Abstract
Stimulatory antiplatelet derived growth factor receptor α (PDGFRA) antibodies have been associated with extensive chronic graft-versus-host disease (cGVHD). We performed a phase 1 dose escalation trial of imatinib in corticosteroid-dependent/refractory cGVHD to assess the safety of imatinib and test the hypothesis that abrogation of PDGFRA signaling can ameliorate the manifestations of cGVHD. Fifteen patients were enrolled. Mean follow-up time was 56.6 weeks (range, 18-82.4 weeks). Imatinib 400 mg daily was associated with more frequent moderate to life-threatening adverse events than 200 mg daily. The main adverse events were nausea, edema, confusion, diarrhea, liver function test elevation, fatigue, and myalgia. The overall response rate was 40% (6 of 15). The treatment failure rate was 40% (6 of 15). Twenty percent (3 of 15) of subjects had stable disease. Of 4 subjects with phospho-PDGFRA and phospho-PDGFRB immunohistochemistry studies before and after treatment, inhibition of phosphorylation was observed in 3 but correlated with response in one. Anti-PDGFRA antibodies were observed in 7 of 11 evaluable subjects but correlated with clinical activity in 4. We conclude that cGVHD responds to imatinib through multiple pathways that may include PDGFRA signal transduction. This study is registered at www.clinicaltrials.gov as #NCT00760981.
Introduction
Chronic graft-versus-host-disease (cGVHD) is the allogeneic reaction of the donor immune system against recipient body tissues that can result in fibrosis of the skin and connective tissues as well as other manifestations, including ocular and oral cavity sicca.1,2 It is the major complication of allogeneic hematopoietic cell transplantation and can result in life-long disability and pain if not adequately treated or controlled.3 Although several studies have demonstrated a negative correlation between cGVHD and quality of life, one study has reported that patients with resolved, inactive cGVHD have healthcare outcomes similar to those patients who did not develop cGVHD after allogeneic hematopoietic cell transplantation.4-9
The primary treatment for cGVHD is corticosteroids with or without calcineurin inhibitors.10,11 Approximately 50% of cGVHD fails to respond to these regimens. Several second-line therapies with efficacies between 40% and 60% have been reported. However, no consensus exists as to the best intervention for patients failing primary therapy, making the characterization and development of new therapeutic modalities a research priority.12-15
Stimulatory anti–platelet-derived growth factor receptor-α (PDGFRA) antibodies were retrospectively identified in all 22 patients with extensive cGVHD in a previously reported multicenter study.16 In vitro, these antibodies induced PDGFRA phosphorylation and reactive oxygen species generation, and increased α-actin and collagen expression. These processes have been associated with systemic scleroderma, an autoimmune disease that shares many phenotypic manifestations with cGVHD.17-20
Imatinib is currently approved for the treatment of Philadelphia chromosome-positive chronic myelogenous leukemia, Philadelphia chromosome-positive acute lymphoblastic leukemia, gastrointestinal stromal tumor, dermatofibrosarcoma protuberans, FIP1L1-PDGFRA hypereosinophilic/chronic eosinophilic syndrome, aggressive systemic mastocytosis without the D816V c-KIT mutation, and PDGFR mutation-associated myelodysplastic/myeloproliferative syndromes.21-28 These disorders are associated with aberrant tyrosine kinase activity. Imatinib inhibits the phosphorylation of the tyrosine kinases PDGFR, c-KIT, BCR-ABL, DDR1, and DDR2. PDGFR is a heterodimer of 2 homologous polypeptides, PDGFRA and PDGFRB. The IC50 of PDGFR is 0.039μM.29 In pharmacokinetic studies, the trough serum concentration achieved by administration of imatinib 400 mg daily was 1.46μM.30 Given its proven safety in humans and ability to inhibit PDGFR phosphorylation, we and others hypothesized that imatinib would be an effective treatment for cGVHD based on the rationale that some of the phenotypes of cGVHD may arise from stimulation of the PDGF receptor by anti-PDGFRA antibodies, leading to a signal transduction cascade resulting in tissue fibrosis.
