Abstract
Mice carrying a conditional prothrombin knockout allele (fIIlox) were established to develop an experimental setting for exploring the importance of thrombin in the maintenance of vascular integrity, the inflammatory response, and disease processes in adult animals. In the absence of Cre-mediated recombination, homozygous fIIlox/lox mice or compound heterozygous mice carrying one fIIlox allele and one constitutive-null allele were viable. Young adults exhibited neither spontaneous bleeding events nor diminished reproductive success. However, the induction of Cre recombinase in fIIlox mice using the poly I:C-inducible Mx1-Cre system resulted in the rapid and near-complete recombination of the fIIlox allele within the liver, the loss of circulating prothrombin, and profound derangements in coagulation function. Consistent with the notion that thrombin regulates coagulation and inflammatory pathways, an additional early consequence of reducing prothrombin was impaired antimicrobial function in mice challenged with Staphylococcus aureus peritonitis. However, life expectancy in unchallenged adults genetically depleted of prothrombin was very short (∼5-7 days). The loss of viability was associated with the development of severe hemorrhagic events within multiple tissues, particularly in the heart and brain. Unlike the constitutive loss of either clotting or platelet function alone, the conditional loss of prothrombin is uniformly not compatible with maintenance of hemostasis or long-term survival.
Introduction
Proteolytic conversion of prothrombin (fII) to the active serine protease thrombin is central to thrombus formation and the local containment of blood loss after vascular injury. Thrombin-mediated proteolysis directly controls platelet activation through the protease-activated receptors (eg, PAR-1, -3, and -4),1-3 converts fibrinogen to fibrin, and enhances thrombus stability through the proteolytic activation of factor XIII (fXIII) and thrombin-activated fibrinolysis inhibitor (TAFI).4 The seminal regulatory role of thrombin is further emphasized by the fact that this key protease both positively regulates further thrombin generation through the activation of coagulation factors XI, VIII, and V (fXI, fVIII, and fV, respectively) and negatively controls thrombin generation through the thrombomodulin-dependent activation of the natural anticoagulant, protein C.4 Beyond the intense, long-term interest in thrombin-mediated proteolysis in the control of hemostasis, there has been increasing interest in the ability of thrombin to regulate a wide spectrum of physiologic and pathologic processes that have little to do with traditional hemostatic plug formation, including embryonic development, tissue repair, the inflammatory response, and pathologic processes (eg, malignancy, host defense, and inflammatory diseases).
The concept of a broad biologic importance of thrombin was underscored by early developmental studies in gene-targeted mice lacking prothrombin,5,6 factors that participate in thrombin generation (eg, tissue factor [TF], fVII, fV, and fX),7-11 or selected thrombin substrates (eg, PAR-1).1 Notably, the loss of prothrombin or thrombin-mediated proteolysis resulted in frequent mid-gestation developmental failures, whereas the loss of either fibrinogen, platelet function, or both appears to be uniformly compatible with developmental success.12-14 Diverse biologic roles of prothrombin are also implied by the extraordinary variety of cells known to express thrombin-activated PARs (eg, endothelial cells, fibroblasts, and smooth muscle cells, among others)1,3,15-17 and the multiple cellular responses observed when cells are challenged with thrombin, including changes in proliferation, migration, apoptosis, adhesion, process outgrowth, and cytokine and growth factor expression/release.3,18-20
The broad biologic actions of thrombin can be appreciated in part by the diversity of thrombin substrates. Changes in cellular function after the engagement of fibrin matrices by integrin and nonintegrin receptors are undoubtedly one mode of thrombin action in vitro and in vivo. Thrombin-initiated PAR-1 and -4 signaling is also an important modifier of cellular function. Many other thrombin substrates have been tied to important biologic processes, including protein C,21 complement factors (eg, C5),22 immune modulators (eg, osteopontin),23 hepatocyte growth factor (HGF) activator,24 carboxypeptidase TAFI,25 and fXIII. A particularly strong link has emerged between thrombin targets and the regulation of the inflammatory response and inflammatory disease. This has been strengthened by recent findings indicating that activated protein C (APC) has potent anti-inflammatory properties that are distinct from its anticoagulant activity.21,26,27 Furthermore, thrombin activation of PARs on platelets, endothelial cells, and other cells can lead to the production of an impressive array of cytokines and chemokines, as well as changes in adhesive properties important in leukocyte trafficking and/or activation.28-33
Progress in defining the precise contribution of prothrombin and thrombin-mediated proteolysis in tissue remodeling/repair, antimicrobial response, inflammatory disease, and other complex processes has been hampered by the inability to generate viable prothrombin-deficient adult mice.5,6 The constitutive loss of prothrombin (fII−/−) results in partial mid-gestation embryonic lethality. The small fraction of fII−/− offspring surviving to term develop fatal hemorrhagic events shortly after birth and thus provided no means of exploring the role of prothrombin in adults. To investigate in greater detail the contribution of prothrombin/thrombin to physiologic processes and diseases in vivo in adult animals, we have generated mice carrying a conditional knockout allele for prothrombin (fIIlox). Although a substantial constitutive reduction (eg, 10%-20% of normal) in circulating prothrombin was found to be compatible with long-term survival, the consequences of a more profound diminution in circulating prothrombin were severe, including reduced capacity for microbial clearance and fatal hemorrhagic events, particularly in the heart and brain.
Methods
Transgenic mice
The prothrombin conditional knockout allele was generated using a targeting vector constructed by introducing the following 4 elements into plasmid pKO v901 (Stratagene, La Jolla, CA) that was modified to include loxP sites adjacent to the polylinker AscI site: (1) a 3.7-kb hypoxanthine-guanine phosphoribosyl transferase (HPRT) minigene (AscI cassette; Stratagene), (2) a 2-kb herpes simplex virus thymidine kinase minigene (RsrII cassette; Stratagene), (3) a 0.7-kb “short arm” complementary to intron 9 and exon 10 sequences of the mouse prothrombin gene, and (4) a 6.6-kb “long arm” that draws together a 5′ portion of the mouse prothrombin gene, a portion of the mouse prothrombin cDNA, and a 3′ portion of the hGH gene (KpnI cassette). The 0.7-kb “short arm” gene fragment was generated by polymerase chain reaction (PCR) amplification using primers complementary to intron 9 (5′-GATCGGATCCTGGACTGATCTAC-3′) and exon 10 (5′-GTACAGAATGCAGTCGACAGCAGTGAG-3′), but with nucleotide substitutions designed to introduce nonnative BamHI and SalI sites (underlined). The “long arm” was constructed from 3 major components. The first component was a 4.4-kb KpnI-AgeI fragment of the mouse prothrombin gene (isolated from a 129 strain genomic DNA library34 ) that extends 1.2 kb upstream of exon 1 to the AgeI sequence within exon 5. This was modified to introduce a third LoxP site downstream of a unique SmaI site within intron 2. The second component of the “long arm” was a 1.5-kb portion of the mouse prothrombin cDNA extending from the internal AgeI site to 46 nucleotides downstream of the translation stop codon where a nonnative HindIII site was introduced. The final component of the “long arm” was a 0.7-kb fragment containing the hGH gene polyadenylation addition signal.35 The final targeting vector was linearized at a unique NotI site downstream of the short arm and used to electroporate E14TG2a embryonic stem cells36 as described previously.37 Stable transfectants were established under hypoxanthine/aminopterin/thymidine (HAT) and gancyclovir selection37 and screened for homologous recombination events by PCR across the short arm. Here, a primer complementary to the HPRT cassette (5′-ATGCTCCAGACTGCCTTG-3′) was used in combination with a primer complementary to the mouse prothrombin gene, but outside of those sequences included in the targeting vector, (5′-GAGCGATATCGCGGTCTAGGTTCTC-3′; complementary to exon 11) yielding a 1.3-kb PCR product. Homologous recombination was confirmed by a second similar strategy (HPRT and exon 12—5′-CACCACCTGCAGGACGCTGGGCTGT-3′—specific primers; yielding a PCR product of 1.6 kb). Germline-competent chimeric founder mice were generated using 3 embryonic stem cell isolates confirmed to have incorporated the targeting vector by homologous recombination by both PCR and Southern blot analyses. Mice carrying the prothrombin conditional knockout allele were backcrossed 6 generations to C57BL/6J before detailed analyses and were routinely identified by PCR analysis of DNA obtained from ear biopsies using primers complementary to prothrombin intron 2 that flanked the LoxP insertion (forward primer F1: 5′-GCTCTTCCCCTTTTCAGACTTTCAC-3′; reverse primer B1: 5′-ACCTTATCCTTCCCTTACCACCTG-3′), which yield products of 402 bp and 438 bp with the wild-type and mutant allele templates, respectively. All experiments were approved by the Cincinnati Children's Hospital Research Foundation Animal Care and Use Committee and complied with National Institutes of Health guidelines.
