytopenia, and 2% for anemia.
Discussion
When this study was initiated, imatinib had not yet been approved by the regulatory agencies for the treatment of CML, and IFN plus ara-C was considered a standard of care. This study of imatinib in CP CML after IFN failure was the basis for the first approval of imatinib in CML. The goal of this study was to assess the benefit of imatinib in patients with CP CML whose disease had progressed while on IFN or who were intolerant to IFN. The patient population in this study is referred to as “late CP,” to be differentiated from patients in the IRIS study who were newly diagnosed and received imatinib as first-line therapy for CML. Classically, late CP refers to patients whose CML disease duration was 12 months or longer; most patients in this study fit this criterion and all had failed IFN therapy. Patients with “early CP” in IRIS have a better prognosis due to a lower overall CML burden and less time for the development of resistance to treatment. Thus, this long-term follow-up of patients who had failed initial therapy with IFN, and who had a more advanced late CP disease state, indicates a significant activity for imatinib in a population with a relatively poor-prognosis CML.
The data from this study confirm that imatinib prolongs survival in patients with CP CML after failure of IFN. The estimated 6-year OS rate was 76% (Figure 2). This should be compared with historical mortality rates per year of 15% to 20%,14,15 and median survival durations of 3 to 4 years. In an analysis from a single study comparing a similar study group with historical patients who had failed IFN but who did not receive imatinib subsequently, the estimated 4-year survival rates were 86% with imatinib and 43% with other modalities (P < .001). The survival advantage with imatinib was confirmed by multivariate analysis (hazard ratio, 0.19; P < .001).16
Response rates to imatinib therapy in this population were high and responses were durable. Of 199 patients still on study treatment at data cutoff, 192 patients (96%) had achieved a MCyR during treatment and 185 patients (93%) remained in MCyR at data cutoff. The degree of cytogenetic response at 12 months was associated with PFS and OS (Figure 3). Patients could be divided into 2 groups with different 6-year OS rates. Little distinction can be made in the estimated 6-year OS between patients with CCyR and PCyR, or even minor cytogenetic response at 12 months (P = .019 for MCyR vs minor cytogenetic response). Patients with minimal or no cytogenetic response within 12 months have a markedly worse OS (Figure 4). These data suggest that achieving at least a minor cytogenetic response at 12 months of therapy may be a prognostic indicator for survival in patients with late CP CML, as described previously.16
A number of patients, particularly those who developed MCyR and CCyR after being on imatinib therapy for more than 12 months, required dose escalation of imatinib to either 600 mg/d or 800 mg/d. It is possible that the response rate, in particular the major molecular response, may be improved with higher starting doses of imatinib.17,18 An ongoing clinical trial, the Tyrosine Kinase Inhibitor Optimization and Selectivity Study (TOPS), is evaluating the use of higher (800 mg/d) doses of imatinib as first-line therapy for CP CML, using major molecular response as the primary end point.
This 6-year follow-up continues to demonstrate excellent tolerability of imatinib. No additional serious safety concerns were identified with longer-term follow-up. In particular, drug-related cardiotoxicity was rare, as previously reported.19 The higher rate of myelosuppression noted earlier in the course of therapy likely reflects the higher disease burden at that time. As imatinib is eliminating the malignant clone, there is a lag in the development of normal hematopoietic precursors to repopulate the bone marrow and reconstitute the blood counts. Once normal hematopoiesis has been restored, the incidence of imatinib-associated myelosuppression decreases. In addition, there is no reported increase in serious laboratory abnormalities over time.
In summary, the benefit of imatinib continues to be observed even after more than 6 years of treatment for patients with CP CML after IFN failure. Imatinib remains remarkably safe and well tolerated. A majority of patients achieved a cytogenetic response, with a prolongation of OS. A subset of patients with “suboptimal response”—failure to achieve major cytogenetic response by 12 months—appears to benefit from continuation of therapy with imatinib.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: H.M.K. was the principal investigator (United States), performed research, enrolled and treated patients, and wrote the paper; A.H. was the principal investigator (Europe), performed research, enrolled and treated patients, and contributed to the paper; B.D., C.S., F.G., C.A.S., J.C., D.W.N., C.G., R.M.S., J.G., T.F., S.G.O., J.J.R., and M.T. performed research and enrolled and treated patients; M.M. and T.K. designed research and analyzed data.
Conflict-of-interest disclosure: All authors, except M.M. and T.K., received research grants from Novartis. Authors M.M. and T.K. are employees of Novartis Pharmaceuticals Corporation.
Correspondence: Hagop Kantarjian, Chairman and Professor of Medicine, Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: hkantarj@mdanderson.org.