The Italian transplant group has prospectively treated 19 subjects with sclerotic cGVHD with imatinib 50 to 200 mg daily in a phase 1 trial and observed that imatinib was well tolerated and that 79% of participants experienced improvement in their cGVHD status by 6 months.31 Magro et al have reported a 50% response rate after a median of 5.9 months of therapy in a retrospective study.32 Here, we present the results of 15 subjects enrolled in a phase 1 trial of imatinib for corticosteroid-dependent/refractory cGVHD with a median follow-up of 56.6 weeks. The primary study aim was to determine the safety of imatinib. The secondary study aim was to assess the clinical efficacy of imatinib along with laboratory correlative studies. We examined the pharmacodynamic effect of imatinib on PDGFR in cGVHD affected skin with immunohistochemical studies. To test the hypothesis that antibodies against PDGFRA stimulate PDGFR signal transduction resulting in a sclerotic/fibrotic phenotype, we determined whether purified antibodies could induce PDGFRA phosphorylation with an in vitro fibroblast bioassay. Finally, we identified clinical trial subjects with anti-PDGFRA antibodies and correlated the presence of antibody with the results of imatinib treatment.
Methods
Clinical trial
Patients with corticosteroid refractory or dependent cGVHD were eligible. We defined corticosteroid refractory as progressive clinical manifestations despite a corticosteroid dose equivalent to prednisone 0.5 mg/kg per day for 1 month, and corticosteroid dependent as the inability to taper corticosteroids below a dose equivalent to prednisone 0.25 mg/kg per day for 3 months. In addition to corticosteroids, one other systemic treatment for cGVHD was allowed. The dosing regimen of the corticosteroid and other therapy was required to be stable for ≥ 30 days before study enrollment. Other eligibility criteria included cGVHD manifestations that could be followed by physical or laboratory examination, an absolute neutrophil count > 500/μL, hematocrit > 26%, platelet count > 20 000/μL, total bilirubin < 1.5× the upper limit of normal, and aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase < 2.5× the upper limit of normal.
This protocol was approved by the Institutional Research Boards and Scientific Review Committees of Stanford University and the Fred Hutchinson Cancer Research Center. Informed consent was obtained in accordance with the Declaration of Helsinki.
Subjects were treated with imatinib 200 mg daily followed by dose escalation to 400 mg daily after 4 weeks if no side effects were experienced and complete resolution of cGVHD manifestations did not occur. Because the safety of imatinib was unproven, the first 6 participants were enrolled following an incremental accrual plan in which the first 3 participants were observed for at least 4 weeks for toxicities before the second 3 participants could be enrolled. In turn, the second 3 participants were observed for at least 4 weeks for toxicities before the trial could be fully opened to accrual.
Participants received imatinib 200 to 400 mg daily in 100-mg increments as tolerated. Mandatory clinical evaluations were performed at 1, 2, 4, and 6 months. Each participant's cGVHD was evaluated at each visit by the same attending physician and the same mid-level practitioner or bone marrow transplant fellow. After 6 months, clinical evaluations were performed at the attending physician's discretion. Leukocyte, erythrocyte, platelet, and serum electrolyte measurements were performed 1 week after any imatinib dose adjustments to monitor for side effects. Fifteen patients were enrolled between September 24, 2008 and August 14, 2009: 12 from Stanford University and 3 from the Fred Hutchinson Cancer Research Center. Subject characteristics are presented in Table 1. The median duration of follow-up was 56.6 weeks (range, 18-82.4 weeks).