Conditional recombination of the mutant prothrombin allele
C57BL/6-inbred mice carrying the poly I:C–inducible Mx1-Cre recombinase transgene38 were obtained and genotyped per protocol (The Jackson Laboratory, Bar Harbor, ME). The induction of hepatic Cre recombinase expression was done by intraperitoneal injection of poly I:C (GE Healthcare Bio-Sciences, Little Chalfont, United Kingdom) prepared in phosphate-buffered saline. Dosing regimens varied in individual experiments from a single injection at 0.05 μg per g body weight (200 μL at 5 μg/mL per 20 g mouse) to 5 μg per gram of body weight (200 μL at 0.5 mg/mL per 20 g mouse). Hepatic recombination of the conditional prothrombin allele was established by a combination of PCR and Southern blot analyses with purified liver genomic DNA. Multiplex PCR was used for detecting the wild-type allele (fIIWT), intact fIIlox allele, and Cre-excised fIIlox (fIIΔCre) allele using primers F1 and B1 together with the additional primer B2: 5′-ATGAGTCACGTAAGCTCGCTTGGC-3′ (Figure 1A). The fIIWT, fIIlox, and fIIΔCre templates generated distinct 402-, 438-, and 318-bp PCR products, respectively. Southern analysis was done with liver genomic DNA digested with HindIII (Figure 1A).5 The 0.9-kb hybridization probe (Figure 1) was generated by PCR using genomic DNA as a template and the following primers: 5′-ATTATGCTGACGGTGGGAGCCCCAGCCC-3′ and 5′-GATTGTCCTGACTCTCTCTGGGTTAATATT-3′.
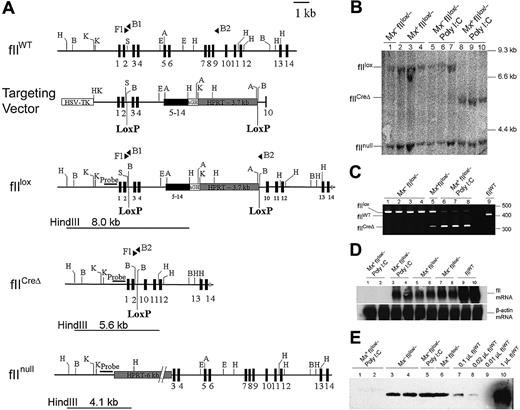
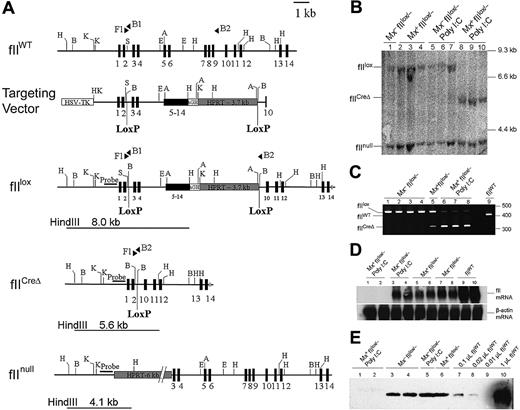
Generation of mice carrying the conditional prothrombin knockout allele (fIIlox) and Cre-mediated recombination. (A) Organization of the wild-type prothrombin gene (fIIWT), the fIIlox targeting vector, the modified prothrombin allele carrying LoxP sites (fIIlox), the Cre-disrupted mutant prothrombin allele (fIICreΔ), and the previously described constitutive prothombin null (fIInull) allele. Exons are indicated as numbered boxes. The position and orientation of diagnostic PCR primers used are indicated with solid triangles. The location and size of HindIII fragments used to identify the fIIlox, fIICreΔ, and fIInull alleles by Southern blot analysis are indicated by lines below each allele. The relative position of the DNA fragment used as a hybridization probe in Southern blot analyses is highlighted with a solid bar. (B) Southern blot of HindIII-digested liver genomic DNA prepared from individual fIIlox/− mice carrying (Mx+) or lacking (Mx−) the Cre transgene. Hepatic DNA was isolated from either untreated mice or animals given a single intraperitoneal injection of poly I:C (5 μg/g). Note that near-complete gene recombination was observed in individual Mx+fIIlox/− mice 5 days after treatment with poly I:C (lanes 8-10), whereas the floxed allele was largely intact in Cre transgene-negative animals (Mx−) regardless of any prior exposure to poly I:C. (C) Complementary multiplex PCR analysis of genomic liver DNA from individual fIIlox/− adults after poly I:C treatment. The PCR products derived from the fIIWT allele, the intact fIIlox allele, and the Cre-recombined fIIlox allele (fIICreΔ) were 402 bp, 438 bp, and 318 bp, respectively. Note that only trace recombination was observed in Mx+fIIlox mice in the absence of poly I:C, whereas the fIIlox allele was largely disrupted after poly I:C treatment in Mx+ animals. (D) Northern blot analysis of total liver RNA isolated 5 days after poly I:C administration to Mx− and Mx+ fIIlox/− mice illustrating the selective loss of detectable fII mRNA in Mx+fIIlox mice. (E) Western blot analysis of plasma prothrombin in unchallenged fIIlox/− adults and animals challenged 5 days previously with poly I:C. Note that plasma prothrombin was approximately 10% to 20% of normal in unchallenged fIIlox/− mice and was reduced to immunologically undetectable levels in poly I:C-treated Mx+fIIlox/− mice. H indicates HindIII; B, BamHI; K, KpnI; S, SmaI, E, EcoRI.
