Abstract
Pretreatment characteristics and outcome of patients treated with induction regimens containing high-dose ara-C (HiDAC) at M. D. Anderson Cancer Center refractory to 1 cycle of induction were compared with similar patients achieving a complete response (CR). Among 1597 patients treated with HiDAC-based induction from 1995 to 2009, 285 were refractory to 1 cycle. Median age was 59 years (range, 18-85 years). Induction regimens included HiDAC with anthracyclines (n = 181; 64%) or HiDAC with nonanthracycline chemotherapy (n = 104; 36%). Refractory patients were older (median age, 59 vs 56 years; P < .001), more likely with unfavorable cytogenetics (P < .001) and antecedent hematologic disorder (P < .001), and had a higher presentation white blood cell count (P = .04), but not a higher incidence of FLT3 mutations (P = .85), than those achieving CR. Forty-three patients (22%) responded to salvage (35 CR and 8 CR without platelet recovery). With a median follow-up of 72 months (range, 27-118 months) in responders, 11 are alive. Nineteen patients (7%) were alive and in CR for at least 6 months, including 9 who underwent allogeneic stem cell transplantation. On multivariate analysis, severe thrombocytopenia, leukocytosis, increasing marrow blast percentage, unfavorable cytogenetics, and salvage not including allogeneic stem cell transplantation were associated with a worse survival. Alternative strategies are needed for these patients.
MedscapeCME Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://cme.medscape.com/journal/blood; and (4) view/print certificate. For CME questions, see page 6153.
Disclosures
The authors, the Associate Editor Martin S. Tallman, and the CME questions author Charles P. Vega, University of California, Irvine, CA, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Analyze variables that predict a poor response to high-dose cytarabine induction chemotherapy in AML
Evaluate variables that predict a worse response to salvage therapy for induction failure of AML, and identifiy the rate of complete response to salvage therapy
Describe factors that reduce overall survival among patients who do not respond to induction chemotherapy for AML
Release date: December 23, 2010; Expiration date: December 23, 2011
Introduction
The progress in the understanding of the biology of neoplastic transformation in patients with acute myeloid leukemia (AML) has led to significant advances in the treatment of selected patients with well-defined cytogenetic and molecular characteristics.1,2 However, current therapeutic strategies remain unsatisfactory for most patients, particularly those with adverse cytogenetic abnormalities and those with more advanced age.3 The majority of younger patients and a significant proportion of older patients with AML achieve a complete remission (CR) after the initial induction regimen. However, relapse remains the major cause of failure and an obstacle to achieving long-term cure in most patients.4 Several predictors of outcome for patients in first relapse have been identified and include the duration of the first CR, cytogenetics at diagnosis, age at relapse, and whether a prior allogeneic transplantation was performed in the first CR.5 Among these, the length of first CR appears to be of major significance.5,6 Primary refractory disease, variably defined as failing to achieve a CR after 1 or 2 cycles of standard combination chemotherapy, accounts for a significant proportion of patients with expected poor long-term outcome. As inherent in the definition, patients with primary refractory AML have leukemic cells that are primarily resistant to the effects of cytotoxic, DNA-interactive chemotherapy and, as such, would be candidates for investigational strategies focused on alternative cytostatic or cytotoxic mechanisms. Those at the highest risk of induction failure include patients with adverse karyotype, therapy-induced AML, and AML evolving from other hematologic disease, such as myelodysplastic syndrome or myeloproliferative disorders.4
Standard treatment of AML has included the combination of standard-dose ara-C (100 or 200 mg/m2 daily for 7 days) and an anthracycline, variably daunorubicin or idarubicin. Recent studies have suggested a significant benefit for escalation of dose of the anthracycline in particular subsets, both in younger and older adults.7,8 Few studies have examined the role of escalating the dose of ara-C in induction. A meta-analysis of these studies concluded that induction therapy with high-dose ara-C (HiDAC) improved long-term disease control and overall survival in adults younger than 60 years.9 Therefore, some centers, including ours, have adopted this strategy for the frontline therapy of younger patients and have reported high CR rates of 70%-80% in prior trials using this strategy. Clearly, failing to respond to such regimens with high-dose anthracyclines or HiDAC is likely to have different prognostic implications than failing to achieve CR after the more traditional, less intensive regimens. Information on patients who are refractory to such HiDAC-based induction is limited. We therefore undertook this study to examine the characteristics and outcome of patients with AML who failed to achieve CR after 1 cycle of such a HiDAC-based regimen. We chose to exclude patients achieving CR without platelet recovery (CRp) and partial response (PR) as well as those who received > 1 course of HiDAC-based induction.