The primary endpoint was the safety of daily imatinib administration. Adverse events were graded according to Common Terminology Criteria for Adverse Events Version 3.0 criteria. The secondary endpoint was the clinical effect of imatinib on cGVHD. Responses were graded according to the Hopkins scale for cGVHD assessment modified to exclude longstanding irreversible lesions.33 This scale scores cGVHD responses in 6 domains (lichenoid/erythematous rash, scleroderma, fascial involvement, oral symptoms, oral examination findings, and liver disease) on a 4- to 5-point scale. A major response was defined as a decrease by 2 in any domain without worsening in the others. A minor response was defined as a decrease by 1 in any domain without worsening in the others. Stable disease was defined as the absence of clinically significant changes from baseline. Progressive disease was defined as worsened manifestations of cGVHD requiring the initiation of new immunosuppressive medications. Subjects who experienced mild worsening of cGVHD symptoms not requiring the initiation of a new immunosuppressive medication continued treatment with imatinib while prednisone was temporarily increased; prednisone was dosed at the discretion of the attending physician and continued until symptoms resolved or progression of symptoms required initiation of a new immunosuppressant. Responses were also scored according to National Institutes of Health consensus criteria for cGVHD.34
Clinical responses were assessed at 24 weeks and last follow-up. Treatment failure was defined as progressive disease at any time or discontinuation of imatinib before 24 weeks because of toxicity, noncompliance, or withdrawal of consent for any reason. Overall response was defined as a major or minor response. Subjects with stable disease were classified as nonresponders. Subjects were required to complete 24 weeks of therapy to be considered clinically evaluable. Subjects with progressive disease at any time were considered clinically evaluable.
Surrogates for clinical response were reduction in daily prednisone dose and change in symptom burden as measured by the Lee cGVHD symptom scale.5 For subjects receiving a dose > 0.5 mg/kg prednisone at study entry, a 50% dose reduction was required to be considered clinically significant. For those receiving 0.5 to 0.25 mg/kg prednisone, a 25% dose reduction was required; and for those receiving ≤ 0.25 mg/kg prednisone, a total of 10 mg/day or less, or < 0.0625 mg/kg per day, was required. A change more than or equal to 7 points on the Lee cGVHD scale was considered clinically significant. Surrogate endpoints were correlated with clinical responses using the Spearman rank correlation coefficient. Evidence for clinical activity of imatinib was defined as a major response, minor response, or stable disease with a clinically significant reduction in daily prednisone requirement.
Correlative studies
Primary staining for PDGFRA, phosphorylated PDGFRA (polyclonal, 1:300 dilution, Santa Cruz Biotechnology), PDGFRB, and phosphorylated PDGFRB (polyclonal, 1:400 dilution, Santa Cruz Biotechnology) was performed on formalin-fixed, paraffin-embedded tissue sections of skin biopsy specimens obtained before and after 24 weeks of imatinib treatment. Secondary staining using rabbit antigoat alkaline phosphatase conjugate was followed by color development with Vulcan Fast Red Chromagen. Sections were counterstained with Automated Hematoxylin (Dako North America, S3301). Dermal layer spindle cell (fibroblast) staining for phosphorylated and nonphosphorylated PDGFRA and PDGFRB was graded on a 0 to 3 scale: 0 indicates no staining; 1, > 25% staining; 2, 25% to 75% staining; and 3, > 75% staining. Immunohistochemical staining was independently interpreted twice in a blinded manner and the grading averaged.
Anti-PDGFRA antibodies were detected by immunoblot and confirmed by ELISA using the extracellular fragment of PDGFRA (R&D Systems). PDGFRA extracellular fragment in carbonate solution was deposited overnight at 4°C onto 96-well ELISA plates (Nunc). Wells were blocked with 2% milk/TBST solution followed by probing with subject plasma diluted 1:50 in 2% milk/TBST. Secondary detection was through monoclonal alkaline phosphatase-conjugated goat antibody directed against the Fc portion of human IgG. P-nitrophenyl phosphate substrate color development occurred over 50 minutes and was detected at 405 nm.
The ability of anti-PDGFRA antibodies to stimulate PDGFRA (defined by autophosphorylation) was determined using an in vitro human fibroblast bioassay. Fibroblasts (CRL-2091, ATCC) natively expressing PDGFRA were plated at 1 million cells per 2.2-cm-diameter well and grown to confluence. Fibroblasts were starved 24 hours in serum-free media. A total of 400 μg protein G purified antibody from each subject with anti-PDGFRA antibody detectable by ELISA or Western blot was incubated with the fibroblasts for 5, 60, and 720 minutes. Fibroblasts were lysed in ice-cold Triton-X 100 buffer with sodium vanadate. Lysates were immunoprecipitated with anti-PDGFRA antibody (C-20, Santa Cruz Biotechnology), separated by electrophoresis, transferred onto nitrocellulose, and probed for phosphorylated tyrosine residues with mouse monoclonal antiphosphotyrosine antibody (4G10, Millipore).