Generation of mice carrying the conditional prothrombin knockout allele (fIIlox) and Cre-mediated recombination. (A) Organization of the wild-type prothrombin gene (fIIWT), the fIIlox targeting vector, the modified prothrombin allele carrying LoxP sites (fIIlox), the Cre-disrupted mutant prothrombin allele (fIICreΔ), and the previously described constitutive prothombin null (fIInull) allele. Exons are indicated as numbered boxes. The position and orientation of diagnostic PCR primers used are indicated with solid triangles. The location and size of HindIII fragments used to identify the fIIlox, fIICreΔ, and fIInull alleles by Southern blot analysis are indicated by lines below each allele. The relative position of the DNA fragment used as a hybridization probe in Southern blot analyses is highlighted with a solid bar. (B) Southern blot of HindIII-digested liver genomic DNA prepared from individual fIIlox/− mice carrying (Mx+) or lacking (Mx−) the Cre transgene. Hepatic DNA was isolated from either untreated mice or animals given a single intraperitoneal injection of poly I:C (5 μg/g). Note that near-complete gene recombination was observed in individual Mx+fIIlox/− mice 5 days after treatment with poly I:C (lanes 8-10), whereas the floxed allele was largely intact in Cre transgene-negative animals (Mx−) regardless of any prior exposure to poly I:C. (C) Complementary multiplex PCR analysis of genomic liver DNA from individual fIIlox/− adults after poly I:C treatment. The PCR products derived from the fIIWT allele, the intact fIIlox allele, and the Cre-recombined fIIlox allele (fIICreΔ) were 402 bp, 438 bp, and 318 bp, respectively. Note that only trace recombination was observed in Mx+fIIlox mice in the absence of poly I:C, whereas the fIIlox allele was largely disrupted after poly I:C treatment in Mx+ animals. (D) Northern blot analysis of total liver RNA isolated 5 days after poly I:C administration to Mx− and Mx+ fIIlox/− mice illustrating the selective loss of detectable fII mRNA in Mx+fIIlox mice. (E) Western blot analysis of plasma prothrombin in unchallenged fIIlox/− adults and animals challenged 5 days previously with poly I:C. Note that plasma prothrombin was approximately 10% to 20% of normal in unchallenged fIIlox/− mice and was reduced to immunologically undetectable levels in poly I:C-treated Mx+fIIlox/− mice. H indicates HindIII; B, BamHI; K, KpnI; S, SmaI, E, EcoRI.
Northern blot analysis of hepatic prothrombin mRNA
Total hepatic RNA was extracted using STAT-60 (Iso-Tex, Friendswood, TX). Prothrombin mRNA was detected as described previously39 using a 32P-radiolabeled 1.1-kb XhoI-EcoRV fragment derived from the coding portion of the murine fII cDNA.40 Blots were imaged on a Storm 860 phosphoimager with image acquisition and quantitative analysis accomplished using Storm Scanner Control 5.03 and ImageQuant 5.2, respectively (GE Healthcare Bio-Sciences).
Hematologic profiles, immunologic analysis of prothrombin, and bleeding times
Whole blood, drawn from the inferior vena cava of anesthetized mice into a one-tenth volume of 0.1 M citrate, was used for complete blood cell analysis or processed to prepare plasma. Prothrombin was immunologically detected by Western blot analysis12 using either a rabbit antihuman prothrombin serum (Nordic Immunological Laboratories, Tilburg, The Netherlands) known to cross-react with murine prothrombin or a goat antimurine thrombin activation peptide I (Santa Cruz Biotechnology, Santa Cruz, CA). These were used in conjunction with appropriate biotinylated secondary antibodies and a streptavidin-horseradish peroxidase detection system (Vector Laboratories, Burlingame, CA) and chemiluminescent substrate (Pierce Chemical, Rockford, IL). Prothrombin times (PTs) and activated partial thromboplastin times (aPTTs) were determined by the use of a STA-Hemostasis Analyzer using the STA-Neoplastine Cl and STA-PTT reagents (Diagnostica Stago, Asnieres, France). The thrombin time was determined manually by combining 10 μL plasma with 10 μL of a solution containing 2 units/mL bovine thrombin (Enzyme Research Laboratories, South Bend, IN) and 40 mM CaCl2. Bleeding times were established in ketamine/xylazine-anesthetized mice by excising 3 mm of tail tip and submerging it in Tris-buffered normal saline containing 2 mM CaCl2 at 37°C. The time was recorded until blood flow stopped; observation was terminated at 13 minutes in any animal that failed to stop bleeding.
Histopathology
Harvested tissues were fixed in 10% buffered formalin (Sigma-Aldrich, St Louis, MO) and subsequently embedded in paraffin. Sections were cut and stained with hematoxylin and eosin, Masson trichrome stain, or Prussian Blue (iron deposition). Light microscopy was performed using an Axioplan 2 microscope (Carl Zeiss, Jena, Germany) equipped with an AxiocamHR camera and software (Axiovision 4.6.3; Carl Zeiss). Micrographs in Figures 4 and 6 used a Zeiss 40×/0.75 objective. Micrographs in Figure 5 used a Zeiss 5×/0.16 objective. Images were processed using Adobe Photoshop Elements 4.0 (Adobe Systems, San Jose, CA). Gross pictures were taken with a digital SLR camera (Rebel XT; Canon, Tokyo, Japan).
Clearance of S aureus after peritoneal injection
Cohorts of Mx+fIIlox/lox or Mx−fIIlox/lox animals were given poly I:C (5 μg/g, intraperitoneally) on day 0 to initiate Cre-mediated loss of fII in Mx+ animals. On day 5, overtly normal animals were anesthetized with isofluorane and injected intraperitoneally with 109S aureus (Newman strain) in 1 mL of saline. One hour later, all mice were again anesthetized and the peritoneal cavity was lavaged with 5 mL cold phosphate-buffered saline. Dilutions of retrieved lavage fluids were plated on tryptic soy agar for determination of colony-forming units. Plasma was harvested for determination of the circulating prothrombin concentration by Western blot as described in “Hematologic profiles, immunologic analysis of prothrombin, and bleeding times.”
Statistical analysis
Mouse survival was analyzed using the Kaplan-Meier statistical test. All other statistical comparisons were generated using the Mann-Whitney U test, except hematologic parameters analyzed with the Fisher exact test as indicated in Table 1.
Results
Mice carrying a conditional prothrombin knockout allele
The endogenous prothrombin gene was modified to generate a Cre recombinase-sensitive conditional knockout allele (fIIlox) containing loxP sites in introns 2 and 9 (Figure 1 for gene-targeting strategy). In the absence of Cre recombinase activity, the fIIlox gene retained an intact proximal promoter region and encoded full-length wild-type murine prothrombin, but the gene organization was modified to form a 5 exon minigene, whereby portions of the mouse prothrombin cDNA and 3′ noncoding and flanking portions of the hGH gene were incorporated to both complete the coding sequence and introduce an appropriate polyadenylation motif. Cre-mediated recombination was expected to result in a null allele lacking exons 3 through 9 (mRNA splicing between exon 2 and any of the residual downstream exons would result in a translational reading frame shift). Germline competent mice carrying the mutant allele were established, and heterozygous offspring were backcrossed to C57BL/6 mice for 6 generations before any further analysis. Unlike previously described mice carrying a constitutive prothrombin null allele,5 analysis of offspring from heterozygous fIIlox/+ parental mice revealed that homozygosity for the conditional knockout allele was compatible with both developmental success and growth to adulthood. Heterozygous fIIlox/+ and homozygous fIIlox/lox mice were overtly normal, and animals of both genotypes, regardless of sex, transmitted the mutant allele with typical Mendelian inheritance (data not shown). Furthermore, crosses between compound heterozygotes carrying one copy of the conditional knockout allele and one copy of the constitutive null allele (fIIlox/−) established that the fIIlox allele was transmitted to weaning age offspring with a Mendelian inheritance pattern. Here, given the expected absence5,6 of any viable homozygous fII−/− progeny, fIIlox/− and fIIlox/lox mice were found at near the expected 2:1 ratio (among 561 total progeny raised thus far, 359 were fIIlox/− and 202 were fIIlox/lox). The developmental and/or perinatal success of unchallenged fIIlox/− mice was superior to prothrombin-null fII−/− mice (which are uniformly not viable) and was comparable with previously described39 transgenic mice expressing low levels (∼7%) of human prothrombin. FIIlox/− mice also survived well into adulthood, with the oldest mice now exceeding a year of age. Overt spontaneous hemorrhagic events were never appreciated in either fIIlox/lox or fIIlox/− mice at any age, and females were capable of carrying multiple litters to term (many have delivered 5 or more litters). Thus, carrying either one or 2 intact conditional prothrombin knockout alleles was sufficient to maintain normal development, growth, and reproductive success.