Methods
Patients
Between January 1995 and June 2009, a total of 1597 patients with AML were treated with a HiDAC (defined as ≥ 1 g/m2 per dose) containing regimens for their induction at the University of Texas M. D. Anderson Cancer Center. During the same period, 2129 patients with AML received induction therapy, including 353 who received regimens without ara-C, and 179 who received regimens containing standard-dose ara-C. Among the patients receiving HiDAC induction, 1468 patients received only 1 course of the regimen. This included 894 (61%) who achieved CR and 285 (19%) who were refractory to the first course of therapy. Another 52 (4%) achieved a CRp, 11 (< 1%) achieved a PR, and 226 (15%) died at induction (Table 1). The characteristics of patients with primary refractory disease and those who achieved CR after 1 course of induction are summarized in Table 2. The median age of these patients was 57 years (range, 17-88 years). Their median presenting white blood cell (WBC) and platelet counts were 6.8 × 109 (range, 6.3-394 × 109/L) and 51 × 109/L (range, 3-1355 × 109/L), respectively. Cytogenetics (assessed based on the criteria published by the Cancer and Leukemia Group B10 were diploid or intermediate in 820 patients (70%), unfavorable in 235 (20%), and favorable in 124 (10%). FLT3 mutational status was available in 584 patients; 139 (24%) had mutated FLT3 and 445 (76%) were wild type. Antecedent hematologic disease had been noted in 473 (40%). All patients were treated on clinical trials approved by the M. D. Anderson Cancer Center Institutional Review Board and all reviewed and signed a consent form to participate in the trial in accordance with the Declaration of Helsinki. Herein, we will focus on the 1179 patients who either achieved a CR or were refractory to 1 induction course. Not included in this analysis were 129 patients who received > 1 course (2 or 3 courses) of HiDAC-based chemotherapy; 38 achieved CR and 3 CRp, with 63 patients remaining resistant and 25 dying during the induction courses.
Treatment regimens
Various frontline regimens were used during the specified period (Table 3). They included regimens combining HiDAC with anthracyclines in 765 patients (65%) and HiDAC with nonanthracycline chemotherapy (including fludarabine, clofarabine, topotecan, and/or troxacitabine) in 414 patients (35%). The dose of ara-C in all these regimens was 1-2 g/m2 daily for 3-5 days, with each dose given over 2-3 hours. Standard supportive practices, prevailing at the time of inclusion, such as prophylactic and therapeutic antibiotics and transfusion of blood products, were used to support the patients during the aplastic phase.
Response criteria
CR was defined by the presence of < 5% blasts in the bone marrow (BM) with > 1 × 109/L neutrophils and > 100 × 109/L platelets in the peripheral blood (PB). Patients with CRp achieved the above criteria, with the exception of the platelet count remaining < 100 × 109/L. PR was defined as achieving CR criteria in the PB with BM blast reduction by ≥ 50%, but remaining > 5%.11
Statistical analysis
Differences in subgroups by different covariates were evaluated using the χ2 test or Fisher exact test for categorical variables and the Mann-Whitney U test for continuous variables. CR duration (CRD) was calculated from the time of CR until relapse. Overall survival (OS) was calculated from the time of first salvage until death or last follow-up. Time to event curves were plotted by the Kaplan-Meier method and compared using the log-rank test. Factors evaluated for predicting CR to the salvage therapy and the OS included cytogenetics at diagnosis, therapy-related AML, presence of antecedent hematologic disorder, salvage treatment regimens, and other characteristics at the time of first salvage, such as age, marrow blast percentage, WBC, and platelet count. Logistic regression and Cox proportional hazard models were applied to identify significantly independent factors for CR and OS, respectively. A P value < .05 was considered to be significant.