Results
Safety of imatinib
The disposition of study participants at last follow-up is shown in Figure 1. Imatinib was dose escalated in 12 of 15 subjects. In 3 cases (patients 4, 9, and 12), the dose of imatinib was reduced because of nausea and vomiting, tachycardia and hypertension, and severe fatigue, respectively. Four cases (patients 2, 8, 13, and 14) withdrew study consent because of myalgia, fatigue, diarrhea with failure to thrive, and concerns about impaired wound healing after worsening lower leg ulceration and edema after solar exposure while sailing despite the admonitions of his physician, respectively. Patient 3 experienced progressive cGVHD and was removed from the study. Patient 1 developed cryptogeneic organizing pneumonia after 78 weeks of treatment and having previously discontinued prednisone for 51 weeks. This subject was removed from the study for toxicity. Two subjects (patients 6 and 15) tolerated 400 mg daily but tapered and discontinued imatinib at weeks 77 and 32, respectively, after reaching a response plateau. At week 25 of treatment, patient 6 developed an unidentifiable seasonal viral illness with pulmonary infiltrates on CT scan. Finally, 1 case (patient 7) continues treatment with imatinib 400 mg daily.
Disposition of trial participants. Dose of imatinib received, duration of treatment with imatinib, and reason for discontinuation of imatinib are shown.
Disposition of trial participants. Dose of imatinib received, duration of treatment with imatinib, and reason for discontinuation of imatinib are shown.
Six subjects were treated with 200 mg daily, of which 3 were dose reduced from 400 mg daily and 3 (patients 10, 11, and 5) were not dose escalated because of liver function test (LFT) elevation, nausea, or myalgia, which developed after imatinib started. Patient 4 was removed for noncompliance. Patient 11 was removed for progressive cGVHD. Two subjects (patients 5 and 9) were removed for toxicity. Patient 5 experienced severe (grade 3) vascular insufficiency that was attributed to a preexisting condition and not imatinib after recurrent polycythemia vera was ruled out. Patient 9 developed respiratory failure because of H1N1 infection and died. Finally, 2 subjects (patients 10 and 12) continue treatment with imatinib 200 mg daily.
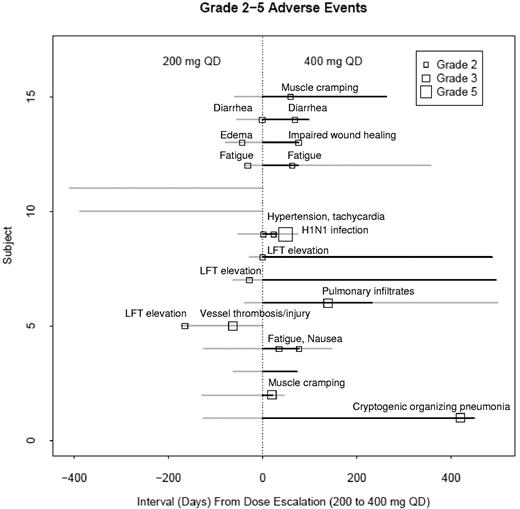
The prevalence of all toxicities possibly, probably, and definitely attributed to imatinib is reported in Table 2, and their relationship to imatinib dose is shown in Figure 2. The median period of observation was 56.6 weeks. No deaths were probably or definitely attributed to imatinib. Grade 1 adverse events were almost twice as prevalent at the 200-mg daily dose as at the 400-mg daily dose (2.7 events/subject vs 1.6 events/subject). Grade 1 toxicities occurring in ≥ 20% subjects were nausea, edema, confusion, diarrhea, LFT elevation, fatigue, and myalgia. These toxicities were self-limited or resolved with minimal symptomatic treatment. In contrast, grade 2 to 5 (moderate to death-associated) adverse events were almost 3 times as prevalent at the 400-mg daily dose than at the 200-mg daily dose (1.17 events/subject vs 0.4 events/subject).