Cre-mediated disruption of the hepatic fII gene and loss of circulating fII
To explore the consequences of a genetic loss of prothrombin in adult mice, fIIlox/− mice were crossed with Mx1-Cre mice carrying an interferon-inducible Cre recombinase transgene. Poly I:C–induced Mx1-Cre mice are known to support rapid and near-complete recombination of loxP-containing target alleles in the liver,38 the primary, if not sole, source of circulating prothrombin in adult animals. Our studies focused on fIIlox/− compound heterozygotes because this genetic background would require just one Cre-mediated recombination event per diploid cell to render it incapable of fII expression. Consistent with published findings,38 Southern blot (Figure 1B) and PCR (Figure 1C) analyses of the prothrombin locus in genomic DNA isolated from the liver of Mx+fIIlox/− mice 5 days after a single injection of poly I:C (5 μg per gram of body weight) revealed near-complete recombination of the fIIlox conditional knockout allele. In contrast, no recombination of the fIIlox allele could be appreciated in liver DNA collected from Cre transgene-negative mice based on Southern blot or PCR analyses, regardless of poly I:C injection. Low levels of recombination of the fIIlox allele were evident in Cre transgene-positive mice in the absence of poly I:C induction based on PCR analysis (lane 5 in Figure 1C), but recombination events in the liver under these conditions were probably rare as they were not detectable by Southern blot analysis. Complementary gene expression studies at both the mRNA level (Figure 1D) and protein level (Figure 1E) established that fIIlox/− mice lacking Cre recombinase had basal levels of prothrombin expression that were less than the expected value of 50% of wild-type. Specifically, quantitation of hepatic prothrombin mRNA by phosphoimager analysis of Northern blot data indicated that prothrombin expression was approximately 25% of wild-type in uninduced fIIlox/− mice. These data suggest that the novel organization of the intact fIIlox allele, including the substitution of 3′ noncoding and flanking sequences, resulted in a reduction in basal hepatic expression at the level of gene transcription and/or mRNA processing/half-life. In retrospect, this outcome is not that surprising given that noncoding polymorphisms in the human gene mRNA result in altered steady-state mRNA levels (eg, the prothrombin G20210A polymorphism41,42 ). Consistent with the hepatic mRNA findings, immunologic analyses of prothrombin in plasma samples collected from Cre-negative fIIlox/− mice (Figure 1E) established that, even in the absence of recombination, these animals had low basal levels of circulating prothrombin (estimated to be 10%-20% of wild-type mice). More impressively, the low levels of prothrombin expression in adult Mx+fIIlox/− mice could be reduced to essentially undetectable levels at both the mRNA and protein level within 5 days of Cre recombinase induction with poly I:C (Figure 1D,E).
Hematologic consequences of genetic elimination of prothrombin in adult mice
Comparative analyses of complete blood counts in whole blood collected from adult Mx−fIIlox/− and Mx+fIIlox/− mice established that these animals retained blood cell profiles that were not appreciably different from wild-type mice, with normal hemoglobin, platelet, and white blood cell counts (Table 1). Coagulation analyses using standard PT and aPTT assays were only modestly prolonged in unchallenged fIIlox/− mice relative to wild-type mice (Table 1). In contrast, both of these prothrombin-sensitive clotting assays revealed a profound loss of clotting function in Mx+fIIlox/− mice 6 days after the deletion of the fIIlox allele. Indeed, neither TF- nor contact-induced activation of the coagulation system resulted in clot formation for as long as the samples were observed. Despite this profound impairment in clotting function, the platelet count in whole blood was not diminished in poly I:C–induced Mx+fIIlox/− mice relative to wild-type controls (Table 1), suggesting that, in the absence of any significant hemorrhagic event, the simple loss of coagulation function resulted in no major ongoing platelet consumption. There was also no significant fibrinogen consumption in fIIlox/− mice carrying very low prothrombin. Here, prompt clot formation was observed in plasma after the addition of exogenous bovine thrombin (ie, standard TT assays) in both poly I:C–induced Mx+fIIlox/− and control mice (Table 1). Not surprisingly, the profound diminution in circulating prothrombin observed in Mx+fIIlox/− mice 5 days after poly I:C injection resulted in the inability to effectively control bleeding in a standard tail tip excision assay; none of 7 mice tested stopped bleeding over a 13-minute observation period, whereas all of 8 wild-type mice tested in parallel were able to terminate blood loss in 1.2 plus or minus 0.4 minutes (P < .001 vs Cre-induced fIIlox/− mice). Despite the absence of appreciable spontaneous bleeding events, a modest extension of bleeding times was anticipated in Cre-negative fIIlox/− mice based on their low basal levels of circulating prothrombin. Indeed, 11 of 16 Cre-negative fIIlox/− mice successfully controlled blood loss in an average time that was only modestly longer than wild-type animals (2.4 ± 1.0 minutes; P < .002 vs wild-type mice). However, the remaining 5 mice, although obviously successful in slowing blood loss, ultimately failed to fully stop blood flow and exhibited a pattern of delayed resumption in bleeding (presumably reflecting unstable thrombus formation). Thus, the low level of circulating prothrombin observed in fIIlox/− mice appeared to be compatible with event-free survival, but it was low enough to be biologically meaningful in at least some persons challenged with a relatively large mechanical injury.
Loss of prothrombin in adult mice results in fatal hemorrhagic events
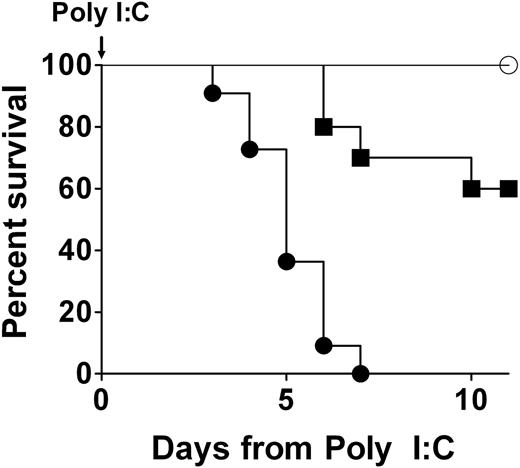
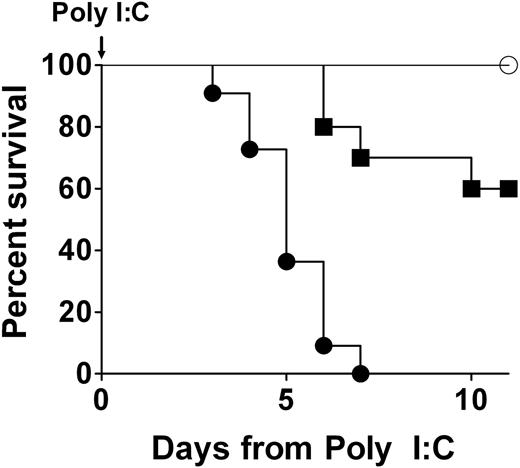
Any phenotypic consequences of a conditional disruption of the prothrombin gene in adult mice would not be expected to be immediately obvious, as circulating prothrombin would be expected to decline as a function of both prothrombin mRNA and protein half-life. Consistent with this view, induction of Cre in Mx+fIIlox/− mice resulted in no overt pathology for several days; however, this was followed by a dramatic loss of mouse viability (Figure 2). Induction of Cre expression with a single intraperitoneal injection of 1 to 5 μg of poly I:C per gram of body weight resulted in uniform death of Mx+fIIlox/− mice by 7 days, regardless of mouse sex (Figure 2; and data not shown). This was repeated more than 10 times with similar results. Even as little as 0.05 μg of poly I:C per gram of body weight was found to be life-threatening to Cre transgene-positive adult mice, but at this dose less than half died over a 2-week observation period (Figure 2). In contrast, no deaths were observed in cohorts of Cre transgene-negative fIIlox/− mice treated with any of a wide spectrum of dosing regimens of poly I:C. No losses or appreciable phenotypes were observed in Mx−fIIlox/− mice challenged with a single intraperitoneal injection of poly I:C at 5 μg per gram (Figure 2) or even 3 injections at twice that dose administered over a period of 5 days.