Results
Characteristics of patients with primary refractory disease
Among the patients with AML treated with a HiDAC-containing regimen, 285 (19%) were refractory to the first cycle of induction. Patients with primary refractory disease were older (median age, 59 vs 56 years) than those who achieved CR (P < .001). They also had a higher presenting WBC and a lower presenting platelet count (P = .04 and .02, respectively; Table 2). Patients with primary refractory disease were also more likely to have had antecedent hematologic disease and unfavorable cytogenetics (P < .001 for both). Furthermore, they were more likely to have therapy-related AML than the responding patients (P = .03). Interestingly, among the patients with available FLT3 mutational status, there was no difference between the primary refractory patients and those achieving a CR (P = .85). There was also no difference by the induction regimen between these 2 groups when considering the combination of HiDAC with an anthracycline, compared with HiDAC, and a nonanthracycline agent (P = .58; Table 4). On multivariate analysis, unfavorable cytogenetics, older age (≥ 50 years), severe thrombocytopenia (< 30 × 109/L), and antecedent hematologic disease were predictive of lack of response to first cycle of induction therapy (data not shown). Mutated FLT3 did not predict for a lower likelihood of response; this is consistent with prior observational reports suggesting no influence of FLT3 mutational status on achievement of CR.12
Response and survival outcome in patients with primary refractory disease
Among the 285 patients with primary refractory disease after the first cycle of induction, 197 (69%) received salvage therapy, which included combination chemotherapy in 111 (39%) with or without ara-C, single-agent therapy, such as hypomethylating agents, in 63 (22%), or allogeneic stem cell transplantation (without further chemotherapy before transplantation) in 23 (8%; Table 5). One hundred ninety-two patients had follow-up information and were available for response assessment. Only 35 (18% of total treated) achieved a CR with salvage therapy; 8 patients (4%) had CRp and 3 (2%) had a PR; and 111 (56%) patients were resistant and 35 (18%) died. The factors that were associated with a lower likelihood of achieving a CR with the salvage treatment included thrombocytopenia at salvage (P = .002), higher marrow blast percentage at salvage (P = .03), unfavorable cytogenetics at presentation (P = .001), presence of antecedent hematologic disorder (P = .008), and therapy not including allogeneic stem cell transplantation (P < .001; Table 6). Multivariate stepwise analysis further identified all the above factors, except for marrow blast percentage, as having an independently worse effect on achieving CR with first salvage (Table 7). Of note, there was a selection bias for the patients proceeding directly to an allogeneic stem cell transplantation with a significantly lower blast percentage in these patients (P = .002).
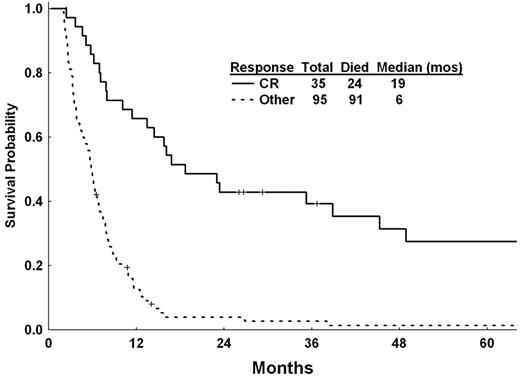
The median survival of the 285 patients with primary refractory disease was 3.8 months (Figure 1A). The median CR duration for the 35 patients who achieved CR was 9.1 months (Figure 1B). As expected, the outcome of these patients was significantly better than those who did not achieve a CR, with a median survival of 19 versus 6 months, respectively (excluding patients who died within 2 months from the first salvage therapy; Figure 2). Severe thrombocytopenia, leukocytosis, and increasing marrow blast percentage at salvage, as well as unfavorable cytogenetic, and salvage not including allogeneic stem cell transplantation, were associated with a significantly worse survival (all P < .05; Table 6). Multivariate stepwise analysis identified platelet count of < 30 × 109/L at salvage (P = .001), WBC ≥ 10 × 109/L at salvage (P = .007), increasing marrow blast percentage at salvage (P < .001), and unfavorable cytogenetics at presentation (P < .001) as significant for survival (Table 7). Salvage strategies did not independently affect OS after adjustment for the other covariates—in particular, the significant association of allogeneic stem cell transplantation with a lower blast percentage (P = .02). With a median follow-up of 72 months (range, 27-118 months) for the patients responding to salvage, 11 patients are currently alive. Nineteen patients (7% of the total population with primary refractory disease) were alive and in CR for at least 6 months, including 9 who underwent an allogeneic stem cell transplantation as their salvage therapy.
OS after salvage chemotherapy. (A) For the 285 patients with primary refractory AML (to 1 cycle of HiDAC containing induction). (B) Complete remission duration for the 35 patients who achieved a CR after salvage chemotherapy
OS after salvage chemotherapy. (A) For the 285 patients with primary refractory AML (to 1 cycle of HiDAC containing induction). (B) Complete remission duration for the 35 patients who achieved a CR after salvage chemotherapy
Landmark (2-month) analysis of OS after salvage for the responders (achieving CR) and nonresponders.
Landmark (2-month) analysis of OS after salvage for the responders (achieving CR) and nonresponders.