Adverse event severity in relation to imatinib dose and duration of imatinib treatment. Grade 2, 3, and 5 adverse events occurred more frequently on imatinib 400 mg than 200 mg daily. No grade 4 adverse events occurred. Only those adverse events possibly, probably, or definitely attributed to imatinib are shown. Gray represents 200 mg imatinib daily; and black, 400 mg imatinib daily.
Adverse event severity in relation to imatinib dose and duration of imatinib treatment. Grade 2, 3, and 5 adverse events occurred more frequently on imatinib 400 mg than 200 mg daily. No grade 4 adverse events occurred. Only those adverse events possibly, probably, or definitely attributed to imatinib are shown. Gray represents 200 mg imatinib daily; and black, 400 mg imatinib daily.
cGVHD responses to imatinib
At 24 weeks, the overall response rate based on Hopkins criteria was 33% (5 of 15): one participant achieved a major response (patient 11) and 4 achieved a minor response (patients 2, 4, 7, and 12). Treatment failure was observed in 33% (5 of 15) of subjects, including 2 who withdrew consent (patients 13 and 14), 2 who required drug discontinuation because of toxicity (patients 5 and 9), and one who developed progressive disease (patient 3). The 5 remaining participants had stable disease (patients 1, 6, 8, 10, and 15).
At last follow-up, the overall response rate was 40% (6 of 15). This included 2 subjects who achieved a major (patient 12) or minor response (patient 7) and continued imatinib, one who achieved a major response but tapered imatinib because he reached a response plateau (patient 6), and 3 who achieved a major or minor response but discontinued imatinib because of noncompliance or withdrawal of consent (patients 2, 4, and 8). The overall treatment failure rate was 40% (6 of 15). This included 2 subjects with progressive disease (patients 3 and 11), 2 who withdrew consent before 24 weeks (patients 13 and 14), and 2 who were removed from the trial before 24 weeks for toxicity (patients 5 and 9). The 3 remaining patients were classified as stable disease (patients 1, 10, and 15), including one (patient 1) who was removed from the study after developing cryptogenic organizing pneumonia (COP) during the 78th week of therapy and one (patient 15) who tapered imatinib without disease progression.
Details about organ system responses in patients who received at least 24 weeks of imatinib as well as those with progressive disease are presented in Table 3. At baseline, the most common manifestations were cutaneous (9/11) and oral (9/11). Subjects commonly had lichenoid, sclerodermatous, and fascial disease with 5 of 9 having all 3 and 8 of 9 having fascial, sclerodermatous, or both. At last follow-up, improvement in skin/connective tissue disease was seen in 5 of 9 subjects, with 4 of these 5 having sclerotic/fasciitic disease. Improvement in oral disease was seen in 4 of 9 subjects. Participants with total bilirubin > 1.5 times the upper limit of normal were excluded from this study. Six of 11 participants had other abnormal LFTs at baseline possibly related to cGVHD, although drug effect and occult infection could not be excluded. At last follow-up, 3 of 6 had normal LFTs. Ocular cGVHD was present in 8 of 11 participants. At baseline, the average sum of ocular symptom scores by the Lee scale was 9.5 (range, 0-19). There was no statistical difference in the ocular scores at last follow-up (average score, 6.25; range, 2-10). Lung cGVHD was present in one subject (patient 4). At baseline, peak flow and FEV1 were 2 and 2.96 L (75% predicted), respectively, compared with 2.61 and 3.28 L (84% predicted) at last follow-up. During this interval, the subject tapered his daily prednisone dose from 25 mg to 5 mg. Esophageal strictures secondary to cGVHD were present in one participant (patient 10). This subject underwent bougienage twice before starting imatinib and again after 8 weeks of treatment.