A profound genetic diminution of prothrombin in adult mice is not compatible with long-term survival. Representative survival profiles in Mx+fIIlox/− mice after a single intraperitoneal injection of either 0.05 μg/g (cohort of 10; ■) or 5 μg/g (cohort of 11; ●) of poly I:C to induce hepatic recombination of the prothrombin floxed allele. A parallel survival analysis using Mx−fIIlox/− animals lacking the Cre recombinase transgene, but treated with 5 μg/g of poly I:C, is shown for comparison (cohort of 5; ○). A dose-dependent loss in viability was observed in poly I:C–treated Mx+fIIlox/− mice, whereas no deaths were observed in Mx−fIIlox/− animals treated with poly I:C. Gross analyses of tissues at autopsy revealed severe hemorrhage within multiple tissues of Cre-induced mice, including heart, pleural cavity, and brain. Identical results were obtained in multiple independent experiments of the same design. Kaplan-Meier analysis established a statistically significant difference in survival in Cre transgene-positive and Cre transgene-negative fIIlox/− mice after administration of 5 μg/g poly I:C (P < .002).
A profound genetic diminution of prothrombin in adult mice is not compatible with long-term survival. Representative survival profiles in Mx+fIIlox/− mice after a single intraperitoneal injection of either 0.05 μg/g (cohort of 10; ■) or 5 μg/g (cohort of 11; ●) of poly I:C to induce hepatic recombination of the prothrombin floxed allele. A parallel survival analysis using Mx−fIIlox/− animals lacking the Cre recombinase transgene, but treated with 5 μg/g of poly I:C, is shown for comparison (cohort of 5; ○). A dose-dependent loss in viability was observed in poly I:C–treated Mx+fIIlox/− mice, whereas no deaths were observed in Mx−fIIlox/− animals treated with poly I:C. Gross analyses of tissues at autopsy revealed severe hemorrhage within multiple tissues of Cre-induced mice, including heart, pleural cavity, and brain. Identical results were obtained in multiple independent experiments of the same design. Kaplan-Meier analysis established a statistically significant difference in survival in Cre transgene-positive and Cre transgene-negative fIIlox/− mice after administration of 5 μg/g poly I:C (P < .002).
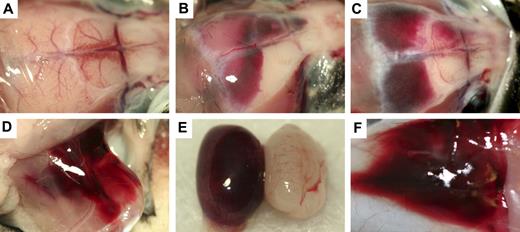
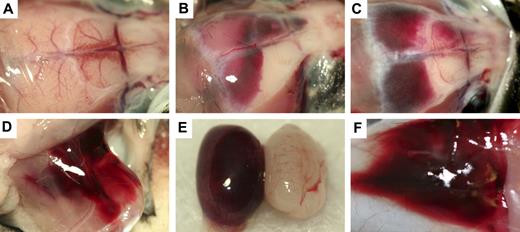
The probable cause of morbidity and mortality in poly I:C-induced Mx+fIIlox/− mice could be readily appreciated in the gross and microscopic evaluations of tissues. Gross inspection of 39 animals found moribund or dead after Cre induction revealed that nearly all had patchy darkened areas in the heart that reflected cardiac hemorrhagic events (see next paragraph). A substantial fraction of these animals presented with appreciable free blood in the pleural cavity (18; 46%), intracranial hemorrhage (18; 46%), or focal hemorrhage within skeletal muscle tissue (7; 18%). Other less common sites of bleeding included the skin, spinal canal, bowel, and testes (Figure 3). Five Mx+fIIlox/− mice had lungs harvested after inflation fixation at 5 days after poly I:C. All of these mice were found to have developed cardiac hemorrhage, but none of these mice exhibited evidence of pulmonary hemorrhage. In contrast, none of 20 age-matched 2- to 3-month-old Mx−fIIlox/− control mice evaluated in parallel exhibited any gross evidence of bleeding in any tissue, regardless of exposure to poly I:C.
Gross hemorrhage in Mx+fIIlox/− mice after Cre-mediated recombination of the fIIlox allele. (A) Normal brain in a control mouse. (B,C) Dural-based hemorrhage in Mx+fIIlox/− animals after administration of poly I:C. CNS hemorrhage was observed in dural, ventricular, and parenchymal areas of poly I:C-treated Mx+fIIlox/− mice. Additional sites of hemorrhage after recombination of the fIIlox allele include thigh (D), testes (E), and skin (F). Mx+fIIlox/− treated with poly I:C would often have multiple sites of subcutaneous hemorrhage in addition to other more life-threatening areas of hemorrhage.
Gross hemorrhage in Mx+fIIlox/− mice after Cre-mediated recombination of the fIIlox allele. (A) Normal brain in a control mouse. (B,C) Dural-based hemorrhage in Mx+fIIlox/− animals after administration of poly I:C. CNS hemorrhage was observed in dural, ventricular, and parenchymal areas of poly I:C-treated Mx+fIIlox/− mice. Additional sites of hemorrhage after recombination of the fIIlox allele include thigh (D), testes (E), and skin (F). Mx+fIIlox/− treated with poly I:C would often have multiple sites of subcutaneous hemorrhage in addition to other more life-threatening areas of hemorrhage.