Discussion
Relapse is the most important obstacle to achieving long-term cure in patients with AML. Prognostic factors for patients with relapsed AML include age at relapse, cytogenetics at diagnosis, and duration of the first CR.5,6 The latter has been shown in several studies to be the most important parameter to predict the achievement of a second CR and, subsequently, disease-free and overall survival.5,6 At the extreme is primary refractory AML when CR was never achieved. As such, these patients are expected to have a dismal prognosis. However, prior reports have shown that a proportion of these patients could be salvaged with regimens containing HiDAC when standard-dose ara-C was used for induction.13 Here, we examined the outcome of patients who received HiDAC as a component of their induction regimen and were refractory to it. Only 35 (18%) patients achieved a CR with a median CR duration of 9 months; another 8 (4%) achieved CRp for an overall response rate of 22%. Unfavorable cytogenetics at presentation, presence of antecedent hematologic disorder, and salvage treatment with nontransplantation strategies were associated with a worse CR rate.
Clearly, the potential for salvaging patients who are refractory to 1 cycle of HiDAC-based induction is limited, and only approximately one-quarter of these patients can be rescued with the available salvage strategies. Further studies to identify biologic factors responsible for lack of response to the standard cytotoxic regimens and to develop and design new agents and strategies to overcome such resistance are needed. It is interesting to note that patients who proceeded directly to an allogeneic stem cell transplantation had an improved survival on univariate but not multivariate analysis, suggesting a selection bias, which was confirmed by the significantly lower marrow blast percentage in patients who received transplants (P = .002).
Historically, the definition of primary refractory AML has not been uniform due to the different interpretations of the definition of CR and the number of cycles and type of chemotherapy needed to achieve it. With the evolution of the definition of CR and the more recent recognition of subtleties in defining a morphologic CR, such as the role of persisting circulating blasts despite achievement of < 5% marrow blast,14 and the importance of normalization of PB counts,15 it has become easier to arrive at uniform and universal definitions of failure of induction therapy.11 It is clear that failure based on persistence of > 50% blasts in the marrow is different from failing to meet the definition of CR due to the presence of few circulating blasts or persistent thrombocytopenia.14,15 The exact time to assess response after the induction course has not been standardized. Traditionally, it has been customary by many groups to perform a BM examination on days 10-14 or 21-28 to assess for the presence of residual leukemia after the induction chemotherapy, and arbitrary definitions have been used to determine the need for a second induction course, depending on the persistence of leukemic cells at these time points. However, the lack of uniformity and the absence of certainty on the percentage of blasts that is clearly indicative of disease resistance make these determinations less than ideal. Many patients with residual marrow blasts on a day 14 marrow achieve a CR, although the probability of this decreases with increasing blast percentage.16
Similarly, whether patients should fail at least 2 cycles of chemotherapy before being considered as primary failure is debatable, but it is generally accepted that patients who have failed only 1 cycle of standard-dose chemotherapy will do better than those who have not responded to 2 or more cycles. In fact, a reduction of BM blasts to 5%-15% (or a PR) after the first cycle is associated with a high chance of achieving CR after a second course of chemotherapy.17 Although, in this study, the overall survival was inferior for the patients with PR, there was no difference in relapse rate in patients achieving CR after the first or second course.17 Thus, although other studies have not demonstrated such a likelihood of a better outcome for the patients achieving PR after the first cycle than for those with primary refractory disease, the mere possibility of achieving a CR with the second course of chemotherapy further mandates having a more uniform definition of primary refractory disease. This is particularly important as the second course in many patients may include HiDAC (defined arbitrarily as ≥ 1 g/m2 per dose) in patients who received standard dose ara-C for their induction. Regimens containing HiDAC are generally considered as the best salvage option for patients with primary refractory disease or those with a short first CR.13
Using more traditional regimens containing lower doses of ara-C, it may be possible to have a better outcome if the patients destined to be unresponsive were identified early (eg, by a day 14 BM) and treated with a second similar course of induction. Rowe et al18 have recently reported that among patients with AML treated in 6 studies by the Eastern Cooperative Oncology Group, those who achieved a CR after 1 or 2 courses of chemotherapy had a similar outcome. This further illustrates the need to standardize the definition of primary refractory disease, as clearly lack of response to a single course of standard-dose ara-C cannot be equated to unresponsiveness to HiDAC-containing induction regimens with different prognostic implications for the 2 scenarios. This study had the limitations associated with retrospective studies. However, the use of a HiDAC-based regimen for treating the majority of patients with AML (75%) treated during the study period reduced the likelihood of selection biases. Further prospective studies comparing HiDAC and standard-dose ara-C regimens and the role of 1 or 2 cycles of induction may shed further light to the outcome of these patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: F.R. and H.K. designed the study, treated patients, provided material, analyzed the data, and wrote the manuscript; J.C., S.F., S.O., G.G.-M., S.V., F.P.S.S., and M.d.L. treated patients, provided material, and reviewed the manuscript; and J.S., M.B., and S.P. analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Farhad Ravandi, Department of Leukemia, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: fravandi@mdanderson.org.