Temporal relationship between clinical assessments, surrogates of response, and imatinib dose
The onset of clinical responses to imatinib was correlated with the dose of imatinib to determine whether the 200-mg dose was adequate for PDGFRA inhibition, as suggested by preclinical pharmacodynamic studies (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Initial responses to imatinib were observed in 4 subjects (patients 3, 4, 10, and 11) while on the 200-mg dose without prior treatment at the 400-mg dose. One subject (patient 6) achieved a response while on the 200-mg dose but was immediately treated with the 400-mg dose beforehand. Initial responses to imatinib were observed in 3 subjects (patients 7, 8, and 12) only after escalation to the 400-mg dose. These data suggest that the 200 mg daily dose of imatinib may be clinically active.
Correlation of clinical assessments with daily corticosteroid requirements and cGVHD symptom burden
Clinical assessments were correlated with serial changes in the daily prednisone requirement and the Lee cGVHD symptom burden score to corroborate cGVHD responses (Table 4; supplemental Figure 1). Of the clinically evaluable subjects, we found that cGVHD responses segregated into 4 groups: (1) improvement accompanied by a clinically significant (patients 4, 6, 7, and 12) or nonsignificant (patient 8) decrease in corticosteroid dose; (2) progression accompanied by increasing doses of corticosteroids (patients 3 and 11); (3) stable disease accompanied by a clinically significant decrease in corticosteroid dose (patients 1 and 10); and (4) stable disease without clinically significant changes in corticosteroid dose (patients 2 and 15). Correlation between reduction in corticosteroid dose and clinical assessments was statistically significant (P < .01) in patients 6, 7, and 12, whereas correlation between reduction in Lee symptom score and clinical assessments was statistically significant (P < .01) in patients 6, 7, and 8. Groups 1 and 3 identify a subset of clinically evaluable patients in whom imatinib may have had clinical activity.
Pharmacodynamic effects of imatinib on PDGFRA phosphorylation
Immunohistochemical staining for PDGFRA, phosphorylated PDGFRA, PDGFRB, and phosphorylated PDGFRB was performed for 4 skin-involved subjects with paired skin biopsies collected before and after treatment with imatinib (Table 5; Figure 3). Differences in cell density between biopsies were controlled for by calculating the ratio of phosphorylated receptor to total receptor. PDGFRB phosphorylation was decreased in patient 6, who experienced a major response, and PDGFRA phosphorylation was decreased in patient 1, who had stable disease and a clinically significant reduction in daily corticosteroid dose. These data are consistent with imatinib affecting cGVHD by interruption of PDGFR signal transduction. In contrast, no phosphorylation changes occurred in patient 12, who experienced a major response, suggesting that imatinib may also affect cGVHD by binding to its other receptor targets. Finally, PDGFRB phosphorylation was decreased in patient 11 who ultimately developed progressive cGVHD. Patient 11 experienced flares of cGVHD after viral infections with subsequent improvement on imatinib suggesting that, although imatinib may have been exerting inhibitory effects at PDGFR leading to response, other ongoing immune inputs occurred, driving cGVHD progression.
Pharmacodynamic effects of imatinib. *Antiphosphorylated PDGFRA staining (original magnification × 400) before (A) and after (B) imatinib treatment (patient 1) and *antiphosphorylated PDGFRB staining (original magnification × 400) before (C) and after (D) imatinib treatment (patient 11). Microscope: Olympus BX45; camera: Olympus DP25; acquisition software: CellSens Standard (Olympus).
Pharmacodynamic effects of imatinib. *Antiphosphorylated PDGFRA staining (original magnification × 400) before (A) and after (B) imatinib treatment (patient 1) and *antiphosphorylated PDGFRB staining (original magnification × 400) before (C) and after (D) imatinib treatment (patient 11). Microscope: Olympus BX45; camera: Olympus DP25; acquisition software: CellSens Standard (Olympus).