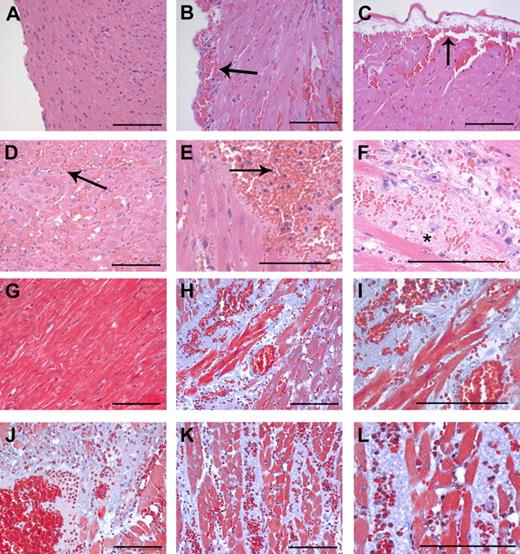
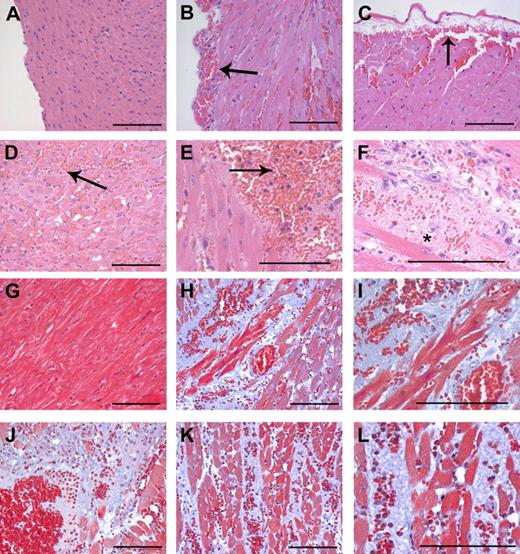
Microscopic analysis of heart tissue sections from both moribund and dead (n = 28) Mx+fIIlox/− mice 4 to 7 days after induction of prothrombin gene disruption with poly I:C confirmed that intramuscular and subcapsular cardiac hemorrhage occurred with virtually 100% phenotypic penetrance (Figure 4). In contrast, microscopic evidence of hemorrhage was never encountered in heart sections from unchallenged Mx+fIIlox/− (n = 7), Mx−fIIlox/− (n = 7), or poly I:C-challenged Mx−fIIlox/− (n = 7) mice. The subcapsular hemorrhagic lesions (see Figure 4 for representative lesions) imply that at least one source of free blood in the pleural cavity of Mx+fIIlox/− mice is cardiac hemorrhage. Microscopic evaluation of heart sections stained with hematoxylin and eosin and Masson trichrome suggests that multiple reactive changes are widespread in the hearts of Cre-induced Mx+fIIlox/− mice, including muscle ischemia/necrosis, neutrophil infiltrates and early granulation tissue, and disruption of muscle architecture by amorphous plasma and matrix proteins (Figure 4). Again, no such changes were appreciated in heart sections prepared from the 7 Cre-negative fIIlox/− mice evaluated.
Intramuscular cardiac hemorrhage, necrosis, and inflammatory infiltrates are uniformly associated with Cre-mediated loss of prothrombin in Mx+fIIlox/− mice. Representative hematoxylin/eosin-stained heart sections from a wild-type mouse (A) and Mx+fIIlox/− mice 4 to 6 days after poly I:C induction of hepatic Cre recombinase (B-F). Note that loss of prothrombin resulted in widespread intramuscular cardiac hemorrhage (B-F) as well as frequent subepicardial bleeding events (B,C). Large areas of the myocardium exhibited evidence of hemorrhage, infiltration of neutrophils, and ischemia/necrosis (D,E; arrows). (F) High-power view of hemorrhagic lesion with severe disruption of tissue architecture. *Ischemia/necrosis within myocardium often resulted in the formation of large acellular regions. Representative Masson trichrome-stained heart sections from a wild-type mouse (G) and Mx+fIIlox/− mice 4 to 6 days after poly I:C induction of hepatic Cre recombinase (H-L). In addition to focal hemorrhage, the normal cardiac structure in prothrombin-depleted mice was often disrupted by widespread areas of amorphous plasma and matrix proteins (blue regions) (H-L). (I) A high-powered view of the region in panel H illustrating free red blood cells and the disruption of the muscle fibers. (J) Hemorrhagic lesion where ischemic/necrotic myocardium has been replaced by amorphous proteinaceous exudate. (K) Areas of hemorrhage and ischemia often tracked between tissue planes to disturb large regions of the heart. (L) High-power view of the region in panel K showing hemorrhage, neutrophils, and disruption of muscle fibers. Scale bars represent 100 μm.
Intramuscular cardiac hemorrhage, necrosis, and inflammatory infiltrates are uniformly associated with Cre-mediated loss of prothrombin in Mx+fIIlox/− mice. Representative hematoxylin/eosin-stained heart sections from a wild-type mouse (A) and Mx+fIIlox/− mice 4 to 6 days after poly I:C induction of hepatic Cre recombinase (B-F). Note that loss of prothrombin resulted in widespread intramuscular cardiac hemorrhage (B-F) as well as frequent subepicardial bleeding events (B,C). Large areas of the myocardium exhibited evidence of hemorrhage, infiltration of neutrophils, and ischemia/necrosis (D,E; arrows). (F) High-power view of hemorrhagic lesion with severe disruption of tissue architecture. *Ischemia/necrosis within myocardium often resulted in the formation of large acellular regions. Representative Masson trichrome-stained heart sections from a wild-type mouse (G) and Mx+fIIlox/− mice 4 to 6 days after poly I:C induction of hepatic Cre recombinase (H-L). In addition to focal hemorrhage, the normal cardiac structure in prothrombin-depleted mice was often disrupted by widespread areas of amorphous plasma and matrix proteins (blue regions) (H-L). (I) A high-powered view of the region in panel H illustrating free red blood cells and the disruption of the muscle fibers. (J) Hemorrhagic lesion where ischemic/necrotic myocardium has been replaced by amorphous proteinaceous exudate. (K) Areas of hemorrhage and ischemia often tracked between tissue planes to disturb large regions of the heart. (L) High-power view of the region in panel K showing hemorrhage, neutrophils, and disruption of muscle fibers. Scale bars represent 100 μm.
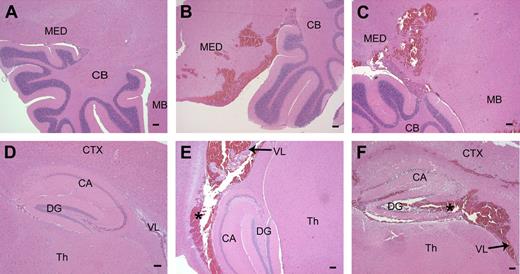
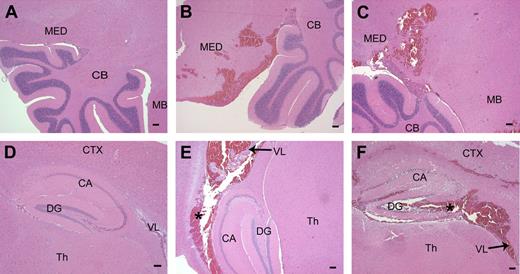
Microscopic analysis of brain sections confirmed that hemorrhage within the central nervous system (CNS) was commonplace in adult Mx+fIIlox/− mice genetically depleted of prothrombin (11 of 21 mice evaluated microscopically; 53%) but was never encountered in Cre-negative control mice (Figure 5). Although CNS bleeding often appeared to be dural based (Figure 3), microscopic analysis of tissue sections established that ventricular and parenchymal hemorrhage events were commonplace, with parenchymal bleeding events frequently encountered in the medulla, cerebellum, and hippocampus (Figure 5).
Parenchymal hemorrhage in the brain after disruption of the fII gene. (A) Normal hindbrain and cerebellum from a control mouse. (B,C) Brain sections taken from Mx+fIIlox/− mice 4 to 6 days after poly I:C. A frequent site of hemorrhage in the brain parenchyma was the medulla and the hindbrain near the cerebellum. (D) Normal hippocampal formation in a wild-type mouse. (E,F) Hemorrhage was observed in and around the hippocampal region and into the lateral ventricles in mice with conditionally deleted fII. *Areas of hemorrhage. MED indicates medulla; CB, cerebellum; MB, midbrain; CTX, cortex; CA, Ammon horn; DG, dentate gyrus; Th, thalamus; VL, lateral ventricle. Scale bars represent 100 μm.
Parenchymal hemorrhage in the brain after disruption of the fII gene. (A) Normal hindbrain and cerebellum from a control mouse. (B,C) Brain sections taken from Mx+fIIlox/− mice 4 to 6 days after poly I:C. A frequent site of hemorrhage in the brain parenchyma was the medulla and the hindbrain near the cerebellum. (D) Normal hippocampal formation in a wild-type mouse. (E,F) Hemorrhage was observed in and around the hippocampal region and into the lateral ventricles in mice with conditionally deleted fII. *Areas of hemorrhage. MED indicates medulla; CB, cerebellum; MB, midbrain; CTX, cortex; CA, Ammon horn; DG, dentate gyrus; Th, thalamus; VL, lateral ventricle. Scale bars represent 100 μm.
Complementary studies of Mx−fIIlox/− mice evaluated at more than a year of age also revealed neither gross nor microscopic evidence of hemorrhage or other pathologies in any major organ systems except the heart. Consistent with prior findings in aged mice with life-long deficits in other key hemostatic factors (eg, TF,43 fX,44 fVII,45 fXIII46 ), the hearts of aged Mx−fIIlox/− mice exhibited evidence of both hemosiderin deposition and cardiac fibrosis (modest and focal; Document S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Like low TF mice47 and fXIII-deficient animals,46 cardiac fibrotic changes were generally more appreciable in year-old Mx−fIIlox/− males but also observed in older Mx−fIIlox/− females. No overt clinical disorders or appreciable loss of fertility was noted in older Mx−fIIlox/− mice of either gender relative to control mice.
Prothrombin-deficient mice exhibit an impediment in microbial clearance
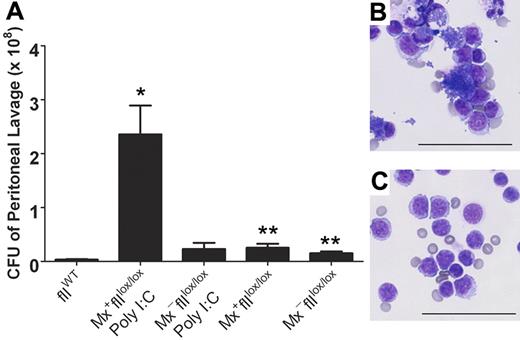
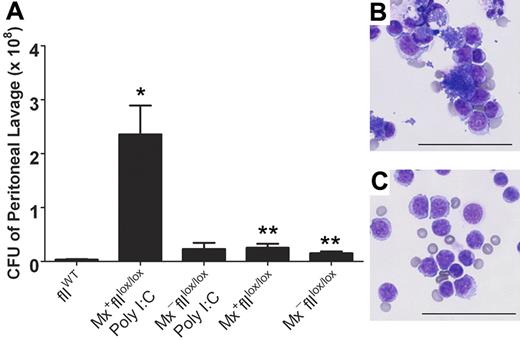
The capacity to conditionally eliminate prothrombin in adult mice provided a novel opportunity to explore the prevailing notion that thrombin regulates both coagulation and inflammatory pathways. Therefore, we evaluated the consequence of prothrombin depletion on bacterial clearance in a model of S aureus–mediated peritonitis. Here, cohorts of Mx+fIIlox/lox and Mx−fIIlox/lox mice were treated in parallel with 5 μg/g of poly I:C, and 5 days later these mice and control animals were challenged with an intraperitoneal injection of 1 × 109 colony-forming units (CFU) of S aureus. One hour after the injection, peritoneal lavage fluid was collected and evaluated for the number of S aureus CFU. Like mice with mutations in fibrinogen, a profound diminution of clearance was observed in the prothrombin-depleted Mx+fIIlox/lox animals relative to both wild-type and fIIlox/lox controls (Figure 6). Consistent with these findings, microscopic analysis of peritoneal lavage fluid revealed abundant free bacteria within the lavage fluid as well as bacteria-engorged and disrupted leukocytes in the Mx+fIIlox/lox animals depleted of prothrombin. In contrast, very few bacteria were noted in the control animals (Figure 6). Western analysis of plasma obtained from the Mx+fIIlox/lox animals that received poly I:C confirmed that the depletion of prothrombin was accomplished (data not shown).
Diminished clearance of S aureus after peritoneal infection in mice that lack prothrombin. (A) Determination of the number of CFU in peritoneal lavage fluid harvested from fIIloxand control mice 1 hour after the intraperitoneal injection of 1 × 109 CFU of bacteria. Values are mean plus or minus SEM. Whereas a modest trend toward decreased bacterial clearance was observed in fIIlox/lox animals in the absence of Cre recombination, the more profound diminution of circulating prothrombin associated with hepatic induction of Cre in Mx+fIIlox/lox mice (5 days after poly I:C treatment) resulted in a dramatic impairment in the ability to clear peritoneal S aureus (*P < .008 vs wild-type; **P < .02 vs wild-type). Representative cytospins of peritoneal lavage fluid collected 1 hour after infection from a Cre-induced Mx+fIIlox/lox animal (5 days after poly I:C treatment) (B) and a wild-type mouse challenged in parallel (C). Note that cytospin preparations from animals lacking prothrombin (B) were characterized by both abundant clusters of free S aureus and bacteria-engorged and disrupted phagocytes, whereas parallel preparations from control mice were largely clear of bacteria. Bars represent 50 μm.
Diminished clearance of S aureus after peritoneal infection in mice that lack prothrombin. (A) Determination of the number of CFU in peritoneal lavage fluid harvested from fIIloxand control mice 1 hour after the intraperitoneal injection of 1 × 109 CFU of bacteria. Values are mean plus or minus SEM. Whereas a modest trend toward decreased bacterial clearance was observed in fIIlox/lox animals in the absence of Cre recombination, the more profound diminution of circulating prothrombin associated with hepatic induction of Cre in Mx+fIIlox/lox mice (5 days after poly I:C treatment) resulted in a dramatic impairment in the ability to clear peritoneal S aureus (*P < .008 vs wild-type; **P < .02 vs wild-type). Representative cytospins of peritoneal lavage fluid collected 1 hour after infection from a Cre-induced Mx+fIIlox/lox animal (5 days after poly I:C treatment) (B) and a wild-type mouse challenged in parallel (C). Note that cytospin preparations from animals lacking prothrombin (B) were characterized by both abundant clusters of free S aureus and bacteria-engorged and disrupted phagocytes, whereas parallel preparations from control mice were largely clear of bacteria. Bars represent 50 μm.
Discussion
Thrombin plays a premier role in thrombus formation and thrombotic disease through the engagement of a wide array of substrates (eg, fibrinogen, fXI, fVIII, fV, fXIII, protein C, thrombin-activated fibrinolysis inhibitor), receptors (eg, PARs, thrombomodulin, GPIbα), and inhibitors (eg, antithrombin III, heparin cofactor II).4 However, thrombin-mediated signaling events also control fundamental cellular functions, independent of hemostasis, in a large number of diverse cell types (eg, endothelial cells,15 fibroblasts,16 smooth muscle cells17 ). Thrombin-mediated proteolysis appears to be important to development, tissue repair, malignancy, inflammatory processes, and antimicrobial function. The embryonic and perinatal failure of mice constitutively lacking either prothrombin or key proteins controlling thrombin generation/activity has been a significant impediment to detailed genetic analyses of thrombin in physiologic and pathologic processes in adult animals. Here, we used a gene-targeting approach to define the consequences of a genetically imposed loss of prothrombin in postnatal mice. Unlike the prothrombin null mice, mice carrying an intact fIIlox conditional knockout allele uniformly developed to term, survived the perinatal period, and remained viable well into adulthood. FIIlox/− mice also maintained normal reproductive success and never developed overt bleeding events, despite a significant basal reduction in prothrombin levels and a modest alteration in clotting times and tail-tip bleeding times in the absence of Cre induction. However, real-time analyses of thrombus formation in vivo in conditional knockout mice will be required to precisely define the consequences of both modest and acute prothrombin deficiency on overall thrombus formation and stability. Induction of Cre recombinase in adult fIIlox mice resulted in rapid and near-complete recombination of the fIIlox allele within the liver, the loss of circulating prothrombin, and profound derangements in coagulation function. Adult mice that were profoundly depleted of prothrombin rarely survived longer than a week, and the loss of viability was associated with the development of severe hemorrhagic events within multiple tissues. However, the loss of prothrombin appeared to compromise longer than hemostasis. An early consequence of reducing prothrombin (before overt hemorrhage) was impaired antimicrobial function in mice challenged with S aureus peritonitis. These data strongly suggest a differential requirement for thrombin-mediated proteolysis in maintaining vascular integrity in various organ systems and support the prevailing notion that thrombin controls both hemostatic and inflammatory processes. Finally, these data show that the loss of prothrombin, unlike the loss of either coagulation- or platelet-function alone, is not compatible with survival in adult animals.