Correlation of response with anti-PDGFRA antibodies
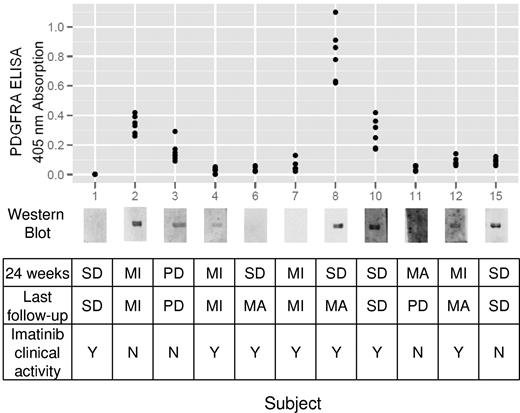
The presence of anti-PDGFRA antibodies was determined in plasma samples obtained before imatinib treatment (Figure 4). When pretreatment samples were not available, plasma samples taken 1 week after initiation of imatinib were used. Anti-PDGFRA antibodies were detected by immunoblotting in 7 of 11 participants. These findings were confirmed by ELISA, which in general correlated a high absorption reading with a high-intensity chemiluminescent immunoblot band, although there were some exceptions, such as patient 15 in whom a high-intensity band did not correlate with comparable ELISA readings.
Anti-PDGFRA antibody status and clinical responses. Immunoblot detection of anti-PDGFRA antibodies and ELISA confirmation are shown with corresponding clinical outcomes for each subject. MI indicates minor response; MA, major response; PD, progressive disease; and SD, stable disease.
Anti-PDGFRA antibody status and clinical responses. Immunoblot detection of anti-PDGFRA antibodies and ELISA confirmation are shown with corresponding clinical outcomes for each subject. MI indicates minor response; MA, major response; PD, progressive disease; and SD, stable disease.
Agonistic antibodies have been hypothesized to bind to PDGFRA and induce tyrosine phosphorylation and further downstream events resulting in a cGVHD phenotype. Given imatinib's antagonistic effects at PDGFRA, we expected that participants who respond to imatinib would have anti-PDGFRA antibodies. Of the 7 participants found to have anti-PDGFRA antibodies before treatment, 4 demonstrated evidence for imatinib clinical activity, 2 did not respond to imatinib, and 1 had progressive disease. The agonistic ability of these antibodies to induce PDGFRA phosphorylation was tested in an in vitro fibroblast bioassay to determine whether there was a qualitative difference between anti-PDGFRA antibodies found in imatinib responders versus nonresponders. No PDGFRA phosphorylation could be detected by immunoblot after 5, 60, and 720 minutes of antibody exposure, whereas robust phosphorylation was evident after 5 minutes of 12.5 μg PDGF-BB incubation (data not shown). These findings suggest that response to imatinib is not restricted to patients with detectable anti-PDGFRA antibodies and that these anti-PDGFRA antibodies do not stimulate PDGFRA auto-phosphorylation.
Discussion
Fifteen patients with steroid-dependent/refractory cGVHD were treated with 200 mg or 400 mg imatinib daily in this phase 1 dose escalation trial. A dose of 200 mg was found to be safe with fewer serious adverse events and fewer participants withdrawing consent or requesting a dose reduction compared with the 400-mg dose. At a median of 56.6 weeks of follow-up, the overall response and treatment failure rates were similar at 40% (6 of 15). Four of the 6 responders had sclerotic/fasciitic disease, consistent with sclerotic cGVHD responses in 2 reported trials.31,32 cGVHD responses were observed with both 200- and 400-mg doses. Moreover, the 200-mg response rate may be underestimated because of the scheduled escalation from 200 mg to 400 mg. This suggests that 200 mg may be an adequate dose for cGVHD treatment. Equivalency to the 400-mg dose, however, cannot be concluded without direct comparison in a randomized trial. Taken together, these data support testing the 200-mg dose in future phase 2 trials for cGVHD.
Our toxicity results differ from a previously reported prospective trial of imatinib for cGVHD.31 We observed a prevalence of nonhematologic adverse events rather than primarily hematologic adverse events. Our study cohort had a longstanding history of cGVHD and immunosuppressant use; thus, they were more likely to have other comorbidities and increased susceptibility to side effects. Moreover, the cGVHD in our cohort appeared to be more refractory, as suggested by the high number of previous therapies for control of the disease. Finally, the 400-mg dose of imatinib used in the dose escalation schema of this trial was higher than previously reported in the cGVHD setting.