The survival profile of Cre-negative fIIlox/− mice illustrates that circulating prothrombin can be diminished substantially without significant hemostatic consequences and may even hold some phenotypic benefits in the context of selected challenges (eg, acute thrombotic insults, malignancy). This finding is compatible with an earlier finding39 that transgenic mice expressing constitutively low levels (∼7% of normal) of human prothrombin and no endogenous murine prothrombin survive well into adulthood without experiencing major bleeding events. However, the consequences of imposing a profound deficit in circulating prothrombin in unchallenged adult mice are rapid and fatal. The most prominent and highly penetrant pathology observed was hemorrhagic events within the heart and brain, but evidence of bleeding was also often appreciated in the pleural cavity, skeletal muscle, and skin. Some of these tissues were also sites of bleeding and other pathologic changes in adult mice expressing low levels (∼1%) of either TF or fVII,43,45 reduced activity of fX,44 or deficient in fXIII.46 In our study, pulmonary hemorrhage was not observed, as was seen in the low TF mice, but this may merely be a reflection of earlier and acute bleeding events elsewhere in conditional prothrombin-deficient mice. However, like mice with deficits in TF, fVII, fX, and fXIII, mice carrying low levels of fII in the absence of Cre recombination developed an age-dependent process of modest cardiac hemorrhage (as evidenced by hemosiderin deposition) and modest fibrosis that was evident in most, albeit not all, mice by 1 year of age. However, the most significant distinction between prior findings in low TF, fVII, and fX mice and Mx+fIIlox/− mice is that Cre-induced depletion of prothrombin in the latter results in profound cardiac pathologies, including subepicardial and myocardial hemorrhage, ischemia, inflammatory cell infiltrates, and diffuse plasma and/or matrix protein deposition, regardless of gender, and within just days rather than over many months.
The acute development of cardiac pathologies in fIIlox mice after loss of circulating prothrombin offers some insight into the mechanism underlying the cardiac phenotype previously described in low TF and low fVII mice. The present findings suggest that the development of hemosiderin deposits and fibrosis in older low TF/fVIII mice is probably a result of a chronic impediment in a thrombin-coupled process rather than the loss of a thrombin-independent TF/fVII signaling pathway. An attractive working hypothesis for the rapid development of cardiac pathologies in prothrombin-deficient adults is that the collapse of hemostatic function results in catastrophic hemorrhage in the heart under repetitive mechanical stress. Consistent with the concept of a broad failure of hemostasis, it is notable that the individual loss of any PAR, including PAR-1 or -4, does not result in cardiac pathologies in unchallenged adult mice. Similarly, the individual loss of fibrinogen also does not lead to spontaneous heart disease. Rather, the combination of hemostatic derangements associated with loss of either prothrombin or the capacity for thrombin activation appears to underlie hemorrhage and secondary fibrosis in the heart.
The proposal that tissues under recurrent mechanical stress may have a special requirement for an effective hemostatic system may provide an explanation for why some tissues (eg, heart, skeletal muscle, skin), but not others (eg, liver, spleen, kidneys), are prone to bleeding in the context of genetic or pharmacologic disruption of hemostatic factors. The need to have effective control of chance vascular breaks may also provide a rationale for the high levels of TF expression observed in heart, lung, and other tissues.43,48 The brain is a particularly rich source of TF and a previously recognized site of hemorrhage in older mice expressing low levels of TF/fVII. The finding that rapid-onset, multifocal hemorrhagic events in the brains of mice after conditional elimination of prothrombin again points to the critical role of TF/fVII in the control of thrombin generation in the brain. Like the heart, no brain pathologies have been recognized in adult mice lacking any of the PARs, suggesting that a failure of thrombin signaling alone does not account for CNS hemorrhage. At first glance, a failure at the level of loss of hemostatic function may appear to be equally improbable because the skull-protected brain would not seem to be subjected to recurrent mechanical insult. However, it should be noted that surgical exposure of the brain reveals an easily visible pulsation of brain tissue with every heartbeat, a phenomenon that cannot be readily appreciated on gross inspection of most other organs.49 Therefore, it is conceivable that the unique, flexible, and highly vascularized structure of brain tissue may demand special hemostatic controls to counter chance vascular breaks associated with pulsatile motion. The potential importance of thrombin in brain vasculature integrity is further emphasized by the fact that thrombomodulin, the endothelial thrombin receptor that redirects thrombin activity away from procoagulant substrates, is distinctly limited within the brain vasculature. More detailed studies of the role of thrombin in the brain, particularly disease processes involving disruption of the neurovascular unit, may be highly revealing.
Antithrombotic strategies being developed at the level of fVIIa, fXa, thrombin, and other coagulation factors probably offer therapeutic benefits in multiple contexts, including acute thrombotic events (eg, stroke, myocardial infarction), inflammatory diseases, and cancer. However, the present studies suggest that aggressive and/or long-term pharmacologic strategies to limit thrombin generation or thrombin-mediated proteolysis may hold special risks in certain organs, particularly heart and brain. This is reinforced by the high incidence of spontaneous intracranial hemorrhage associated with certain anticoagulation or thrombolytic protocols.50 However, the fact that remarkably low prothrombin levels are well tolerated suggests that only the most aggressive forms of intervention must be approached with caution. The finding that prothrombin levels can be readily manipulated in fIIlox mice and reduced by more than 90% without acute loss of vascular integrity or viability provides a novel experimental opportunity to explore the prothrombin dependence of virtually any physiologic and pathologic process. Of particular interest will be defining the precise contribution of prothrombin to tumor progression, vessel wall disease, sickle cell disease, and inflammatory joint, lung, bowel, and CNS disease.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Kelley Barney and Elizabeth Blevins for their outstanding technical assistance, Joseph Palumbo for his supportive advice, and Tanya Mullins for her helpful suggestions.
This work was supported by National Institutes of Health grants HL085357 (J.L.D.), AR049822 (J.L.D.), and T32 HD043005 (E.S.M) and the National Hemophilia Foundation Clinical Fellowship Award (E.S.M.).
National Institutes of Health
Authorship
Contribution: E.S.M., S.J.D., and J.L.D. designed research; E.S.M., K.W.K., M.A.S., K.E.T., M.J.F., J.M.U., and W.S. performed research; E.S.M., M.J.F., D.P.W., and J.L.D. analyzed data; and E.S.M., M.J.F., and J.L.D. prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jay L. Degen, Children's Hospital Research Foundation, Children's Hospital Medical Center, CHRF Room 2042, 3333 Burnet Avenue, Cincinnati, OH 45229-3039; e-mail: jay.degen@cchmc.org.