The lower overall response rate to imatinib in our study compared with previous reports may have been because of several factors, including differences in the assessment systems and in the refractoriness or severity of cGVHD between the studies. Two methods were used in our study to grade responses with the Hopkins scale yielding higher response rates than the National Institutes of Health consensus cGVHD criteria (data not shown). This may be because of greater sensitivity to sclerotic changes (the predominant feature in this trial's population) as recently reported by Jacobsohn et al.35 Because 5 of 6 criteria for the Hopkins score are based on physical examination or reported symptoms, assessments of response to imatinib are limited by an element of subjectivity. Assessing treatment response in cGVHD is complicated: the best tool for assessing cGVHD is being actively investigated, and the great variability in response evaluation makes appropriate comparisons between studies difficult.
Anti-PDGFRA antibodies were present in a smaller proportion of our subjects compared with a previous retrospective study of anti-PDGFRA antibody frequency.16 We found no correlation between anti-PDGFRA antibodies and response to imatinib. We also did not observe any induction of PDGFRA phosphorylation by these antibodies when tested by an in vitro fibroblast bioassay. These findings do not support the hypothesis that agonistic antibodies against PDGFRA are involved in the pathogenesis of cGVHD. Other investigators have similarly been unable to detect any PDGFR-stimulating ability in anti-PDGFR antibodies in scleroderma.36,37 Methodologic laboratory issues may have contributed to the differences between our study and published studies; anti-PDGFRA antibodies and phosphorylation changes in PDGFRA were directly detected with specific antibodies rather than indirectly detected by measuring reactive oxygen species generated by secondary processes occurring after PDGFRA stimulation.16
In a limited number of patients, PDGF receptor phosphorylation in skin was reduced after imatinib treatment in the context of improving cGVHD. Taken with the antibody studies discussed in the preceding paragraph, these data suggest that the PDGF receptor may yet play a role in cGVHD pathogenesis, activated not by agonistic anti-PDGFRA antibodies but by a still-to-be-discovered stimulus that imatinib abrogates at the receptor level. In the same context of improving cGVHD, no changes in PDGF receptor phosphorylation after imatinib treatment were also observed. Given the heterogeneous nature of cGVHD, this might be explained by the inhibitory action of imatinib on its other receptor targets, which may play a role in cGVHD pathogenesis.
The known targets of imatinib and their IC50 values are: KIT (27nM), PDGFR (39nM), DDR1 (43nM), DDR2 (141nM), ABL (649nM), and the metabolic enzyme NQO2 (1000nM).29,38-40 Based on published pharmacokinetic data, a dose of imatinib 200 mg would partially inhibit ABL, which has a role in fibrogenesis, theoretically justifying higher dosing.41,42 Future investigations could focus on the role of ABL in cGVHD to determine whether a lower dose of imatinib can be used with fewer side effects. Finally, these alternative signal cascades could reconcile the discrepancy between our results and previously reported results, which show anti-PDGFRA antibody-induced reactive oxygen species production, possibly because of non–PDGF-mediated signaling pathways. Because the targets of imatinib are known, these alternative pathways could represent an investigative and therapeutic handle onto the pathophysiology of cGVHD for testing in future clinical trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this study and all the BMT nurses, patient coordinators, and staff at Stanford University and the Fred Hutchinson Cancer Research Center who made this work possible, as well as Dr Philip L. McCarthy for reviewing the manuscript and helpful discussion.
This work was supported by Novartis. G.L.C. was supported by a T32 BMT training grant (Stanford University, PI Dr Robert Negrin) and a postdoctoral fellowship with D.B.M.
Authorship
Contribution: G.L.C. and D.B.M. implemented the trial and correlative studies, analyzed data, and wrote the manuscript; S.A. and M.E.D.F. contributed patients and reviewed the manuscript; J.M.O. performed research management; E.C.C. performed experiments; L.J.J. and J.A.S. contributed patients; J.Q. performed pathologic review; and A.M. provided statistical support.
Conflict-of-interest disclosure: D.B.M. received honoraria for scientific advising from Novartis. The remaining authors declare no competing financial interests.
Correspondence: David B. Miklos, 300 Pasteur Dr, MC 5623, Stanford, CA 94309; e-mail: dmiklos@stanford.edu.